We investigated the clinical role of left ventricular ejection fraction (LVEF) in patients with cancer treated with phase I clinical trials.
The overall survival of patients with LVEF ≤ 35% was shorter compared to those with LVEF between 35% and 50%, which was similar to those with LVEF ≥ 50%.
Echocardiography would improve patient selection for enrollment in phase I clinical trials.
Keywords: biomarker, cardiac dysfunction, malignancy and phase I trial
Abstract
Background
New targeted agents may cause acute cardiac events. The purpose of our study was to investigate the incidence and the prognostic significance of left ventricular ejection fraction (LVEF) in phase I trials.
Patients and methods
Between October 2008 and September 2011, the records of 1166 consecutive patients with advanced cancer treated in the Phase I Clinic who underwent echocardiography were retrospectively reviewed.
Results
Most of the patients were White (78%), and the most common tumor types were colorectal cancer and melanoma. Of 1166 patients, 177 (15.2%) patients had an LVEF of <50%. No difference in overall survival (OS) between patients with LVEF ≥ 50% and patients with LVEF < 50% was seen (median OS 7.4 versus 7.0 months, P = 0.84). Patients with LVEF ≤ 35% had shorter survival compared with those with LVEF between 35% and 50% (median 4.2 versus 8.0 months; P = 0.005). In multivariate analysis of patients with LVEF < 50%, independent factors predicting longer survival were LVEF > 35%, ≤2 prior systemic therapies, ≤2 metastatic sites, and normal lactate dehydrogenase and albumin levels.
Conclusion
Echocardiography would improve patient selection for enrollment in phase I clinical trials. These data suggest that it is safe to treat patients with LVEF between 35% and 50%.
introduction
Targeted cancer therapies are showing promise in the treatment of patients with cancer in the setting of phase I clinical trials, but concerns have been raised about their potential cardiotoxicity [1]. Phase I clinical trials are designed to determine the dose-limiting toxicity (DLT) and the maximum tolerated dose (MTD) of new agents or the phase II recommended dose. Some targeted agents may cause acute cardiac events, including left ventricular dysfunction [2]. The use of trastuzumab, a monoclonal antibody against human epidermal growth factor receptor 2 (HER2), is associated with improved clinical outcomes in breast cancer [3] and gastro-esophageal junction adenocarcinoma [4], but in selected patients it is associated with a decrease in left ventricle (LV) systolic function [3]. In addition, the use of anti-vascular endothelial growth factor (VEGF) agents may be associated with cardiotoxicity. Selected Food and Drug Administration-approved anti-VEGF agents, bevacizumab, sunitinib, and sorafenib are associated with grade 3–4 LV systolic dysfunction in 0.3%, 1.4%, and 0.05% of patients, respectively [2]. The use of vascular-disrupting agents has also been associated with cardiovascular events [5, 6].
The occurrence and the outcomes of LV dysfunction caused by standard chemotherapy were reported previously [7–9]. However, the clinical outcomes of patients with advanced cancer and low left ventricular ejection fraction (LVEF) treated in phase I clinical trials have not been systematically analyzed. Therefore, we analyzed consecutive patients with advanced cancer who were treated in our phase I clinic and had undergone echocardiography. The study objectives were to determine the incidence of LV systolic dysfunction, as defined by LVEF < 50%, to describe the clinical characteristics of patients with LVEF < 50%, and to compare their outcomes to those of patients with LVEF ≥ 50%.
methods
patients
We retrospectively reviewed the medical records of 1166 consecutive patients who (a) were referred to the Department of Cardiology for echocardiography and (b) were treated in the Phase I Clinical Trials Program from October 2008 through September 2011. Echocardiography was carried out as a screening procedure for patient participation in a clinical trial or as part of the standard of care. Patient characteristics and laboratory tests were recorded at the time of first echocardiogram (Supplementary data, available at Annals of Oncology online).
results
patient characteristics
The demographic characteristics and tumor histology subtypes of patients included in the study are summarized in Supplementary Table S1, available at Annals of Oncology online. The median age of 1166 patients was 60 years (range 16–82). Overall, 51.2% of patients were older than 60 years and 50.6% were women. The majority (77.8%) of patients were White. The most common tumor subtypes were colorectal cancer and melanoma, which reflects the pattern of referral to the Phase I Clinic. All patients with colorectal cancer received a bevacizumab-containing regimen before referral to the Phase I Clinic.
