Abstract
Psychologists have long distinguished between prospective and retrospective timing to highlight the difference between our sense of duration during an experience in passing and our sense of duration in hindsight. Humans and other animals use prospective timing in the seconds-to-minutes range in order to learn durations, and can organize their behaviour based upon this knowledge when they know that duration information will be important ahead of time. By contrast, when durations are estimated after the fact, thus precluding the subject from consciously attending to temporal information, duration information must be extracted from other memory representations. The accumulated evidence from prospective timing research has generally led to the hippocampus (HPC) being casted in a supporting role with prefrontal–striatal, cortical or cerebellar circuits playing the lead. Here, I review findings from the animal and human literature that have led to this conclusion and consider that the contribution of the HPC to duration memory is understated because we have little understanding about how we remember duration.
Keywords: context, hippocampus, episodic memory, time cells, interval timing, temporal sequence
1. Introduction: the hippocampus is critical to remembering experiences
In our everyday life, we perceive events as unfolding in relation to other events that precede, co-occur or follow. Despite this complex and seemingly endless stream of information, we are able to later bring to mind events and the context of ‘when’ they occurred in our past. The ability to recover the temporal context of a past event together with related events that occurred around that time falls under the domain of episodic memory, and is largely connected to what is meant by ‘remembering’ in the word's everyday use [1]. Indeed, one of the hallmarks of episodic remembering is the temporal organization of the events that compose a memory for a distinct experience. In this way, episodic memory supports remembering the temporal order of events that occurred, and provides a foundation for a mental chronology of our personal history.
There is overwhelming evidence from animal and human studies that the hippocampus (HPC) is critical to episodic memory [2–4]. For example, humans with selective bilateral hippocampal damage sustained relatively early in life have a robust impairment in episodic memory, but show spared semantic memory [2]. Moreover, patients that develop acute, transient lesions of the HPC (specifically, area CA1) are amnesic for personal experiences with a flat retrograde gradient that extends decades into the past [5]. Accordingly, fMRI studies show that the HPC is involved in remembering personal experiences and the order in which they occurred on an autobiographical timeline [6,7].
The HPC also contributes to the temporal organization of memories on a much finer timescale. Hippocampal damage dramatically impairs remembering the order of events separated by durations in the range of seconds to minutes. For example, Mayes et al. [8] reported that patients with selective bilateral hippocampal damage could recall whether they read a single word from a recently presented word list and, in another paradigm, could identify whether two words had been presented together as a pair on a recently presented list. However, they were impaired in memory for the order in which two words were presented within a list, as well as the order of the lists. Thus, hippocampal damage can leave intact the ability to recognize the recent occurrence of single words or word pairs in isolation, while impairing the ability to remember the order in which they occurred in relation to one another. Fortin et al. [9] developed a task to assess the rat's memory for the temporal order of odours and demonstrated a similar effect in rats with complete hippocampal lesions. Human functional imaging studies have confirmed and extended upon the major conclusions from these lesion studies. For example, hippocampal activation during sequence learning is related to the length of the ‘empty’ duration between two events that must be bridged for the successful encoding of their association [10]. Moreover, there is greater activation in the HPC when subjects correctly encode and remember the temporal order of events in an experience [11–13]. Taken together, these results support the idea that the HPC contributes to the temporal organization of events in episodic memory, and that such a function can support accurate judgements about when events occurred in relation to one another.
Because the content and organization of episodic memory is fundamentally temporal in nature, it seems only natural to ask how time is represented in the HPC? [14] This is a challenging question if for no other reason than time is a complex cognitive phenomenon, and different kinds of temporal information may contribute to organizing event sequences in memory. In this regard, research in temporal cognition has generally distinguished among distinct yet related aspects of psychological time, such as duration and succession among others [15,16]. In the light of this issue on interval timing, my central focus is to consider the contribution of the HPC to memory of durations ranging from seconds to minutes. In studies of episodic memory, time is typically conceived as an ever-changing, multi-dimensional background stimulus—i.e. context—in which events are embedded in memory [17]. Consequently, repeated occurrences of an event can be remembered as distinct occurrences, even if the events are otherwise identical across repetitions. In these studies, the duration between events during encoding, or between encoding and retrieval may be manipulated as an independent variable, but often the end game is to evaluate how and whether the events are remembered (but see [18] for example). By contrast, at the forefront of interval timing research is to understand how durations are perceived and coded into memory—subjective duration is the dependent variable. These two fields have mainly evolved in parallel from one another, and it is my hope that this review is encouraging more integration.
