Abstract
Aims
We sought to determine the degree of anticoagulation reversal required to mitigate bleeding, and assess the feasibility of using pegnivacogin to prevent ischaemic events in acute coronary syndrome (ACS) patients managed with an early invasive approach. REG1 consists of pegnivacogin, an RNA aptamer selective factor IXa inhibitor, and its complementary controlling agent, anivamersen. REG1 has not been studied in invasively managed patients with ACS nor has an optimal level of reversal allowing safe sheath removal been defined.
Methods and results
Non-ST-elevation ACS patients (n = 640) with planned early cardiac catheterization via femoral access were randomized 2:1:1:2:2 to pegnivacogin with 25, 50, 75, or 100% anivamersen reversal or heparin. The primary endpoint was total ACUITY bleeding through 30 days. Secondary endpoints included major bleeding and the composite of death, myocardial infarction, urgent target vessel revascularization, or recurrent ischaemia. Enrolment in the 25% reversal arm was suspended after 41 patients. Enrolment was stopped after three patients experienced allergic-like reactions. Bleeding occurred in 65, 34, 35, 30, and 31% of REG1 patients with 25, 50, 75, and 100% reversal and heparin. Major bleeding occurred in 20, 11, 8, 7, and 10% of patients. Ischaemic events occurred in 3.0 and 5.7% of REG1 and heparin patients, respectively.
Conclusion
At least 50% reversal is required to allow safe sheath removal after cardiac catheterization. REG1 appears a safe strategy to anticoagulate ACS patients managed invasively and warrants further investigation in adequately powered clinical trials of patients who require short-term high-intensity anticoagulation. Clinical Trials Registration: ClinicalTrials.gov NCT00932100.
Keywords: Acute coronary syndromes, Anticoagulation reversal, REG1
Introduction
Anticoagulant therapy is a cornerstone of treatment for patients with acute coronary syndromes (ACS) and is vital to the safe conduct of percutaneous coronary interventions (PCI); however, more effective antithrombotics inherently increase bleeding. Novel approaches that maintain or improve efficacy without increasing bleeding are needed.
Aptamers are single-stranded nucleic acids that form unique sequence-dependent three-dimensional structures.1,2 Aptamers are unique in that a complementary nucleic acid controlling agent can bind through Watson–Crick base pairing, alter the conformation of, and inactivate its target aptamer.2–4 As the degree of reversal is directly related to the molar ratio of administered components, reversal of aptamer activity can be titrated to the patient's clinical condition.
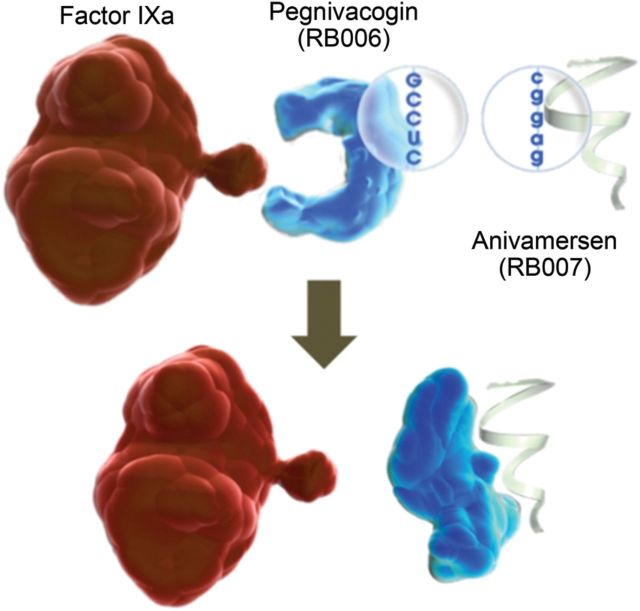
The REG1 system consists of pegnivacogin, a single-stranded RNA factor IXa inhibitor, and its complementary reversal agent, anivamersen, which binds to and inactivates pegnivacogin with rapid kinetics (Figure 1).5 REG1 has been studied in a series of Phase 1 studies4–7 and a Phase 2a feasibility study in a small cohort (n = 22) of stable patients undergoing elective PCI.8 These studies demonstrated that in stable patients, pegnivacogin administered at doses of ≥0.75 mg/kg completely inhibits factor IX activity and that femoral sheath removal after 100% reversal appeared safe. The relationship between the degree of reversal and bleeding and the feasibility of using REG1 to prevent ischaemic events in patients with ACS undergoing PCI have not been established.
Figure 1.
The REG1 anticoagulation system, consisting of pegnivacogin (RB006) and its complementary controlling agent anivamersen (RB007).
The RADAR (A Randomized, Partially Blinded, Multicenter, Active-Controlled, Dose-Ranging Study Assessing the Safety, Efficacy, and Pharmacodynamics of the REG1 Anticoagulation System in Patients with ACSs) trial was designed to: (1) determine the degree of anivamersen-mediated pegnivacogin reversal required to mitigate bleeding and allow early sheath removal following cardiac catheterization and (2) assess the feasibility of using the factor IX inhibitor pegnivacogin to prevent ischaemic events in patients with ACS managed with an early invasive approach.9 An early substudy was included to confirm near complete factor IXa inhibition with the selected pegnivacogin dose in this ACS population.4,10
Methods
Study population and design
The design of RADAR has been previously published.9 RADAR was a Phase 2b, international, adaptive design, partially blinded, dose-ranging clinical trial. The protocol was approved by institutional review boards or ethics committees at each institution and all patients provided written informed consent prior to participation.