Of the 1166 patients who had an echocardiogram, 177 (15.2%) patients had an LVEF of <50%. The median ages of patients with LVEF < 50% and LVEF ≥ 50% were 63.4 and 57.0 years, respectively (P = 0.0079). Eighteen percent of men, compared with 13% of women, had an LVEF of <50% (P = 0.033). There was no statistical difference in LVEF levels by race (P = 0.39). Patients with sarcoma, renal cell carcinoma, pancreatic cancer, and thyroid cancer had the highest proportions of LVEF < 50% (23%, 22%, 20%, and 20%, respectively).
patients with LVEF < 50%
The median overall survival (OS) increases dramatically between LVEF of 30% and LVEF of 40% (Figure 1A), therefore; an LVEF of 35% provides a convenient cut point within the group of patients with LVEF < 50%. Characteristics of patients with LVEF ≤ 35% versus those of patients with LVEF between 35% and 50% are listed in Supplementary Table S2, available at Annals of Oncology online. Of the 177 patients with LVEF < 50%, 32 (18.1%) had an LVEF of ≤35% and 145 (81.9%) had an LVEF between 35% and 50%.
Figure 1.
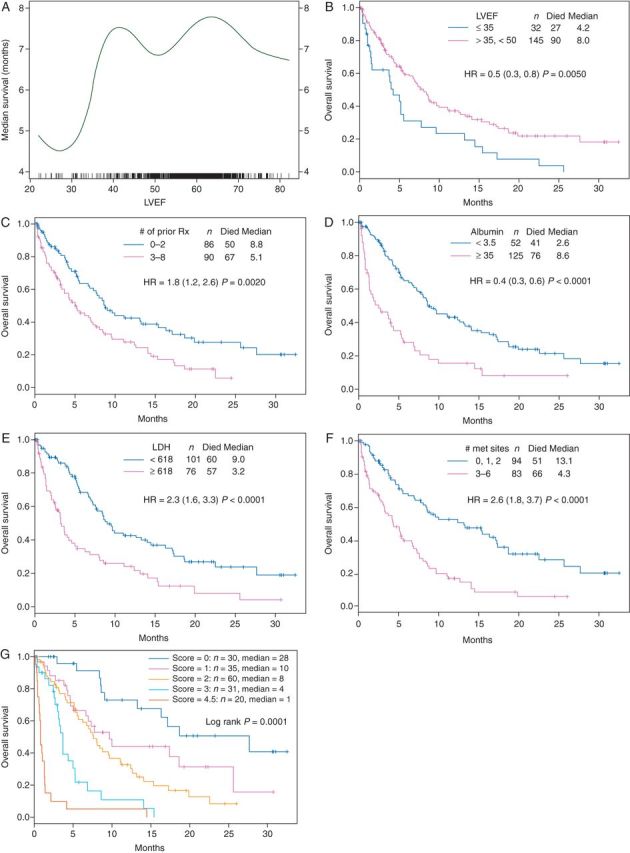
(A) The median OS curve by LVEF. (B) The Kaplan Meier OS curve by LVEF. (C) The Kaplan–Meier curve of OS by the number of prior treatments. (D) The Kaplan–Meier curve of OS by the albumin level. (E) The Kaplan–Meier curve of OS by the LDH level. (F) The Kaplan–Meier curve of OS by the number of metastatic sites. (G) The Kaplan–Meier curve of OS by the different risk scores.
A higher proportion of patients older than 60 years had an LVEF of ≤35% compared with those 60 years or younger (22.9% versus 10.3%, respectively; P = 0.034). The rates of other cardiac risk factors—hypertension, diabetes, dyslipidemia, coronary artery disease, smoking history, and body mass index (BMI) > 30—were not significantly different between the two LVEF groups. There was also no statistical difference between the two groups (LVEF < 35% versus LVEF 35%–50%) in the prior use of potentially cardiotoxic therapy (anthracyclines, trastuzumab, sunitinib, interferon, or radiation therapy).