Considerable evidence from interval timing research has led to the HPC being cast in a supporting role for duration memory, with prefrontal–striatal, cortical and cererbellar circuits playing the lead [19–21]. This is surprising, because a representation of duration in the HPC would preserve in episodic memory the temporal separation among events that occurred in the learning experience to enhance their distinction and facilitate judgements about their temporal order. Here, I suggest that this supporting role is largely earned because of a contemporary emphasis on understanding the neural basis of duration memory based upon prospective timing instead of retrospective timing. In the remainder of this review, I discuss the distinction between prospective and retrospective timing, what we know about prospective duration memory, then review the evidence that has supported a secondary role for the HPC in prospective timing. Finally, I elaborate on retrospective duration memory, and discuss some evidence that the HPC plays a more critical role in this process.
2. Prospective and retrospective timing
James [22] asserted that our sense for the flow of time depends on an awareness of change. Along the same line, duration is related to an awareness of the amount of change in or between events. Indeed, we perceive events as persisting in such a way that the amount of time that flows thereafter can be measured as an interval. Duration refers to an interval that marks the beginning and end of an event, or can refer to an interval between two distinct events (which may be filled with other events).
It is important to recognize that psychologists have long distinguished between prospective and retrospective timing to highlight the difference between the duration of an experience in passing and duration of an experience in hindsight [22–25]. This distinction is captured in the laboratory by using two paradigms that are designed to draw upon each process differently [25]. In prospective paradigms, subjects are always instructed beforehand that they will have to estimate an arbitrary target duration from memory, and tasks differ in how the estimate is reported. In a duration production task, subjects are explicitly informed of a target duration, and to indicate with a verbal or motor response when it has elapsed. Duration reproduction tasks are similar except that subjects instead experience the target duration first, and then reproduce the interval afterwards. A third variant is a duration comparison task. Here, subjects are asked to compare an estimate of a target duration with a standard duration, and report a categorical decision about the comparison (e.g. same or different). Finally, another variant involves allowing the subject to experience a target duration and then asking for a verbal estimate in conventional chronometric units (e.g. seconds). All tasks in which animals are trained to ‘estimate’ duration (see §5) are prospective paradigms. In a retrospective paradigm, subjects are asked to estimate the duration of an experience, but are not aware that they would be asked to do so during the experience. Thus, subjects typically engage in an activity throughout the target duration without knowing they will be asked to estimate the duration of the experience. Later, they are asked to judge duration based upon their memory of the experience. In this way, information about duration is incidentally encoded during the target duration and deployed at some later time to make an estimate.
The difference between prospective and retrospective timing serves as more than a simple heuristic, because there is evidence that each paradigm taps into different psychological processes [26–29]. This is significant, because decades of research have revealed that memory is not a unitary phenomenon, but rather multiple memory systems exist in the brain that can be differentiated by their anatomical circuits and mnemonic operating principles [30–32]. Duration estimates based upon either paradigm may reflect memory that is supported by different neural circuits. However, our current understanding of the HPC's contribution to duration memory is largely as a result of prospective timing experiments. With this in mind, I discuss in §3 what is known about the processes that underlie prospective duration memory, and then I examine the contribution of the HPC to this process based upon human and animal studies (§§4 and 5).
3. Cognitive processes that underlie prospective duration memory
Research on prospective timing has shown that prospective duration memory is systematically less precise for longer durations [33,34]. This so-called scalar property is apparent when subjects are asked to make estimates about durations encoded into memory. For example, human subjects asked to demarcate a target duration with button presses are, on average, accurate but show more variable estimates for longer durations [35]. More interesting, however, is that variability increases linearly with the length of the duration in memory. What this means is that the precision of prospective duration memory is a constant proportion of its accuracy.