Eligibility criteria
Eligible patients were required to have a minimum of 10 min of ischaemic symptoms within 72 h of enrolment associated with (1) dynamic ST-segment changes, or (2) elevated troponin I or T or CK(MB), or (3) a history of coronary artery disease by angiography. In addition, patients had to have planned cardiac catheterization via femoral arterial access within 24 h of randomization. Major exclusion criteria included: (1) ST-segment elevation myocardial infarction (MI); (2) weight >120 kg; (3) prior use of low molecular weight heparin within 3 days or of warfarin, bivalirudin, fibrinolytic agents, or glycoprotein IIb/IIIa inhibitors within 7 days of enrolment; (4) planned use of >7 French sheaths, intraaortic balloon pumps, or other support devices; or (5) an inability to take aspirin or a thienopyridine.
Randomization and study intervention
Using a central interactive voice response system, patients were randomized 3:1 to open-label REG1 with sheath removal 10 min post-procedure or heparin with sheath removal per the standard of care. Aspirin and thienopyridine pre-treatment (clopidogrel or prasugrel) were recommended. Patients not previously treated with thienopyridines were to receive a loading dose (300 or 600 mg of clopidogrel or prasugrel, as indicated). For patients on chronic thienopyridine therapy, the administration of an additional loading dose was left to the discretion of the physician. Glycoprotein IIb/IIIa inhibitors were recommended in patients assigned to heparin. Patients randomized to REG1 received pegnivacogin (1 mg/kg over 1 min intravenously) pre-procedure and were randomized, in a 2:1:1:2 ratio, to a blinded post-procedure dose of anivamersen of 0.075, 0.2, 0.4, or 1 mg/kg to achieve 25, 50, 75, or 100% reversal, respectively.4,7 Doses were chosen based on early phase clinical studies predicting that 1 mg/kg pegnivacogin would reliably achieve near complete factor IXa inhibition and the previously defined relationship between anivamersen dose and the degree of reversal of pegnivacogin activity.4–8 Additional open-label anivamersen (1 mg/kg, a dose sufficient to completely reverse all pegnivacogin activity) could be administered if haemostasis was not achieved after 20 min. Patients who underwent coronary artery bypass graft (CABG) surgery received open-label complete reversal prior to surgery and unfractionated heparin during surgery. Patients randomized to heparin were treated using a standardized recommended dosing algorithm prior to and during catheterization and underwent femoral arterial sheath removal at a time consistent with local standard practice.9 As it is customary to utilize anticoagulation post-procedure for deep vein thrombosis prophylaxis in some locations, this was allowed per local practice in all subjects.
For all patients undergoing sheath removal using manual compression, pressure was maintained for 10 min and, if haemostasis had not been achieved, manual compression was applied for an additional 10 min. Vascular closure devices were allowed 10 min after anivamersen dosing or per the standard of care in heparin patients. An ambulation challenge was recommended within 2 h post-sheath removal in REG1 patients and per the standard of care for heparin patients.
Endpoint definitions
The primary endpoint was total bleeding through 30 days.9 Major bleeding was defined as intracranial, intraocular bleeding or haemarthrosis, access site haemorrhage requiring intervention, ≥5 cm access site haematoma, a reduction in haemoglobin levels of ≥4 g/dL without overt bleeding or ≥3 g/dL with an overt bleeding source, re-operation for bleeding, or need for any transfusion of blood products. Minor bleeding constituted any clinically evident bleeding not meeting the criteria for major bleeding.
The composite ischaemic endpoint was death, non-fatal MI, urgent target vessel revascularization, or recurrent ischaemia in the target vessel distribution through 30 days. Specific procedural complications, including abrupt or threatened coronary artery closure, distal embolization, clot formation on catheters or wires, no reflow, or any other thrombotic complication, were recorded at the time of catheterization.
All bleeding and ischaemic events were adjudicated by an independent clinical events committee blinded to the study treatment using original source documents.
Statistical methods
We projected a 20% absolute rate of total bleeding in the heparin arm and in the REG1 arm with 25% reversal.9 Based on a projected enrolment of 800 patients, the trial had 80% power to detect a 50% relative (10% absolute) reduction in bleeding in the REG1 with 100% reversal arm compared with patients in the heparin or REG1 with 25% reversal arms at an α of 0.05.
The trial employed an adaptive design. The data safety monitoring board (DSMB) reviewed efficacy (bleeding) and safety (ischaemic event) data after enrolment of 100, 200, and 400 patients. Based on pre-specified criteria, the DSMB could recommend the cessation of enrolment into lower reversal REG1 arms if excessive bleeding was observed or into the higher reversal REG1 arms for excessive ischaemic events.9 Patients who would have been randomized to discontinued arms were randomized to the remaining reversal arms, maintaining the same overall sample size and a 3:1 randomization between REG1 and heparin.
Analyses were performed at the Duke Clinical Research Institute with full access to the trial database. All analyses except adverse events were performed on an intention-to-treat basis. Rates of adverse events were analysed according to the actual treatment received. Odds ratios (ORs) and 95% confidence intervals (CIs) were provided for the ischaemic event composite (comparing REG1 with heparin) and for bleeding events (comparing REG1-100% reversal with heparin and REG1-100% reversal with REG1-25% reversal). Statistical testing for these comparisons was performed using Fisher's mid-p test. Events following CABG surgery were not systematically collected and censored per protocol. Evaluation for trends between the reversal dose and decreases in bleeding were performed using a Cochran–Armitage Trend test with a one-sided P-value. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline clinical and demographic characteristics
Between September 2009 and November 2010, 640 patients were randomized at 67 centres in the USA, Poland, Germany, Canada, France, and the Netherlands. Baseline characteristics were well balanced between arms. Patients qualifying for the trial had positive cardiac biomarkers (53.1%), ST-segment changes (25.6%), or a history of coronary artery disease (50.3%) (Table 1).
Table 1.