A higher proportion of patients with low serum albumin levels (26.9%) had an LVEF of <35% compared with those with an LVEF of 35%–50% (P = 0.049). Other factors previously reported to predict clinical outcomes in phase I clinical trials, including Royal Marsden Hospital score, MD Anderson Cancer Center score, Lactate dehydrogenase (LDH) level, number of metastatic sites, number of prior therapy, Eastern Cooperative Oncology Group (ECOG) performance status, gastrointestinal tumor subtypes, and platelet count, were not important.
Appropriate therapy for low LVEF (including digoxin, β-blockers, angiotensin receptor blockers or angiotensin converting enzyme inhibitors, and others) was documented in 62% (n = 109) of patients with LVEF < 50%. Of 177 patients, 23 (13%) were on statin therapy and 154 (87%) were not. Among patients with LVEF < 35%, 84% (n = 27) were reported to receive appropriate therapy for their low LVEF.
Of 177 patients, 52 (29%) had the LDL measurement, 46 (26%) had the high-density lipoprotein (HDL) measurement, 65 (37%) had the troponin measurement, and 36 (20%) had the brain natriuretic peptide (BNP) measurement. Of 32 patients with LVEF < 35%, nine (28%) had BNP tested; of these nine patients, six (67%) had an elevated BNP level. Of 177 patients with LVEF < 50%, 146 (82%) had New York Heart Association (NYHA) class I, 22 (12%) had NYHA class II, and 9 (6%) had NYHA class III disease.
therapy in phase I clinical trials of patients with LVEF < 50%
Of the 177 patients with an LVEF of <50%, 118 (66.7%) underwent echocardiography while receiving treatment on a phase I clinical trial (first-in-human or combination therapy of Food and Drug Administration (FDA)-approved drugs) as part of the clinical trial requirement or as a standard-of-care evaluation, and the remaining 59 (33.3%) patients underwent echocardiography before receiving any treatment, as part of the screening for participation in a clinical trial or a standard-of-care evaluation.
Of the 118 patients who underwent echocardiography while receiving treatment, 97 (82%) were treated on a clinical trial that consisted of targeted therapy as a single agent or in combination. Of the remaining 21 patients (18%), 4 were treated with cytotoxic therapy and 17 with a combination of targeted therapy and cytotoxic agents. Sixty-nine (58.5%) patients were treated on first-in-human clinical trials and 49 (41.5%) patients were enrolled on phase I clinical trials of FDA-approved drugs.
overall survival
The median follow-up of patients with LVEF < 50% (n = 177) and patients with LVEF ≥ 50% (n = 989) was 5.0 and 5.2 months, respectively. There was no statistical difference in OS between patients with LVEF ≥ 50% and patients with LVEF < 50% (median 7.4 versus 7.0 months, respectively, P = 0.84). Subset analysis showed that patients with LVEF between 35% and 50% had longer OS than patients with LVEF ≤ 35% (8.0 versus 4.2 months, P = 0.005; Figure 1B).
On univariate analysis, hypertension, diabetes, dyslipidemia, coronary artery disease, smoking history, and prior use of anthracyclines, interferon, sunitinib, trastuzumab, or chest radiation were not important risk factors for OS (Table 1). Patients with BMI > 30 were found to have longer survival than patients with BMI ≤ 30 (median OS 15.5 versus 5.5 months, respectively; P = 0.033). There was no significant difference in OS between patients reported receiving appropriate treatment of low LVEF and patients who were not receiving any therapy for low LVEF (median 7.6 versus 5.6 months, respectively; P = 0.70).
Table 1.