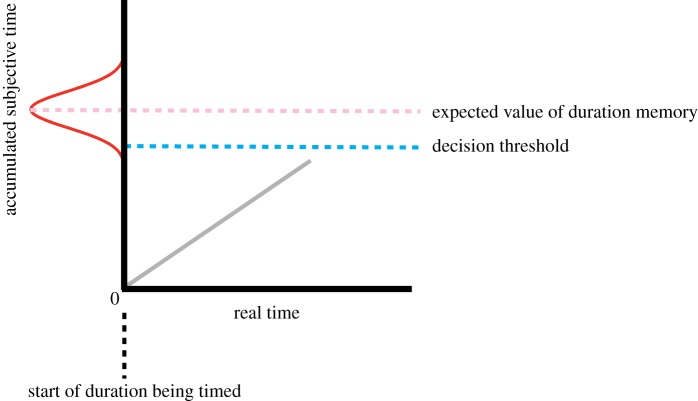
Scalar timing theory (STT) is the dominant psychological model of prospective timing. It was originally developed to account for animal response timing but has also proved valuable for understanding duration memory in humans [33,34,36–38]. From the outset, STT was developed with the scalar property in mind and strongly influenced by normative psychophysics [39]. The scalar property was conceived as arising potentially from several components. Here, I focus on three such sources of variability—the clock-accumulator, memory and the decision threshold [37]. The gist of STT is that the onset of the target duration to be estimated entails the accumulation of pulses emitted by an internal clock. The clock-accumulator is conceived as tightly connected to processing in working memory [37,40,41], and evidence for an independent clock-accumulator and memory component is strongly supported by pharmacological experiments in the animal literature [42–46]. Memory is a long-term store for the expected number of pulses that accumulates across the target duration and is conceived as a distribution. It is assumed that this distribution in memory is based upon extensive experience with the target duration in the past; a product of contents in working memory (clock-accumulator) having been translated into long-term memory many times before. The decision threshold is simply a performance factor that maps the result of a comparison between working memory and long-term memory with a behavioural response. To illustrate how STT is applied to duration production, suppose a signal turns on explicitly marking the beginning of the target duration to be estimated (figure 1). The number of pulses begins to grow in the clock-accumulator. This growing count is compared with a value sampled from long-term memory, and a response is made when these values are ‘close enough’ (i.e. exceeds a decision threshold).
Figure 1.
Cartoon depicting the basics of scalar timing theory. Note that the x- and y-axis refer to real time and accumulated subjective time, respectively. The origin of the plot indicates the beginning of a duration that is being estimated. The Gaussian-shaped distribution (red) on the y-axis indicates the prospective duration memory that is acquired from experience. The upper dotted line (mauve) indicates the expected value of the duration memory. The solid grey line indicates the relationship between the growing magnitude of accumulated subjective time and the passage of real time. The lower dotted line (blue) is the decision threshold that is set in relation to the expected value of duration memory. A response is made when the grey line crosses the decision threshold, or when the current growing duration estimate is ‘close enough’ to the duration from memory. (Online version in colour.)
Therefore, STT provides a quantitative framework by which scalar variation in prospective timing can be isolated to different sources [47–49]. Quantitative modelling of animal and human behaviour in prospective timing tasks points to memory as a significant source of scalar variance [37,50]. This fundamental quantitative property of prospective duration memory places constraints on the underlying cognitive and neural mechanisms. It also provides a useful diagnostic for the normal functioning of duration memory in prospective timing.