Demographic data of enrolled patients
| REG1 25%, reversal (n = 41) | REG1 50%, reversal (n = 117) | REG1 75%, reversal (n = 120) | REG1 100%, reversal (n = 201) | Total REG1 (n = 479) | Heparin (n = 161) | |
|---|---|---|---|---|---|---|
| Age, median (25th, 75th), years | 62.2 (51.4, 70.2) | 63.1 (56.5, 73.2) | 66.7 (56.8, 72.4) | 66.1 (55.9, 72.0) | 64.9 (55.9, 72.1) | 62.5 (55.8, 71.2) |
| Range | 37.0, 82.4 | 38.3, 86.0 | 38.5, 85.5 | 33.7, 83.5 | 33.7, 86.0 | 37.5, 83.4 |
| Male | 25 (61.0) | 83 (70.9) | 79 (65.8) | 136 (67.7) | 323 (67.4) | 114 (70.8) |
| Race | ||||||
| White | 37 (90.2) | 112 (95.7) | 114 (95.0) | 191 (95.0) | 454 (94.8) | 152 (94.4) |
| Black | 4 (9.8) | 2 (1.7) | 4 (3.3) | 6 (3.0) | 16 (3.3) | 6 (3.7) |
| Other | 0 | 3 (2.8) | 2 (1.7) | 4 (2.0) | 7 (1.4) | 3 (1.8) |
| Hispanic/Latino | 2 (4.9) | 1 (0.9) | 2 (1.7) | 2 (1.0) | 7 (1.5) | 2 (1.2) |
| Non-Hispanic/Latino | 39 (95.1) | 116 (99.1) | 118 (98.3) | 199 (99.0) | 472 (98.5) | 159 (98.8) |
| Past medical history | ||||||
| Congestive HF | 4 (9.8) | 14 (12.0) | 11 (9.2) | 20 (10.0) | 49 (10.2) | 16 (9.9) |
| MI | 19 (46.3) | 64 (54.7) | 54 (45.0) | 95 (47.3) | 232 (48.4) | 75 (46.6) |
| Previous PCI | 16 (39.0) | 51 (43.6) | 45 (37.5) | 96 (47.8) | 208 (43.4) | 69 (42.9) |
| Previous CABG | 5 (12.2) | 14 (12.0) | 19 (15.8) | 31 (15.4) | 69 (14.4) | 23 (14.3) |
| Hypertension requiring therapy | 29 (70.7) | 88 (75.2) | 91 (75.8) | 163 (81.1) | 371 (77.5) | 128 (79.5) |
| Diabetes | 18 (43.9) | 47 (40.2) | 32 (26.7) | 60 (29.9) | 157 (32.8) | 42 (26.1) |
| Renal insufficiency | 3 (7.3) | 16 (13.7) | 15 (12.5) | 12 (6.0) | 46 (9.6) | 11 (6.8) |
| Stroke | 5 (12.2) | 7 (6.0) | 6 (5.0) | 4 (2.0) | 22 (4.6) | 5 (3.1) |
| Current tobacco use | 15 (36.6) | 38 (32.5) | 24 (20.0) | 49 (24.4) | 126 (26.3) | 44 (27.3) |
| Enrolment criteria | ||||||
| New or presumably new ST-segment depression | 11 (26.8) | 33 (28.2) | 30 (25.0) | 44 (21.9) | 118 (24.6) | 46 (28.6) |
| Elevated troponin I, T, or CK-MB | 28 (68.3) | 67 (57.3) | 66 (55.0) | 91 (45.3) | 252 (52.6) | 88 (54.7) |
| History of coronary artery disease | 19 (46.3) | 58 (49.6) | 52 (43.3) | 115 (57.2) | 244 (50.9) | 78 (48.4) |
Data are presented as no. (%) unless otherwise indicated. CABG, coronary artery bypass grafting; CAD, coronary artery disease; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Study drug and cardiac catheterization
Of patients randomized to REG1, 98.9% received pegnivacogin. Use of thienopyridines was high and similar in the REG1 and heparin arms (Table 2). Cardiac catheterization was performed in 98% of patients at a median (25th, 75th) of 2.3 (1.5, 3.7), 2.0 (1.3, 3.6), 1.7 (0.9, 2.7), and 2.0 (1.2, 3.4) h after randomization in the 25, 50, 75, and 100% reversal arms, respectively, and at 1.8 (0.9, 2.9) h in the heparin arm. The median (25th, 75th) time from the end of coronary angiography or PCI to sheath removal was 23 (16, 43) min in REG1-randomized patients vs. 184 (11, 316) min in patients randomized to heparin.
Table 2.
Treatment characteristics
| Treatment strategy | REG1 (n = 473) | Heparin (n = 161) |
|---|---|---|
| Study drug | 98.9 | 100 |
| Anivamersen (of patients treated with REG1) | 96.5 | — |
| Aspirin | 98.3 | 97.5 |
| Thienopyridine | 87.9 | 89.4 |
| Clopidogrel | 82.5 | 80.8 |
| Loading dose | 58.0 | 53.4 |
| Loading dose (<300 mg) | 35.3 | 51.1 |
| Loading dose (≥600 mg) | 64.8 | 48.8 |
| Prasugrel | 8.8 | 13.7 |
| Loading dose | 6.1 | 8.1 |
| GP IIb/IIIa inhibitor use after randomization | 9.0 | 15.6 |
| GP IIb/IIIa inhibitor started during catheterization | 8.8 | 15.0 |
| Vascular closure device | 12.9 | 19.4 |
| Management strategy | ||
| Medical therapy | 32.1 | 25.6 |
| PCI | 58.9 | 69.4 |
| CABG | 8.9 | 5.0 |
| Heparin use post-catheterization | 16.6 | 23.9 |
CABG, coronary artery bypass grafting; GP, glycoprotein; PCI, percutaneous coronary intervention.