Survival by cardiac risk factors in 177 patients with LVEF < 50%
| Risk factor | Number of patients (%) | Median survival (months) | 95% CI for median survival | Hazard ratio (HRa) | 95% CI | P-value |
|---|---|---|---|---|---|---|
| LVEF | 0.5 | 0.3–0.8 | 0.005 | |||
| >35% | 145 (82) | 8 | 6.7–10.0 | |||
| ≤35% | 32 (18) | 4.2 | 1.6–9.6 | |||
| Cardiac risk factors | ||||||
| Hypertension | 1 | 0.6–1.5 | 0.95 | |||
| Yes | 95 (54) | 8.8 | 7–13.2 | |||
| No | 82 (46) | 5.2 | 3.7–8.4 | |||
| Diabetes | 0.7 | 0.4–1.2 | 0.2 | |||
| Yes | 30 (17) | 8.1 | 4.7–NR | |||
| No | 143 (83) | 6.9 | 5.2–8.6 | |||
| Dyslipidemia | 1 | 0.6–1.8 | 0.96 | |||
| Yes | 16 (9) | 9.1 | 4.7–25.7 | |||
| No | 161 (91) | 6.9 | 5.2–8.6 | |||
| Coronary artery disease | 1.1 | 0.5–2.2 | 0.86 | |||
| Yes | 26 (15) | 8.1 | 4.2–NR | |||
| No | 151 (83) | 7.0 | 5.2–8.8 | |||
| History of smoking | 0.8 | 0.5–1.3 | 0.31 | |||
| Yes | 46 (26) | 8.1 | 6.3–18.8 | |||
| No | 131 (74) | 6.7 | 4.6–8.6 | |||
| Body mass index | 0.6 | 0.4–0.99 | 0.033 | |||
| >30 | 35 (20) | 15.5 | 9.1–20.0 | |||
| ≤30 | 142 (80) | 5.5 | 4.4–8.0 | |||
| Potentially cardiotoxic prior therapy | ||||||
| Anthracyclines use | 1.2 | 0.7–1.8 | 0.69 | |||
| Yes | 43 (24) | 5.1 | 4.2–13.2 | |||
| No | 134 (76) | 7.6 | 5.7–9.7 | |||
| Interferon use | 0.6 | 0.3–1.3 | 0.17 | |||
| Yes | 16 (9) | 8.6 | 5.2–NR | |||
| No | 161 (91) | 6.9 | 5.1–8.6 | |||
| Sunitinib use | 0.9 | 04–2.2 | 0.76 | |||
| Yes | 8 (4) | 7.4 | 4.2–NR | |||
| No | 169 (96) | 7.2 | 5.4–8.6 | |||
| Trastuzumab use | 0.82 | 0.5–2.7 | 0.82 | |||
| Yes | 9 (5) | 7.6 | 1.4–NR | |||
| No | 168 (95) | 7.0 | 5.3–8.8 | |||
| Radiation therapy to the chest | 1.2 | 0.6–2.2 | 0.64 | |||
| Yes | 21 (12) | 4.2 | 2.8–14.5 | |||
| No | 156 (88) | 7.6 | 5.7–9.6 | |||
| Risk factors in phase I studies | ||||||
| Albumin | 0.4 | 0.3–0.6 | <0.0001 | |||
| ≥3.5 | 125 (71) | 8.6 | 7.6–13.3 | |||
| <3.5 | 52 (29) | 2.6 | 1.4–5.2 | |||
| LDH | 2.3 | 1.6–3.3 | <0.0001 | |||
| <618 | 101 (57) | 9.1 | 7.6–14.5 | |||
| ≥618 | 76 (43) | 3.3 | 2.6–5.3 | |||
| Number of metastatic sites | 2.6 | 1.8–3.7 | <0.0001 | |||
| ≤2 | 94 (53) | 13.2 | 8.4–17.5 | |||
| >2 | 83 (47) | 4.3 | 3.3–6.8 | |||
| Number of prior therapies | 1.8 | 1.2–2.6 | 0.002 | |||
| ≤2 | 86 (49) | 8.8 | 7.3–16.3 | |||
| >2 | 90 (51) | 5.1 | 3.7–7.6 | |||
| ECOG | 1.8 | 1.0–2.9 | 0.025 | |||
| <1 | 28 (16) | 12.4 | 6.2–NR | |||
| ≥1 | 149 (84) | 6.7 | 5.1–8.4 | |||
| Gastrointestinal tumors | 2.4 | 1.5–3.6 | 0.0002 | |||
| Yes | 33 (19) | 4.0 | 1.4–7.0 | |||
| No | 144 (81) | 8.4 | 5.6–12.6 | |||
| RMH Score | 2.8 | 1.9–4.0 | <0.0001 | |||