4. Human studies of prospective duration memory
Many human amnesics have been tested using prospective timing paradigms. In a duration reproduction task, H.M.'s duration estimates up until 20 s were generally accurate [51]. However, for intervals longer than 20 s, H.M. consistently reproduced durations shorter than the target, and the impairment was more pronounced with longer durations. Several studies have since followed up on this finding with reports of patients exhibiting amnesia that is characteristic of hippocampal dysfunction [52]. In one experiment, Perbal et al. [53] presented a case study of a patient (A.C.) who was assessed using various prospective timing paradigms and three intervals (5, 14 and 38 s). Patient A.C. had left hippocampal damage together with damage to some extrahippocampal areas [54] but presented with anterograde and retrograde amnesia for episodic memory, whereas her skill learning, semantic and working memory were generally intact. While A.C. was found to have no impairment in a duration production task, the largest effect observed was using a variant of a duration reproduction task where she reproduced durations shorter than the target duration. There was some suggestions that the scalar property of duration memory was altered, but the effect was inconsistent across tasks. These findings are generally in agreement with another study of a patient who developed dense anterograde amnesia for autobiographical information [55]. Patient B.W. reproduced 30–120 s target durations as shorter in a reproduction task, but showed little impairment for shorter intervals.
Several general conclusions can be made from neuropsychological studies of prospective timing. The most commonly reported prospective timing impairment resulting from hippocampal amnesia is that memory for duration is shortened and is consequently underestimated. Moreover, this change in the content of memory is observed with durations as short as 10 s. There are several reasons why duration underestimation is best described as a memory impairment instead of a faster clock-accumulator, which would also result in duration underestimation. Generally, from the STT standpoint, clock-accumulator speed effects are only apparent if the clock-accumulator speed operating when the target duration is encoded into memory differs from the clock-accumulator speed operating when the subject is actively estimating the target duration. There is no reason to think why this is the case under normal circumstances. There are inconsistencies concerning the generality of the effect on prospective duration memory both within and across studies [56,57], but the differences may be explained by the unselective nature of the hippocampal damage and details of the task, such as the stimulus material used to indicate the target durations (see §5).
While neuropsychological studies can address the necessity of the HPC for prospective timing, they do not address whether the HPC is normally engaged in the process. Such is the advantage of function imaging studies, and the functional imaging of prospective timing has been reviewed on several occasions [58–62]. Across reviews, there are commonly no reports of hippocampal activation, or other medial temporal lobe (MTL) structures typically implicated in episodic memory, in a variety of prospective timing tasks. One exception is a study reported by Harrington et al. [63], who found increased hippocampal activation related to the duration estimate in a duration comparison task. Conversely, a subsequent fMRI experiment that used prospective duration comparison paradigms reported no activation in MTL structures [64]. The conclusion that emerges from these reviews is puzzling in the light of the effect of hippocampal amnesia on prospective timing, and the results from animal studies described in §5. However, there are several reasons why this may be the case. Hippocampal activity is evidently opposing a relatively subtle change in the content of duration memory, which may obscure the detection of hippocampal involvement without the appropriate experimental design. In addition, when and how this content change is taking place is not known; the change may be primarily occurring offline during sleep, for example. Other reasons include the use of highly familiar stimuli with low complexity, short target durations and reliance on a motor response to indicate the duration estimate.
5. Animal studies of prospective duration memory
One major advantage in animal studies of prospective timing is that there is more control over the extent and location of hippocampal damage and more opportunity to fully characterize the effect. Fortunately, many such studies have been undertaken. That animals can encode duration into memory is most apparent when events in their environment reliably predict the occurrence of reward at a later specific time. In animal studies, a motor response, such as a lever press or nose poke, serves in lieu of verbal judgements about duration memory. After a predictive event occurs, the motor response used to obtain reward becomes more likely as time passes, and peaks approximately when the target duration has elapsed. Thus, one indicator of prospective duration memory in animals is response timing, such as a temporal gradient of responding that reaches maximal levels at the target duration.