Glycoprotein IIb/IIIa inhibitor use was low in both arms but modestly higher among patients randomized to heparin. The vast majority of glycoprotein IIb/IIIa inhibitor use after randomization (97%) was started during catheterization or PCI.
Outcomes
Bleeding
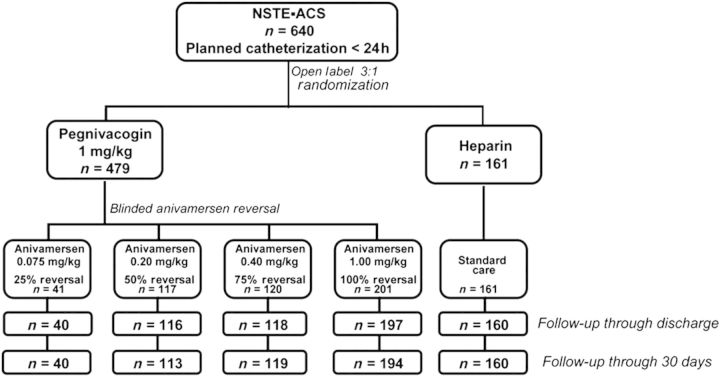
Following the DSMB review of the first 100 patients randomized, enrolment into the 25% reversal arm was stopped due to an excess of bleeding. The final patient distribution and follow-up is summarized in Figure 2.
Figure 2.
Study flow.
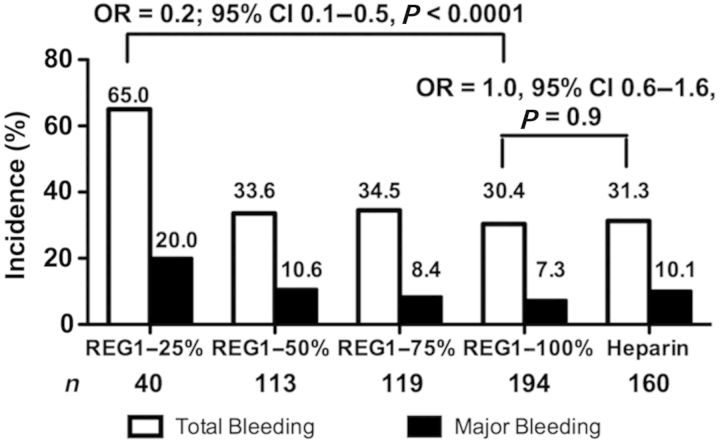
Rates of 30-day total and major bleeding are shown in Figure 3. Bleeding was substantial in the 25% reversal arm. Other than the 25% reversal arm, rates of total bleeding were similar among the REG1 and heparin arms. Major bleeding was lower in a dose-related fashion with higher reversal strategies (P = 0.01 for trend). Open-label anivamersen to effect full reversal was used in 30.8, 5.3, 5.1, and 2.6% of the 25, 50, 75, and 100% reversal arms, respectively. There was a lower rate of total bleeding with REG1 and 100% reversal compared with REG1 and 25% reversal (Figure 3). Similar differences were observed when considering only patients enrolled in the 100% reversal arm prior to the discontinuation of the 25% reversal arm, and after adjusting for baseline differences between the 25 and 100% reversal arms. The rate of total bleeding in the 100% reversal arm was similar to the heparin arm.
Figure 3.
Total and ACUITY major bleeding through a 30-day follow-up. The number and incidence of total and major modified ACUITY bleeding is shown.
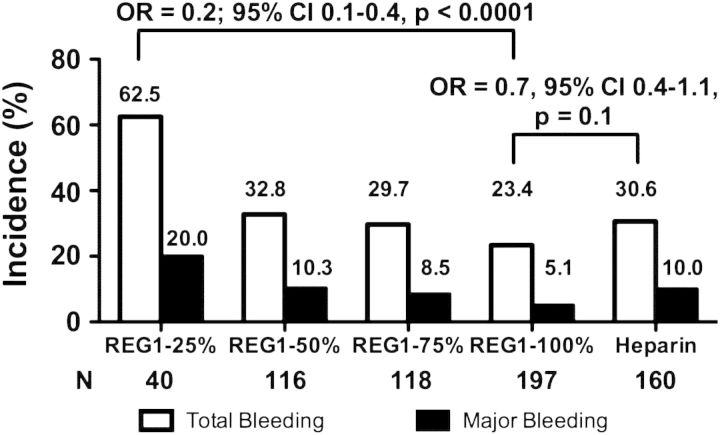
When examining the incidence of bleeding from randomization through hospital discharge, anivamersen dose was directly related to both total and major bleeding (Figure 4). There was a trend towards lower bleeding in the 100% reversal arm compared with heparin (OR 0.7, 95% CI 0.4–1.1; P= 0.10), with a strong relationship between the degree of reversal and major bleeding among REG1-treated subjects (P = 0.001).
Figure 4.
Total and ACUITY major bleeding through hospital discharge. The number and incidence of total and major modified ACUITY bleeding is shown.
Major (89.8%) and total bleeding (88.3%) were predominantly access site-related. One heparin-treated patient suffered an intracranial haemorrhage and major gastrointestinal bleeding occurred in three patients assigned to REG1 and one patient assigned to heparin. A total of eight (1.7%) REG1 and four (2.5%) heparin patients received non-CABG-related transfusions. There were no haemarthroses, or urinary tract or intraocular bleeding events.