| <2 | 111 (63) | 9.7 | 7.8–15.3 | |||
| ≥2 | 66 (37) | 3.2 | 2.1–4.4 | |||
| MD Anderson Score | ||||||
| 0–1 | 56 (32) | 18.7 | 16.3–NR | 1.0 | — | — |
| 2–3 | 98 (55) | 5.4 | 4.4–7.6 | 3.5 | 2.2–5.7 | <0.0001 |
| 4–5 | 23 (13) | 0.8 | 0.7–1.4 | 22 | 12–44 | <0.0001 |
| Platelet count | 0.8 | 03–2.6 | 0.73 | |||
| ≤400 000 | 172 (97) | 6.9 | 5.2–8.6 | |||
| >400 000 | 5 (3) | 16.0 | 7–NR | |||
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; NR, not reached; RMH, Royal Marsden Hospital.
aHR < 1 is associated with longer survival. Significant P values of <0.05 are shown in bold.
In addition, patients who received ≤2 prior therapies had longer OS compared with those who received >2 prior therapies (8.8 versus 5.1 months, respectively; P = 0.002) (Figure 1C). Other factors associated with longer survival in univariate analysis were normal serum albumin levels (versus lower than normal; P < 0.0001; Figure 1D), normal LDH levels (versus higher than normal; P < 0.0001; Figure 1E), 0–2 metastatic sites (versus with >2 metastatic sites; P < 0.0001) (Figure 1F), ECOG performance status 0 (versus ≥1); P = 0.025, (<1 versus ≥1), and gastrointestinal tumor subtypes (versus others, P = 0.0002).
We also examined whether in the patients with LVEF < 50% there was an association between OS and the type of therapy (first-in-human therapy versus FDA-approved drugs in new combinations/non-FDA approved indications). We found no statistically significant difference in OS between the two groups [median survival 7 months, 95% confidence interval (CI) 4–14, versus 8 months, 95% CI 5–16, respectively; Hazard Ratio (HR), 0.8; 95% CI 0.5–1.3; P = 0.41]. Similarly, there was no difference in survival between patients treated with targeted therapy and those treated with chemotherapy combined with targeted agents (data not shown). The median OS durations of patients with NYHA class I versus class II plus III were 7.0 versus 8.1 months, respectively (HR = 1.1, 95% CI 0.7–1.8; P = 0.72). The median OS of patients who were receiving statins was 6.7 versus 7.2 months for patients who were not receiving statins (HR = 0.9, 95% CI 0.57–1.5; P = 0.67).
independent prognostic factors and the prognostic survival score
Among the patients with LVEF < 50%, multivariate analysis demonstrated that the following variables were independent factors predicting longer survival: LVEF > 35% (P = 0.0016), normal albumin level (P = 0.0001), 0–2 metastatic sites (P = 0.0004), 0–2 prior therapies (P = 0.001), normal LDH level (P < 0.0001), and dyslipidemia (P = 0.042; Supplementary Table S3, available at Annals of Oncology online).
