Abstract
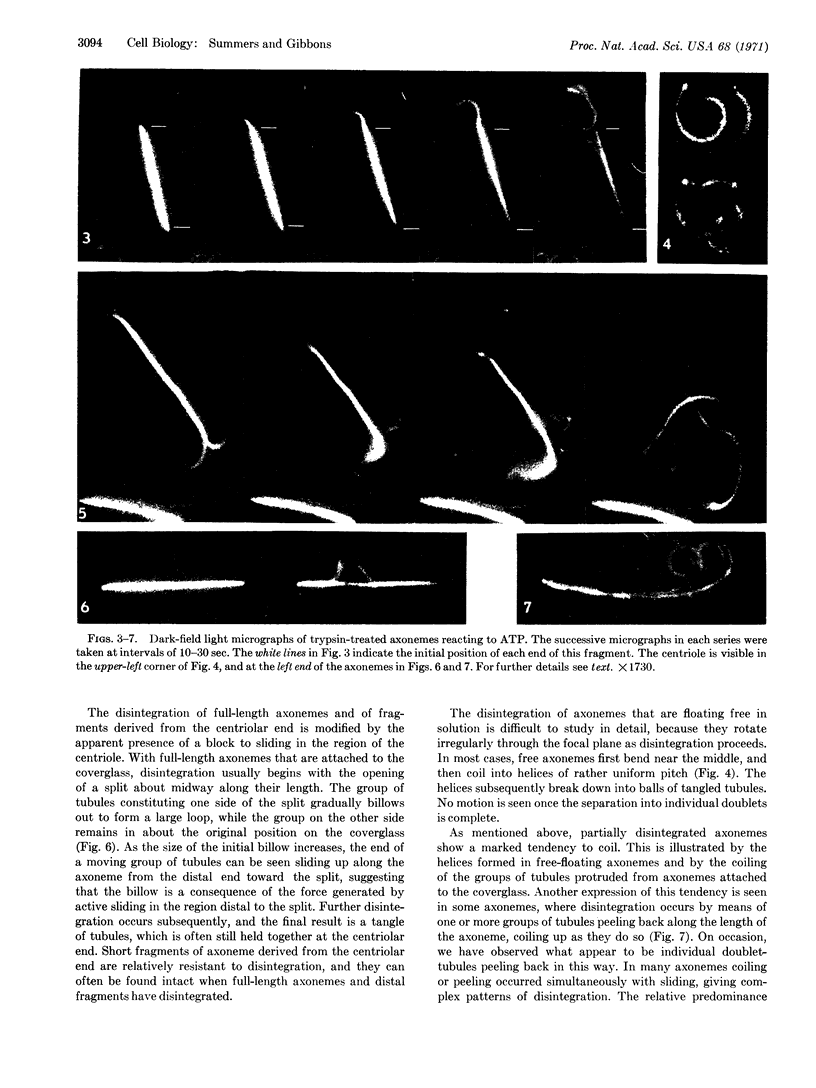
Axonemes isolated from the sperm of the sea urchin, Tripneustes gratilla, were briefly digested with trypsin. The digested axonemes retained their typical structure of a cylinder of nine doublet-tubules surrounding a pair of single tubules. The digestion modified the axonemes so that the subsequent addition of 0.1 mM ATP caused them to disintegrate actively into individual tubules and groups. The nucleotide specificity and divalent-cation requirements of this disintegration reaction paralleled those of flagellar motility, suggesting that the underlying mechanisms were closely related. Observations by dark-field microscopy showed that the disintegration resulted from active sliding between groups of the outer doublet-tubules, together with a tendency for the partially disintegrated axoneme to coil into a helix. Our evidence supports the hypothesis that the propagated bending waves of live-sperm tails are the result of ATP-induced shearing forces between outer tubules which, when resisted by the native structure, lead to localized sliding and generate an active bending moment.
Keywords: motility, microtubule, cilia, sliding filament model, axonemes
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AFZELIUS B. Electron microscopy of the sperm tail; results obtained with a new fixative. J Biophys Biochem Cytol. 1959 Mar 25;5(2):269–278. doi: 10.1083/jcb.5.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura S., Eguchi G., Iino T. Salmonella flagella: in vitro reconstruction and over-all shapes of flagellar filaments. J Mol Biol. 1966 Apr;16(2):302–316. doi: 10.1016/s0022-2836(66)80174-2. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Bend propagation along flagella. Nature. 1966 Jan 8;209(5019):161–163. doi: 10.1038/209161a0. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Bending moments in free-swimming flagella. J Exp Biol. 1970 Oct;53(2):445–464. doi: 10.1242/jeb.53.2.445. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Effects of increased viscosity on the movements of some invertebrate spermatozoa. J Exp Biol. 1966 Aug;45(1):113–139. doi: 10.1242/jeb.45.1.113. [DOI] [PubMed] [Google Scholar]
- GIBBONS I. R., GRIMSTONE A. V. On flagellar structure in certain flagellates. J Biophys Biochem Cytol. 1960 Jul;7:697–716. doi: 10.1083/jcb.7.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS I. R. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J Biophys Biochem Cytol. 1961 Oct;11:179–205. doi: 10.1083/jcb.11.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R. Chemical dissection of cilia. Arch Biol (Liege) 1965;76(2):317–352. [PubMed] [Google Scholar]
- Gibbons I. R. Studies on the adenosine triphosphatase activity of 14 S and 30 S dynein from cilia of Tetrahymena. J Biol Chem. 1966 Dec 10;241(23):5590–5596. [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Lubliner J., Blum J. J. Model for bend propagation in flagella. J Theor Biol. 1971 Apr;31(1):1–24. doi: 10.1016/0022-5193(71)90117-2. [DOI] [PubMed] [Google Scholar]
- Rikmenspoel R., Sleigh M. A. Bending moments and elastic constants in cilia. J Theor Biol. 1970 Jul;28(1):81–100. doi: 10.1016/0022-5193(70)90065-2. [DOI] [PubMed] [Google Scholar]
- Satir P. Studies on cilia. 3. Further studies on the cilium tip and a "sliding filament" model of ciliary motility. J Cell Biol. 1968 Oct;39(1):77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. A. Patterns of ciliary beating. Symp Soc Exp Biol. 1968;22:131–150. [PubMed] [Google Scholar]