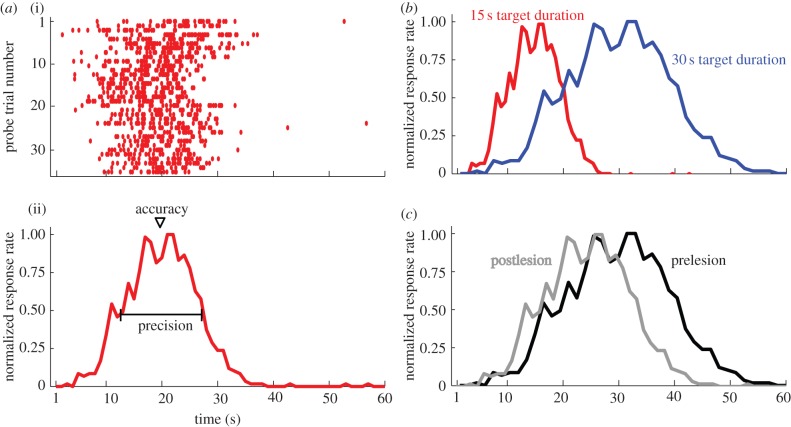
The peak interval (PI) procedure [65,66] is a widely used prospective timing task that has been adapted for a broad range of species, including humans. There are two kinds of trials randomly intermixed in a testing session: fixed duration and probe trials. On a fixed duration trial, a signal (e.g. light) turns on, and an animal is rewarded only if it makes a response (e.g. lever press) after the target duration elapses. Animals learn to obtain reward soon after the target duration elapses. On a probe trial, the animal is not rewarded for responding, and the signal stays on long past the target duration. Response timing data obtained from probe trials (peak functions) are Gaussian-shaped and are generally centred on the target duration (figure 2ai). Two useful diagnostics of duration memory can be extracted from the peak function (figure 2aii). The accuracy of duration memory is indicated by when the highest response rate occurs (peak time). The precision of the duration memory is indicated by the spread of the temporal gradient around the peak (figure 1a). Because the spread is generally linearly proportional to their peak time, peak functions exhibit the scalar property (figure 2b). Consequently, the PI procedure serves as an ideal tool to screen prospective memory.
Figure 2.
The peak interval (PI) procedure is an ideal screen for prospective duration memory. (a) Data from a rat that is trained on the PI procedure [64]. The raster plot (i) depicts lever presses (ticks) during each probe trial. The x-axis is time relative to the beginning of the signal that is timed. Probe trial responses are pooled across trials into 1 s time bins to generate the peak function (ii). The peak function illustrates the trial average response rate and is normalized to range between zero and unity. Because the signal extends well past the target duration (20 s), the accuracy and precision of the prospective duration memory is inferred by the rat's response rate before and after 20 s. (b) This panel depicts two hypothetical peak functions from a single rat that was trained to time one signal using a 15 s target duration (left trace, red) and another signal using a 30 s target duration (right trace, blue). Note that the precision of the peak function for the 30 s target duration is larger in absolute terms, but is the same in relative terms. (c) Depicts two hypothetical peak functions from a rat trained using a 30 s target duration. The black peak function depicts data collected prior to a fimbria–fornix lesion. The grey peak function depicts data several months after the lesion. Note that the leftward shift of the grey peak function indicates that hippocampal lesions cause duration underestimation.
Peak functions from rats with lesions of the fimbria–fornix (one major source of hippocampal input/output) are initially comparable with controls, but gradually shift to earlier peak times over the course of many testing sessions [67,68]. The shift eventually comes to an end, but the earlier peak time persists for as long as testing continues over the course of several months (figure 2c). That duration underestimation is a permanent consequence of hippocampal dysfunction suggests that prospective duration memory is shortened, and is generally consistent with human neuropsychological studies. In addition, the scalar property is preserved in animals with fimbria–fornix lesions. However, a recent study confirmed a dissociation between the effects of dorsal and ventral hippocampal lesions on performance in the PI procedure (see [69]). While dorsal and ventral lesions of the HPC result in a shortened prospective duration memory, the scalar property was violated in rats with ventral, but not dorsal hippocampal lesions. This dissociation is notable, and raises the question of whether it is a consequence of compensatory changes in areas that are known to be involved in prospective timing and are downstream from the ventral HPC, such as the prefrontal cortex [20,58,59]. Hippocampal damage also results in comparable impairments with other prospective timing tasks in which animals estimate duration, such as the differential reinforcement of low response rate (DRL) task [63,70]. In the DRL task, animals learn to space consecutive responses in time; once they make a response, the subsequent response is rewarded only if they wait until the target duration elapses. Animals with hippocampal damage systematically underestimate duration and respond earlier than normal animals [71].
