Abstract
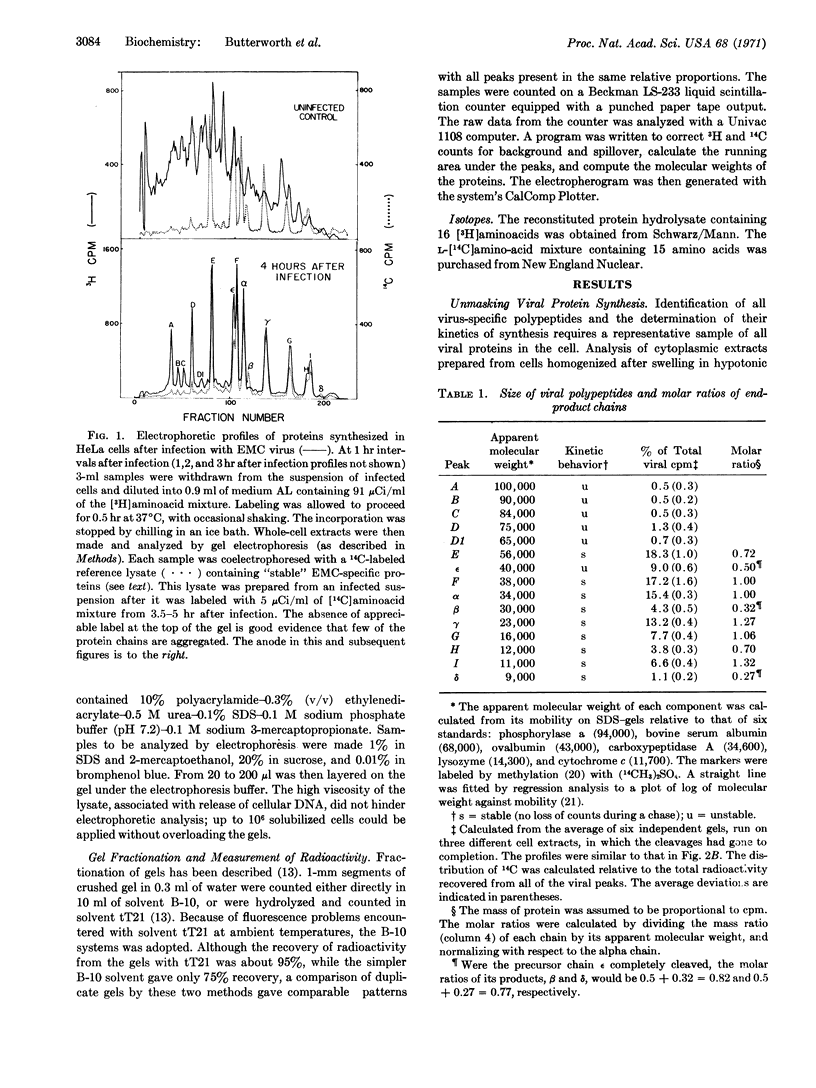
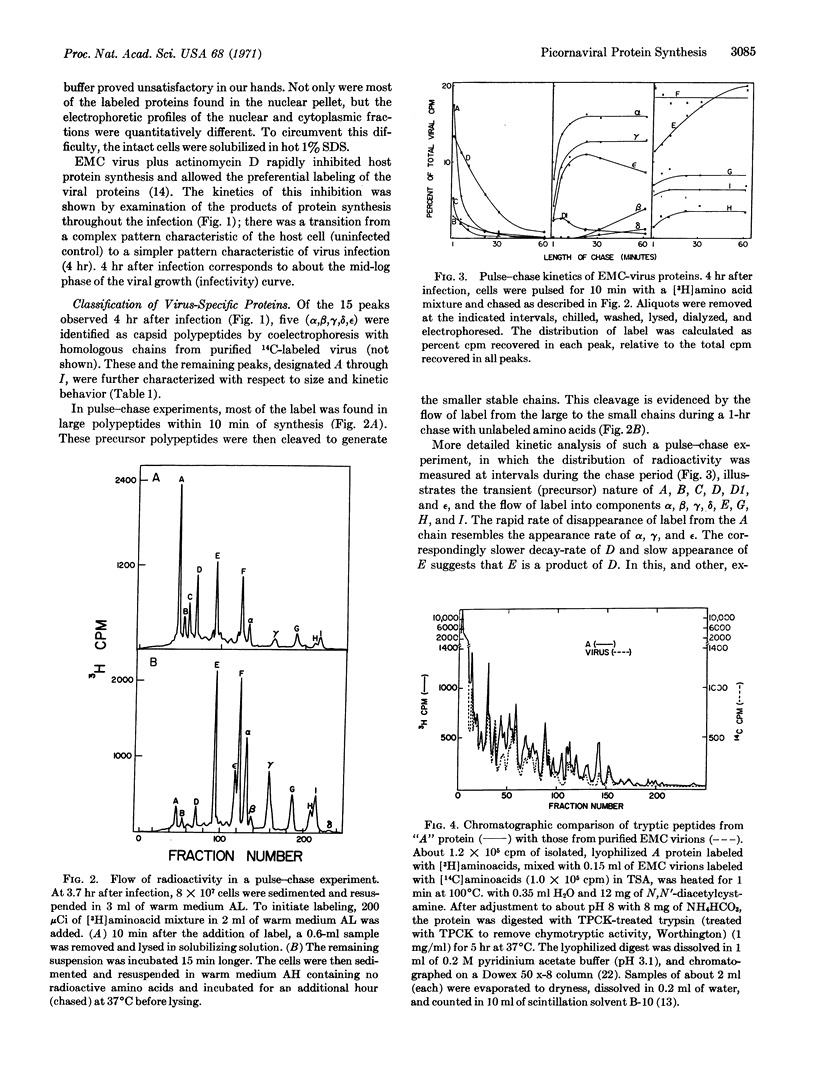
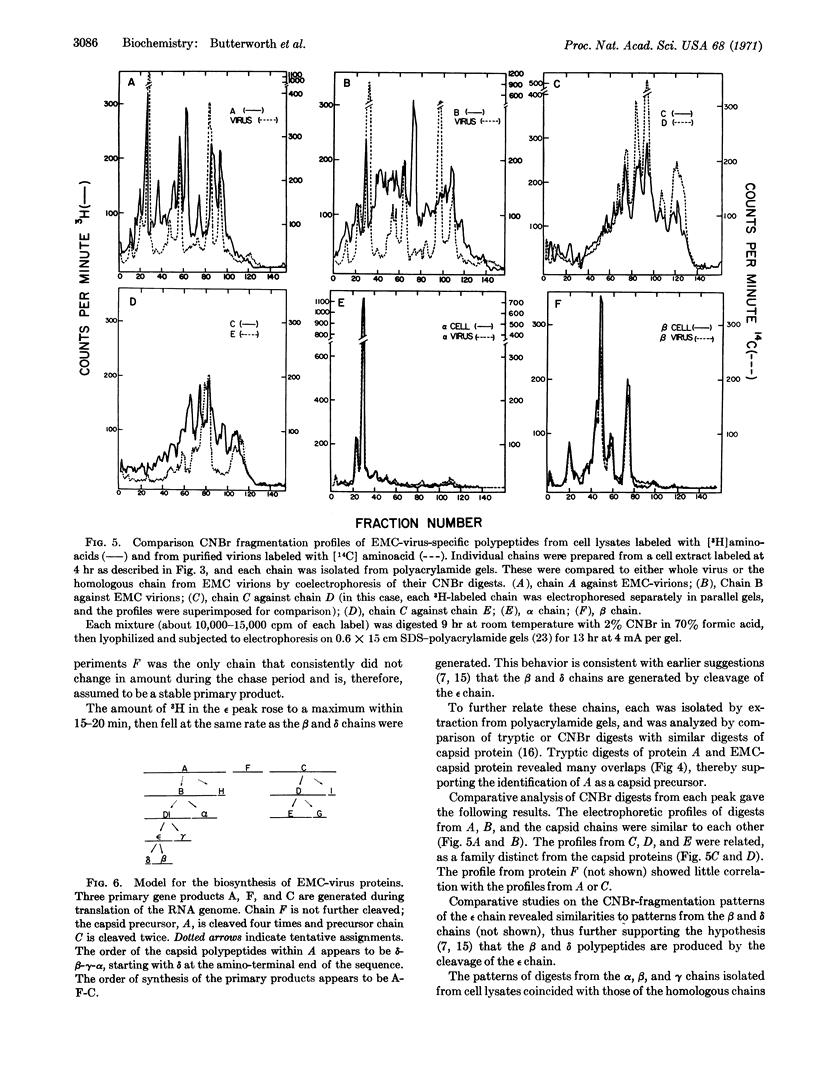
The in vivo synthesis of encephalomyocarditis-specific proteins was studied by labeling the viral proteins with radioactive amino acids under conditions where host-protein synthesis was almost completely inhibited. To assure recovery of all proteins, intact cells were lysed in hot 1% sodium dodecyl sulfate. These lysates were analyzed by quantitative high-resolution electrophoresis on sodium dodecyl sulfate-polyacrylamide gels. This technique allowed the detection and estimation of the molecular weight of 15 virus-specific polypeptides: A, 100,000; B, 90,000; C, 84,000; D, 75,000, D1, 65,000; E, 56,000; ε, 40,000; F, 38,000; α, 34,000; β, 30,000; γ, 23,000; G, 16,000; H, 12,000; I, 11,000; and δ, 9,000. Pulse-chase experiments, in conjunction with cyanogen bromide and tryptic mapping of the isolated polypeptides, indicate that at least three primary gene products (A,F,C), with a cumulative weight of about 220,000, are generated during translation of the RNA