Ischaemic events
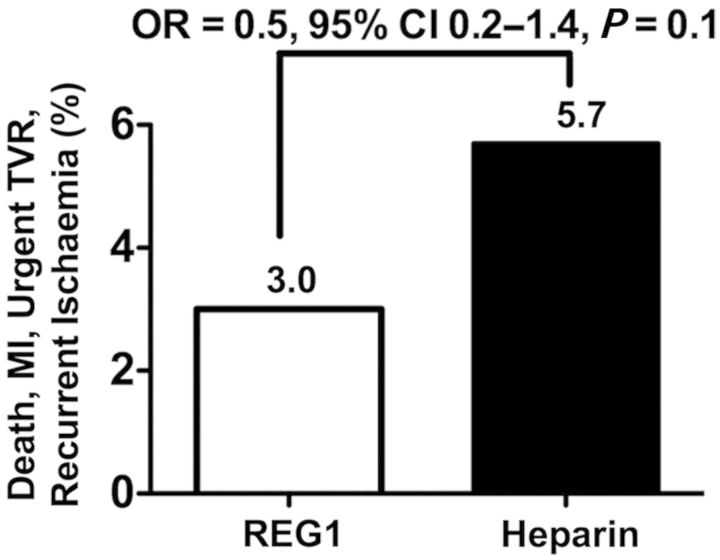
The composite of 30-day death, non-fatal MI, urgent target vessel revascularization, or recurrent ischaemia in the target vessel distribution was numerically lower in patients assigned to REG1 than heparin (Figure 5). Ischaemic events were rare and evenly distributed between the various reversal arms (Table 3). The majority of ischaemic events were non-fatal periprocedural MIs. Target vessel revascularization occurred in <1% of patients and was not related to the degree of reversal. No periprocedural thrombus formation on the wire or catheters was observed in REG1-treated patients, and no patients required transition from pegnivacogin to heparin during the conduct of PCI.
Figure 5.
Incidence of the composite ischaemic endpoint (death, non-fatal myocardial infarction, urgent target vessel revascularization, or recurrent ischaemia in the target vessel distribution) through a 30-day follow-up.
Table 3.
Ischaemic events by treatment arm
| REG1 25% | REG1 50% | REG1 75% | REG1 100% | REG1 overall | Heparin | |
|---|---|---|---|---|---|---|
| Composite | 3 (7.5) | 1 (0.9) | 5 (4.2) | 5 (2.6) | 14 (3.0) | 9 (5.7) |
| Death | 0 | 0 | 1 (0.8) | 0 | 1 (0.2) | 1 (0.6) |
| MI | 3 (7.5) | 1 (0.9) | 4 (3.4) | 4 (2.1) | 12 (2.6) | 7 (4.5) |
| Urgent TVR | 1 (2.6) | 0 | 1 (0.8) | 1 (0.5) | 3 (0.6) | 1 (0.6) |
Values presented as no. (%). There were no recurrent ischaemic events. MI, myocardial infarction; TVR, target vessel revascularization.
Adverse events
The rates of adverse events and serious adverse events (60 and 16% in patients treated with REG1 and 55 and 11% in patients treated with heparin) were similar between REG1- and heparin-treated patients.
Three patients had allergic-like reactions shortly after pegnivacogin administration; two of these reactions were serious. The first patient had a history of allergic urticaria treated with corticosteroids and antihistamines 1 month prior to enrolment. She developed nausea, pruritus, and shortness of breath 5 min after pegnivacogin administration and was treated with corticosteroids, antihistamines, and short-term vasopressor support with resolution of all symptoms within 2 h of onset. The second patient had a history of allergy to radiographic contrast and multiple other allergies. She developed generalized urticaria without respiratory or haemodynamic changes, and was treated with steroids and antihistamines with resolution within 25 min. The third patient had listed allergies to beta-blockers and steroids. After receiving pegnivacogin, she developed dyspnoea and cutaneous tingling. She was treated with corticosteroids, antihistamines, and haemodynamic support after developing tachycardia prompting cardioversion.
Following the third event, enrolment was put on hold. After a review of completed enrolment and aggregate event information, it was felt that the trial had sufficient data to meet its main objectives and enrolment was stopped. To determine if there were additional unrecognized allergic-like reactions, we examined the database for adverse events possibly representing unrecognized milder allergic reactions occurring within 24 h of pegnivacogin administration (Table 4). A full listing of all adverse events reported is available in the online supplementary materials at European Heart Journal online.
Table 4.
Incidence of allergic-like adverse events within 24 h of drug administration
| Adverse events | REG1 (n = 465) | Heparin (n = 163) |
|---|---|---|
| Hives | 0.2 | — |
| Hypotension | 2.4 | 1.9 |
| Rash | — | 0.7 |
| Dyspnoea | 0.9 | — |
Values presented as percentages.
Discussion
RADAR met its primary objectives, defining that at least 50% pegnivacogin reversal is required to allow early sheath removal after cardiac catheterization via a femoral approach and suggesting that near complete inhibition of factor IXa with pegnivacogin, followed by anivamersen-mediated reversal, may provide a strategy that permits effective periprocedural anticoagulation without increased bleeding despite immediate post-procedure sheath removal.
Pharmacodynamic dose verification
Factor IXa activity and thrombin generation are heightened among patients with ACS,11,12 and anticoagulant dosing from stable patients may not translate to more acute settings. We therefore felt it important to verify that dosing based on early phase studies resulted in near complete factor IXa inhibition in RADAR. Our pharmacodynamic substudy demonstrated that pegnivacogin at 1 mg/kg achieves near complete and stable inhibition of factor IXa activity in patients with ACS undergoing cardiac catheterization.10
Bleeding
The primary objective of RADAR was to define the degree of pegnivacogin reversal required to allow prompt femoral sheath removal at the conclusion of cardiac catheterization. RADAR demonstrated that 25% reversal of pegnivacogin anticoagulation is inadequate, while an anivamersen dose that achieves at least 50% pegnivacogin reversal is sufficient to allow early removal of femoral sheaths with bleeding rates that appear similar to those observed with heparin.