Is the HPC engaged in animals performing prospective timing tasks under normal conditions? There are very few animal studies in which hippocampal neural activity is monitored in combination with prospective timing tasks. One experiment focused on single cell recordings of the HPC in rats trained on the DRL task [72]. The activity from some hippocampal neurons gradually decayed after pressing a lever and reached a minimum when the target duration elapsed and the rat pressed the lever again. This profile is consistent with the cells signalling a fading memory trace of the lever press, whose rate of decay would determine when the rat stops waiting and presses the lever (see also [73]). However, there was no obvious relationship between the rate of decay and timing of the rat's second lever press. The remaining class of cells signalled the behaviours of the rat in between lever presses, or the lever press itself. These results show that the HPC signals various features of a DRL task, and that some cells signal temporal information that may be used by downstream areas to track duration and guide when to respond. Other studies have focused on relatively more global electrophysiological signatures of prospective timing by investigating local field potentials in the HPC [74]. These studies adduce evidence that the HPC is relatively more engaged in prospective timing tasks than in non-timing tasks.
6. Hippocampal damage alters the content of prospective duration memory, but does not eliminate it
In animals and humans, the most consistent outcome from hippocampal dysfunction is the shortening of prospective duration memory, and the magnitude of shortening is typically 10–15% of the target duration. In many cases, the scalar property is reported as being intact, which suggests that the precision of prospective duration memory scales appropriately with the accuracy change just as in the normal functioning brain. Nevertheless, more studies in both humans and animals are needed to investigate this topic; delineating the neural circuits that underlie the scalar property is of great importance to fully understand the mechanisms of prospective duration memory. Collectively, the emerging picture from human and animal studies is that prospective duration memory is generally preserved in humans and animals following hippocampal damage. The nature of the impairment indicates that hippocampal function is required for maintaining an accurate prospective duration memory that is otherwise encoded extrahippocampally.
It is important to emphasize that a lesion-induced alteration in the content of duration memory is entirely consistent with some prospective timing studies in animals that reported no effect of hippocampal damage [75,76]. Consider a duration comparison paradigm wherein the animal must make one response for a reward (‘press left lever’) if it experiences a short target duration and an alternative response for reward if it experiences a long target duration (‘press right lever’). Hippocampal damage would result in the shortening of duration memory for both the short and long target duration, and as such choice performance would not be expected to suffer.
Because extrahippocampal structures can largely support normal prospective duration memory, does the HPC disengage during prospective timing? The study of prospective timing is strongly influenced by the psychophysical tradition. As such, many experiments involve the use of a limited number of target durations, low-complexity sensory stimuli (e.g. pure tones) to demarcate the beginning and end of a duration, and a large number of stimulus repetitions using each target duration. Consistent with psychophysical principles, this approach allows for the variability in performance to be decomposed and attributed to different sources that are prescribed by formal models of prospective timing. The quantitative methods are powerful, but the experimental design and ‘steady-state’ assumptions of prospective timing processes are not conducive to detecting hippocampal activation. Indeed, the HPC can be activated in circumstances that require information to be actively maintained across short delays [77,78]. However, the circumstances entail the use of novel or complex material, and in this way, neocortical areas are not in a position to represent the stimuli in support of short-term memory [79].
While it will be crucial to understand precisely how the HPC contributes to prospective duration memory and coordinates with other brain areas to mediate this process [80,81], yet another approach to understanding the representation of duration in the HPC is to consider the case of retrospective timing. Indeed, the role of the HPC in duration memory might be understated simply, because we have little understanding about how we remember the duration of experiences (see also [82]).