genome. Chains A and C then undergo post-translational cleavages, while F remains uncleaved. The proteins generated by the cleavage of A include all of the capsid chains (α, β, γ, δ, ε). Those generated by the cleavage of C include D and E. The chains α, β, γ, δ, E, F, G, H, I, with a cumulative molecular weight of about 230,000, are stable and are produced in about equimolar amounts. A model for the synthesis of, and a cleavage sequence that accounts for, all of the viral polypeptides is proposed.
Keywords: gel electrophoresis, tryptic mapping, cyanogen bromide mapping
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burness A. T. Ribonucleic acid content of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):373–380. doi: 10.1099/0022-1317-6-3-373. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Summers D. F., Maizel J. V. Evidence for ambiguity in the posttranslational cleavage of poliovirus proteins. Virology. 1970 Jul;41(3):408–418. doi: 10.1016/0042-6822(70)90161-3. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Hall L., Rueckert R. R. Infection of mouse fibroblasts by cardioviruses: premature uncoating and its prevention by elevated pH and magnesium chloride. Virology. 1971 Jan;43(1):152–165. doi: 10.1016/0042-6822(71)90233-9. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Synthesis and cleavage of enterovirus polypeptides in mammalian cells. J Virol. 1970 Mar;5(3):358–367. doi: 10.1128/jvi.5.3.358-367.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Roumiantzeff M., Summers D. F., Maizel J. V., Jr In vitro protein synthetic activity of membrane-bound poliovirus polyribosomes. Virology. 1971 May;44(2):249–258. doi: 10.1016/0042-6822(71)90257-1. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Dunker A. K., Stoltzfus C. M. The structure of mouse-Elberfeld virus: a model. Proc Natl Acad Sci U S A. 1969 Mar;62(3):912–919. doi: 10.1073/pnas.62.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scraba D. G., Kay C. M., Colter J. S. Physico-chemical studies of three variants of Mengo virus and their constituent ribonucleates. J Mol Biol. 1967 May 28;26(1):67–79. doi: 10.1016/0022-2836(67)90261-6. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