Assessment of bleeding between REG1- and heparin-treated patients may have been biased by the open-label assignment of these groups. The incidence of minor bleeding between discharge and 30-day follow-up was higher in REG1-treated patients, and did not vary with reversal strategy. Since patients who were randomized to high levels of reversal had normalized coagulation parameters prior to discharge, there may have been ascertainment bias with greater attention to the identification of minor bleeding in patients treated with REG1 compared with those receiving heparin. Major bleeding, which may be less subject to ascertainment bias, appeared more directly related to the dose of anivamersen and was numerically lower in the 100% reversal group compared with heparin. The rate of use of open-label anivamersen to normalize coagulation parameters mirrors these results. These results, however, require confirmation in a larger and preferably blinded clinical trial.
Ischaemic events
Given the availability of reversal with anivamersen, we chose a dose of pegnivacogin that would reliably achieve near complete inhibition of factor IXa. High-dose direct factor Xa inhibition has resulted in improved efficacy but with dose-limiting bleeding.13 RADAR suggests that a strategy of high-level anticoagulation followed by controlled reversal might be attractive in patients with ACS undergoing invasive management. A theoretical concern is that immediate, complete reversal of anticoagulation might result in an increased risk of acute thrombotic complications. Although the number of events is low, we did not observe any increase in acute (<24 h) or short-term (30 days) thrombotic events with higher levels of reversal.
Factor IXa inhibition
Factor IXa is a theoretically attractive target but has not been validated as a clinically effective target for therapeutic anticoagulation.14 Factor IXa is the proximal driver of thrombin generation15 and is directly activated by foreign materials, and increased factor IX activity has been associated with a higher incidence of clinical thrombotic events.11,12 In addition, factor IX concentrations are lower and less variable than those of factor X or thrombin, making consistent high-level target inhibition theoretically more achievable. The results of this study suggest that factor IXa inhibition does represent a viable and potentially effective strategy for anticoagulation. There were no cases of intraprocedural thrombosis, clots formed on equipment, or ‘bail-out’ use of heparin that might suggest inadequate intraprocedural anticoagulation.16
Timing of sheath removal
By design, REG1 patients underwent prompt sheath removal post-procedure, while subjects randomized to heparin had their sheaths removed according to the local standard of care. This resulted in a substantial difference in the time from the end of the procedure to sheath removal between these strategies. Delayed sheath removal has been repeatedly shown to be associated with bleeding.17–19 The use of vascular closure devices reduces the time to sheath removal; however, they have not consistently been shown to reduce bleeding complications and may increase vascular complications.20–22 While radial access also allows early sheath removal, it results in greater patient and operator radiation exposure,23 may not improve cardiac outcomes,24 and is not compatible with procedures requiring larger sheath sizes.
Allergic-like reactions
Ribonucleic acid aptamers are inherently non-immunogenic and extensive analysis in pre-clinical models and early clinical trials5–7 has failed to demonstrate aptamer-mediated complement activation. The nucleic acid component of pegnivacogin is connected to a 40 kD polyethyleneglycol (PEG) chain to increase its stability and prolong its half-life. While antibodies to PEG have been reported,25–27 their incidence is low and first exposure reactions to PEG are uncommon.
Whether the three events observed in RADAR represent an inherent immunogenic property of pegnivacogin, reactions due to patient-specific factors, a random clustering of otherwise rare events, or a manifestation of degradation or contamination in the study drug is under investigation. Investigation of the study drug has not revealed product degradation, lot specificity, or other manufacturing issues as a cause. Each reaction occurred soon (<30 min) after pegnivacogin administration and prior to the administration of contrast. Potential mechanisms of such reactions might include direct complement activation, mast cell activation, or antibody-mediated mechanisms. Extensive primate studies as well as monitoring performed throughout Phase 1 (n = 161) revealed no evidence of complement activation. While mast cell activation has not been specifically evaluated, there were no clinical signs suggestive of pegnivacogin-mediated mast cell activation.
Given the first exposure nature of the observed reactions, antibody-mediated mechanisms would require cross-reactivity between pegnivacogin and a pre-formed antibody. The modifications to and size of the oligonucleotide make first-exposure reactions unlikely. Prior exposure to the PEG component could be a possible culprit. In this regard, the prior history of allergy in each of the subjects might be relevant. If and when pegnivacogin is studied in subsequent clinical trials, systematic screening for allergy history, routine collection of blood samples for analysis, initial enrolment of lower risk patients, and appropriate education and management plans for potential allergic reactions should be considered.
Limitations
The partially open-label design makes comparisons between REG1 and heparin subject to potential bias; however, the primary comparison of bleeding among REG-1 reversal strategies was blinded. As a Phase 2 trial, RADAR was not powered to determine the safety or efficacy of REG1 compared with other anticoagulant strategies. We used a relatively liberal definition of major bleeding to increase our ability to detect differences among groups. Similar definitions have been used in other Phase 228 and Phase 3 studies,29,30 and the use of a stricter definition excluding haematoma as a major bleeding criterion did not affect the results. Finally post-randomization decisions, including administration of open-label heparin, may have both influenced and been influenced by post-randomization bleeding and ischaemic events.
Conclusion
At least 50% anivamersen-mediated reversal of pegnivacogin is required to effectively mitigate bleeding after early femoral sheath removal in invasively managed patients with ACS. Adequately powered randomized clinical trials are warranted to determine the safety and efficacy of factor IXa inhibition with REG1 compared with other anticoagulation strategies in patients who require high-intensity, short-term anticoagulation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The RADAR trial was supported by Regado Biosciences, Inc.