7. Cognitive processes that underlie retrospective duration memory
Models of retrospective timing have only been developed to account for human experimental findings, and eschew clocks and accumulators. Instead, when a person is unexpectedly asked to estimate a target duration in retrospect, they have to remember the experience and feature(s) of the memory are assumed to be diagnostic of its actual duration. For this reason, retrospective duration estimates are referred to as remembered duration [16]. Unlike prospective duration estimates, retrospective estimates do not increase proportionally to the duration of the experience being remembered; therefore prospective timing is generally more accurate than retrospective timing [26]. Moreover, prospective timing is considered more susceptible to changes in attentional resources because it involves actively monitoring duration as it lengthens [16,25,26].
Ornstein [83] proposed one of the earliest cognitive theories of remembered duration, and likened it to a constructive process at the time of retrieval wherein the number of events that were encoded during the experience is related to the length of the remembered duration. In other words, an experience remembered as being filled with more events will also be remembered as being longer in hindsight (cf. [22]). However, many studies since that time have shown that the successful encoding of events that occurs during an experience is not a primary determinant of remembered duration [84–89]. Instead, remembered duration is related to encoding into memory changes in the context1 surrounding the occurrence of events throughout an experience [16]. Some insight into how duration of an experience is estimated retrospectively is gleaned by considering the simple situation in which subjects learn a distinct sequence of events. Here, the experience is defined by the beginning and end of a discrete event. The question of interest is whether the ‘empty’ duration between the two events is incidentally encoded into memory—that is, even when subjects have no explicit instruction that they will need to express this information.
In one experiment [90], subjects studied a long list of words presented one at a time. Some words were repeated, but the number of intervening words (i.e. duration) between the first and second occurrence was manipulated as an independent variable. Critically, the subjects were aware that there would be some word repetitions, but would only later be tested for their memory of the individual words. In testing, subjects who recognized words as having been repeated during study could also indicate their relative separation in time. That is, they remembered that repeated words separated by shorter durations during study occurred closer together in time than repeated words separated by relatively longer durations in study. Thus, duration information was encoded even though subjects had no reason to expect it would later be relevant. Subsequent experiments demonstrated this effect for the occurrence of two words in the list that are conceptually related (e.g. ‘king’ and ‘queen’) [91]. These and other studies yielding similar results [92] led to a proposal that subjects remember the past occurrence of a word and how long ago it happened when they are reminded by the occurrence of the same (or conceptually related) word. As a result, the encoding of memory for the reminding word and its surrounding context includes a reference to the word's past occurrence and prevailing context at that time. This relationship also enables the automatic (but see [93]) encoding of duration as an accessible property of the memory ([90,94]; see also [92,95,96]).
Remarkably, Miller and co-workers have also provided comparable evidence in animals that duration is encoded as part of memories for different event sequences (reviewed in [97]). An illustrative finding is from one experiment by Cole et al. [98] in which there were two groups of animals (figure 3). In the first training phase, a discrete sound stimulus (S1) preceded a shock (X) in both groups, but in one group they were separated by 5 s (group ‘trace’) and in another they were separated by 0 s (group ‘no trace’). In the second phase, for both groups, the S1 was then paired with a novel sound stimulus (S2)—that is, S1 → S2—but there was no intervening delay. Thus, both groups encoded two sequence memories (S1 → X and S1 → S2). The only difference between the two groups was the sequence memory from phase 1—i.e. did a 5 or 0 s delay separate S1 and the shock? Figure 3 illustrates the temporal relationships among events in each sequence memory for both groups. In a final test phase, they found that group ‘trace’ expressed more fear to S2 than group ‘no trace’. This finding demonstrates that each sequence memory includes information about the duration in between two events that compose the sequence, even when there is little evidence for prospective timing by the animals.2 Moreover, the sequence memories were integrated to update S2 with predictive information about the shock based upon duration information that was encoded incidentally, even though the S2 and the shock were never directly experienced together (see figure 3—‘integrated representation’).
Figure 3.