On the basis of the results of the multivariate analysis, we included the individual variables with P < 0.01 to develop a survival predictive score. Therefore, LVEF, albumin, and LDH levels, number of metastatic sites, and number of prior therapies were used to develop the scoring system. The poor prognostic character of each of these factors was given 1 point, with the total score ranging from 0 to 5. Kaplan–Meier survival curves calculated using this scoring system are shown in Figure 1G. The survival outcomes of patients with LVEF < 50% were found to be negatively correlated with their scores (P < 0.0001). Of the 177 patients, 30 (17%) patients were found to have a score of 0, which corresponded to the highest median OS of 27.7 months (95% CI 16.3–NR). The median OS durations of patients with risk scores of 1 (n = 35), 2 (n = 60), and 3 (n = 31) were 9.7 months (95% CI 6.7–NR), 7.6 months (95% CI 5.6–11.1), and 3.7 months (95% CI 3.0–5.3), respectively. Patients with risk score >3 (n = 20) had a median OS of 0.8 months (95% CI 0.7–1.4).
discussion
The use of selected anticancer drugs has been associated with cardiac dysfunction [17]. The most common FDA-approved targeted agents associated with potential cardiotoxity are sunitinib and trastuzumab. The use of sunitinib has been associated with congestive heart failure in 4.1% of patients [18] and with both symptomatic and asymptomatic decrease in LVEF in 1.9% of patients [19]. In patients treated with trastuzumab, the reported incidence of LV dysfunction is 9.8% [20]. The optimal LVEF for participation in clinical trials has not been determined. LVEF of ≥50% has traditionally been used as an optimal cut-off point, with the exception of one study (trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer) in which only patients with a LVEF of ≥55% by echocardiography or multiple gated acquisition scanning were eligible [3].
In this analysis, 15% of patients had an LVEF of <50%, which is relatively high and contributed to the unfavorable characteristics of our patient population. Our patients had advanced solid tumors, unsuccessful standard treatment (median number of prior therapies, 4; range 0–8), and multiple comorbidities. The highest percentage of patients with LVEF < 50% were those with sarcoma (23%), which may be partially attributed to prior anthracycline treatment (80%; 12 of 15 patients with LVEF < 50%), although the number of patients was too small to draw a meaningful conclusion.
In our patient population, LVEF of 35% was the optimal cut-off point. Patients with very low LVEF (≤35%) had shorter survival than patients with LVEF > 35% (Figure 1B). In contrast, compared with patients with LVEF ≥ 50%, patients with LVEF between 35% and 50% had similar survival for the first 10 months and then slightly longer survival after that. It is possible that heart failure was the primary cause of death in patients with very low LVEF (≤35%), whereas cancer was the major cause of death in patients with LVEF > 35%.
Our data show that a higher percentage of patients with LVEF < 50% were male and elderly (>60 years old) compared with patients with LVEF ≥ 50%. In addition, patients with LVEF < 35% were significantly older (>60 years old) than patients with LVEF between 35% and 50%, but no difference was noted in patient sex. These findings demonstrate that male sex and older age are risk factors for CHF in patients with advanced cancer, just as they are in the general population [21].
In the era of personalized medicine, the role of biomarkers is becoming critical for predicting the response to treatment and the overall prognosis of patients with cancer. Our data confirm that previous reported variables predicting overall outcome in the phase I setting [13–15] are still valid in patients with LVEF < 50%. In the multivariate analyses, LVEF < 35%, albumin < 3.5 mg/dl, LDH greater than the upper limit of normal, more than two metastatic sites, and more than two prior therapy regimens were the top five independent adverse factors predicting survival. Based on these findings, we developed a prognostic score that can predict the life expectancy of patients referred to a phase I clinical trial. This score may be used as a good tool for a more refined selection of patients with advanced cancer referred for phase I clinical trials.
Finally, as determination of DLT and MTD are the end points of phase I trials, cardiac assessment should be an important element of the screening process. Traditionally, electrocardiography has been used to assess cardiac safety in phase I clinical trials because it is a simple and cheap test. However, its use has not been shown to prevent major cardiac events in phase I clinical trials [22]. BNP, troponin I and T have shown some benefits in predicting cardiotoxicity in patients treated with anthracyclines [23]. In addition, some experts do emphasize the role of BNP and troponin in stratifying patients undergoing cancer therapy [24]. Of 177 patients with LVEF < 50%, the number of patients who received statins was too small to make clinically meaningful conclusions about the use of statins in this patient group. The patient numbers with HDL, LDL, BNP, and troponin measurements were also too small to assess the effects of these cardiac risk markers on patient outcome. Our study demonstrates that LVEF < 35% is a poor prognostic factor in the phase I setting and confirms that the addition of echocardiography could improve the screening of patients enrolled in phase I clinical trials.