Conflict of interest: T.J.P. receives research salary support through Duke Clinical Research Institute from Regado Biosciences and has received compensation for attending Regado Bioscience's Medical Advisory Board meetings. J.P.V. receives research salary support through Duke Clinical Research Institute from Regado Biosciences. L.H.A. receives research salary support through Duke Clinical Research Institute from Regado Biosciences. R.M. has received compensation for being on Regado Bioscience's Medical Advisory Board. M.G.C. has received consulting honoraria from Regado Biosciences. S.L.Z. is an employee with stock options in Regado Biosciences. R.C.B. receives research salary support through Duke Clinical Research Institute from Regado Biosciences and has received compensation for service on Regado Bioscience's Medical Advisory Board. J.H.A. receives research salary support through Duke Clinical Research Institute from Regado Biosciences and has received compensation for service on Regado Bioscience's Medical Advisory Board. J.D.K., C.B., C.E.B., J.H.C., A.R., M.N., and N.D. have no conflicts to declare. Duke University's institutional conflict of interest is included in online supplementary materials at European Heart Journal online.
Supplementary Material
Acknowledgements
We acknowledge all the investigators and research staff, as well as the patients who contributed to RADAR. In addition, we thank Elizabeth Cook for her editorial assistance with the manuscript.
Appendix
Steering Committee: John H. Alexander (Chair), Duke Clinical Research Institute, Durham, NC, USA; Richard Becker, Duke Clinical Research Institute, Durham, NC, USA; Christoph Bode, MD, University of Freiberg, Freiberg, Germany; Christopher E. Buller, MD, Hamilton General Hospital, Hamilton, Ontario, Canada; Mauricio G. Cohen, MD, University of Miami Miller School of Medicine, Miami, FL, USA; Jan H. Cornel, MD, Medisch Centrum Alkmaar, Alkmaar, the Netherlands; Jaroslaw D. Kasprzak, MD, Medical University of Lodz, Łódź, Poland; Roxana Mehran, MD, Mount Sinai School of Medicine, New York, NY, USA; Gilles Montalescot, MD, Institut de Cardiologie, Pitié-Salpétrière Hospital, Paris, France; Thomas J. Povsic, Duke Clinical Research Institute, Durham, NC, USA; Steven L. Zelenkofske, DO, Regado Biosciences, Basking Ridge, NJ, USA.
Data Safety Monitoring Board: Ronald Waksman, MD, Washington Heart Center (chair); Stefan James, MD, Uppsala Clinical Research Center; Jack Ansel, MD, Lenox Hill Hospital; Vic Hasselblad, PhD, Duke Clinical Research Institute; and Rebecca Torguson, MPH, Washington Heart Center.
References
- 1.Becker RC, Rusconi C, Sullenger B. Nucleic acid aptamers in therapeutic anticoagulation. Technology, development, and clinical application. Thromb Haemost. 2005;93:1014–1020. doi: 10.1160/TH04-12-0790. [DOI] [PubMed] [Google Scholar]
- 2.Becker RC, Povsic TJ, Cohen MG, Rusconi CP, Sullenger BA. Nucleic acid aptamers as antithrombotic agents: opportunities in extracellular therapeutics. Thromb Haemost. 2010;103:586–595. doi: 10.1160/TH09-10-0716. doi:10.1160/TH09-10-0716. [DOI] [PubMed] [Google Scholar]
- 3.Povsic T, Sullenger B, Zelenkofske S, Rusconi C, Becker R. Translating nucleic acid aptamers to antithrombotic drugs in cardiovascular medicine. J Cardiovasc Transl Res. 2011;3:704–716. doi: 10.1007/s12265-010-9230-6. doi:10.1007/s12265-010-9230-6. [DOI] [PubMed] [Google Scholar]
- 4.Povsic TJ, Cohen MG, Chan MY, Zelenkofske SL, Wargin WA, Harrington RA, Alexander JH, Rusconi CP, Becker RC. Dose selection for a direct and selective factor IXa inhibitor and its complementary reversal agent: translating pharmacokinetic and pharmacodynamic properties of the REG1 system to clinical trial design. J Thromb Thrombolysis. 2011;32:21–31. doi: 10.1007/s11239-011-0588-3. doi:10.1007/s11239-011-0588-3. [DOI] [PubMed] [Google Scholar]
- 5.Dyke CK, Steinhubl SR, Kleiman NS, Cannon RO, Aberle LG, Lin M, Myles SK, Melloni C, Harrington RA, Alexander JH, Becker RC, Rusconi CP. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation. 2006;114:2490–2497. doi: 10.1161/CIRCULATIONAHA.106.668434. doi:10.1161/CIRCULATIONAHA.106.668434. [DOI] [PubMed] [Google Scholar]
- 6.Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M, Walder J, Steinhubl SR, Gilchrist IC, Kleiman NS, Vorcheimer DA, Chronos N, Melloni C, Alexander JH, Harrington RA, Tonkens RM, Becker RC, Rusconi CP. Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation. 2008;117:2865–2874. doi: 10.1161/CIRCULATIONAHA.107.745687. doi:10.1161/CIRCULATIONAHA.107.745687. [DOI] [PubMed] [Google Scholar]
- 7.Chan MY, Rusconi CP, Alexander JH, Tonkens RM, Harrington RA, Becker RC. A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J Thromb Haemost. 2008;6:789–796. doi: 10.1111/j.1538-7836.2008.02932.x. doi:10.1111/j.1538-7836.2008.02932.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LH, Greenbaum AB, Fry E, Chan MY, Tonkens RM, Zelenkofske S, Alexander JH, Harrington RA, Rusconi CP, Becker RC. First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous intervention. Circulation. 2010;122:614–622. doi: 10.1161/CIRCULATIONAHA.109.927756. doi:10.1161/CIRCULATIONAHA.109.927756. [DOI] [PubMed] [Google Scholar]
- 9.Povsic TJ, Cohen MG, Mehran R, Buller CE, Bode C, Cornel JH, Kasprzak JD, Montalescot G, Joseph D, Wargin WA, Rusconi CP, Zelenkofske SL, Becker RC, Alexander JH. A randomized, partially-blinded, multicenter, active-controlled, dose-ranging study assessing the safety, efficacy, and pharmacodynamics of the REG1 anticoagulation system in patients with acute coronary syndromes: design and rationale of the RADAR phase IIb trial. Am Heart J. 2011;161:261–268. doi: 10.1016/j.ahj.2010.10.022. doi:10.1016/j.ahj.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Povsic TJ, Wargin WA, Alexander JH, Krasnow J, Krolick M, Cohen MG, Mehran R, Buller CE, Bode C, Zelenkofske SL, Rusconi CP, Becker RC. Pegnivacogin results in near complete FIX inhibition in acute coronary syndrome patients: RADAR pharmacokinetic and pharmacodynamic substudy. Eur Heart J. 2011;32:2412–2419. doi: 10.1093/eurheartj/ehr179. doi:10.1093/eurheartj/ehr179. [DOI] [PubMed] [Google Scholar]
- 11.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: opposite synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. doi:10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 12.Miller GJ, Ireland HA, Cooper JA, Bauer KA, Morrissey JH, Humphries SE, Esnouf MP. Relationship between markers of activated coagulation, their correlation with inflammation, and association with heart disease (NPHSII) J Thromb Haemost. 2008;6:259–267. doi: 10.1111/j.1538-7836.2008.02819.x. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Antman EM, Widimsky P, Ebrahim IO, Kiss RG, Saaiman A, Polasek R, Contant CF, McCabe CH, Braunwald E. Otamixaban for the treatment of patients with non-ST-elevation acute coronary syndromes (SEPIA-ACS1 TIMI 42): a randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2009;374:787–795. doi: 10.1016/S0140-6736(09)61454-9. doi:10.1016/S0140-6736(09)61454-9. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson BI, Dahl OE, Lassen MR, Ward DP, Rothlein R, Davis G, Turpie AG. Partial factor IXa inhibition with TTP889 for prevention of venous thromboembolism: an exploratory study. J Thromb Haemost. 2008;6:457–463. doi: 10.1111/j.1538-7836.2007.02872.x. doi:10.1111/j.1538-7836.2007.02872.x. [DOI] [PubMed] [Google Scholar]
- 15.Monroe DM, Hoffman M, Roberts HR. Transmission of a procoagulant signal from tissue factor-bearing cells to platelets. Blood Coagul Fibrinolysis. 1996;7:459–464. doi: 10.1097/00001721-199606000-00005. doi:10.1097/00001721-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 16.The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464–1476. doi: 10.1056/NEJMoa055443. doi:10.1056/NEJMoa055443. [DOI] [PubMed] [Google Scholar]
- 17.Cantor WJ, Mahaffey KW, Huang Z, Das P, Gulba DC, Glezer S, Gallo R, Ducas J, Cohen M, Antman EM, Langer A, Kleiman NS, White HD, Chisholm RJ, Harrington RA, Ferguson JJ, Califf RM, Goodman SG. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Intervent. 2007;69:73–83. doi: 10.1002/ccd.20897. doi:10.1002/ccd.20897. [DOI] [PubMed] [Google Scholar]
- 18.Gallo R, Steinhubl SR, White HD, Montalescot G. Impact of anticoagulation regimens on sheath management and bleeding in patients undergoing elective percutaneous coronary intervention in the STEEPLE trial. Catheter Cardiovasc Intervent. 2009;73:319–325. doi: 10.1002/ccd.21764. doi:10.1002/ccd.21764. [DOI] [PubMed] [Google Scholar]
- 19.Popma JJ, Satler LF, Pichard AD, Kent KM, Campbell A, Chuang YC, Clark C, Merritt AJ, Bucher TA, Leon MB. Vascular complications after balloon and new device angioplasty. Circulation. 1993;88:1569–1578. doi: 10.1161/01.cir.88.4.1569. doi:10.1161/01.CIR.88.4.1569. [DOI] [PubMed] [Google Scholar]
- 20.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350–357. doi: 10.1001/jama.291.3.350. doi:10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 21.Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J Invasive Cardiol. 2004;16:243–246. [PubMed] [Google Scholar]
- 22.Biancari F, D'Andrea V, Marco CD, Savino G, Tiozzo V, Catania A. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010;159:518–531. doi: 10.1016/j.ahj.2009.12.027. doi:10.1016/j.ahj.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Mercuri M, Mehta S, Xie C, Valettas N, Velianou JL, Natarajan MK. Radial artery access as a predictor of increased radiation exposure during a diagnostic cardiac catheterization procedure. JACC Cardiovasc Interv. 2011;4:347–352. doi: 10.1016/j.jcin.2010.11.011. doi:10.1016/j.jcin.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. doi:10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 25.Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, Hershfield MS. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against Poly(ethylene glucol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther. 2005;8:R12. doi: 10.1186/ar1861. doi:10.1186/ar1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundy JS, Ganson NJ, Kelly SJ, Scarlett EL, Rehrig CD, Huang W, Hershfield MS. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum. 2007;56:1021–1028. doi: 10.1002/art.22403. doi:10.1002/art.22403. [DOI] [PubMed] [Google Scholar]
- 27.Sundy JS, Hershfield Michael S. Uricase and other novel agents for the management of patients with treament-failure gout. Curr Rheumatol Rep. 2007;9:258–264. doi: 10.1007/s11926-007-0041-y. doi:10.1007/s11926-007-0041-y. [DOI] [PubMed] [Google Scholar]
- 28.Steg PG, Mehta SR, Jukem JW, Lip GY, Gibson CM, Kovar F, Kala P, Garcia-Hernandez A, Renfurm RW, Granger CB. RUBY-1: a randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM15)) following acute coronary syndrome. Eur Heart J. 2011;32:2541–2554. doi: 10.1093/eurheartj/ehr334. doi:10.1093/eurheartj/ehr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. doi:10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 30.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. doi:10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.