In conclusion, LVEF should be assessed in patients with cancer enrolled in clinical trials. Patients with LVEF between 35% and 50% had outcomes similar to those of patients with normal LVEF, which suggests that if other cardiac biomarkers are normal, it is safe to treat these patients in phase I clinical trials. Our results demonstrated that albumin and LDH levels, number of metastatic sites, number of prior systemic therapies, and LVEF assessment are independent factors predicting survival in phase I studies, and therefore, their use may improve patient selection for phase I clinical trials. In addition to LVEF assessment, serum levels of BNP and troponin I should be included in the monitoring of patients treated with potentially cardiotoxic anticancer agents. Prospective studies are needed to assess cardiotoxicity of patients with cancer who participate in clinical trials.
funding
This study was funded by the Alberto Barretto Donor Funds granted to Dr. A. M. Tsimberidou, MD, PhD.
disclosure
AMT has a compensated consultant/advisory relationship with CTI (Cell Therapeutics, Inc.). RMW also reports having a compensated consultant/advisory relationship with Eli Lilly & Co., Inc. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors would like to thank Ms Alexandra Adamopoulos for limited data collection and Ms Christine Eberle for assistance with submission of the manuscript.
references
- 1.Tsimberidou AM, Minotti G, Cardinale D. Managing cardiac risk factors in oncology clinical trials. Tex Heart Inst J. 2011;38:266–267. [PMC free article] [PubMed] [Google Scholar]
- 2.Vaklavas C, Lenihan D, Kurzrock R, et al. Anti-vascular endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target? Oncologist. 2010;15:130–141. doi: 10.1634/theoncologist.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 5.Subbiah IM, Lenihan DJ, Tsimberidou AM. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist. 2011;16:1120–1130. doi: 10.1634/theoncologist.2010-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimberidou AM, Akerley W, Schabel MC, et al. Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol Cancer Ther. 2010;9:3410–3419. doi: 10.1158/1535-7163.MCT-10-0516. [DOI] [PubMed] [Google Scholar]
- 7.Ryberg M, Nielsen D, Skovsgaard T, et al. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol. 1998;16:3502–3508. doi: 10.1200/JCO.1998.16.11.3502. [DOI] [PubMed] [Google Scholar]
- 8.Haq MM, Legha SS, Choksi J, et al. Doxorubicin-induced congestive heart failure in adults. Cancer. 1985;56:1361–1365. doi: 10.1002/1097-0142(19850915)56:6<1361::aid-cncr2820560624>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Moreb JS, Oblon DJ. Outcome of clinical congestive heart failure induced by anthracycline chemotherapy. Cancer. 1992;70:2637–2641. doi: 10.1002/1097-0142(19921201)70:11<2637::aid-cncr2820701112>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Folland ED, Parisi AF, Moynihan PF, et al. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–766. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 11.Schiller NB, Acquatella H, Ports TA, et al. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation. 1979;60:547–555. doi: 10.1161/01.cir.60.3.547. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Arkenau HT, Barriuso J, Olmos D, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27:2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 14.Garrido-Laguna I, Janku F, Vaklavas C, et al. Validation of the Royal Marsden Hospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer. 2012;118:1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 15.Wheler J, Tsimberidou AM, Hong D, et al. Survival of 1,181 patients in a phase I clinic: the MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res. 2012;18:2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 17.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 18.Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29:3450–3456. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 19.Ahuja N, Li Q, Mohan AL, et al. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 20.Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 21.He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 22.Naing A, Veasey-Rodrigues H, Hong DS, et al. Electrocardiograms (ECGs) in phase I anticancer drug development: the MD Anderson Cancer Center experience with 8518 ECGs. Ann Oncol. 2012;23:2960–2963. doi: 10.1093/annonc/mds130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–1111. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 24.Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30:3657–3664. doi: 10.1200/JCO.2012.45.2938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