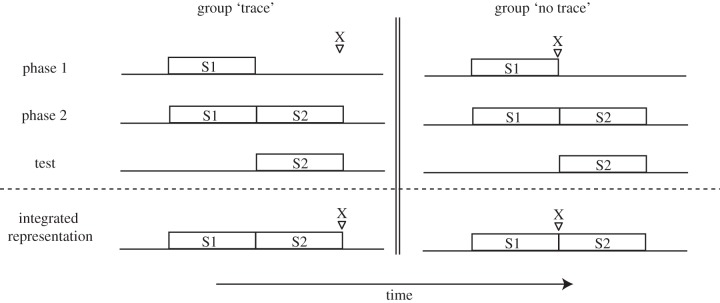
Depiction of the basic training protocol for the experiment. There are two groups: group ‘trace’ and group ‘no trace’, which differ depending on whether there is a 5 or 0 s trace between a sound (S1) and shock (X) during phase 1 of training. During phase 2, both groups received pairings of S1 followed by S2. During the testing phase, both groups were exposed to S2 and group ‘trace’ exhibited more fear. The explanation is best understood through the illustration of what the ‘integrated representation’ of both sequence memories looks like. Note that the presence of a 5 s duration in group ‘trace’ facilitates an inference that the shock (X) immediately follows S2.
These results suggest that duration information can be incidentally encoded while learning a sequence of events, and later be accessed during episodic remembering. That retrospective timing is strongly connected with remembering the temporal context underlying a sequence of events suggests a critical role for episodic memory [99], and by extension the HPC. Indeed, a recent computational model of episodic memory may provide a foundation by which to consider this relationship [100].
8. Retrospective duration memory in the hippocampus
Behavioural experiments show that durations between events are incidentally encoded while humans and animals learn a distinct sequence of events. How does the HPC process temporal information under these circumstances? We have gained much insight into this question from studies that employ techniques to monitor activity from large populations of hippocampal neurons in combination with sequence memory testing. This section is not intended to present an exhaustive review of the many experiments on this topic. I focus on a few selective experimental paradigms that test non-spatial sequence memory. Readers interested in spatial sequence memory are referred to recent reviews on this topic [101,102].
One of the most striking functional dissociations of the HPC is that damage to the structure can grossly impair trace fear and eyeblink conditioning, but have little effect on the delay versions of each task (see [103,104] for extensive reviews). Because duration is encoded during trace fear conditioning without animals engaged in prospective timing, it is of interest to characterize the hippocampal network during this process. With traces as long as 20 s, hippocampal neurons activate at the expected time of the shock when it is omitted ([105]; see also [106]). Different neurons also activate during the trace, as has been reported in other trace conditioning paradigms, but the response profiles of these cells are more heterogeneous across the population.
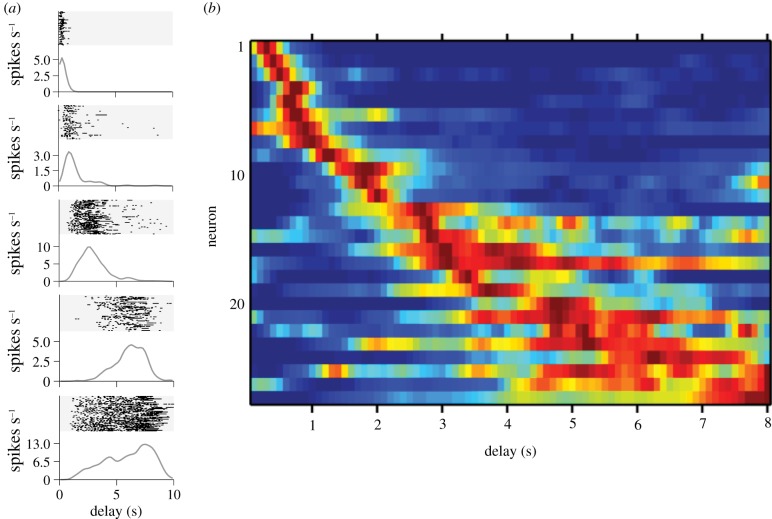
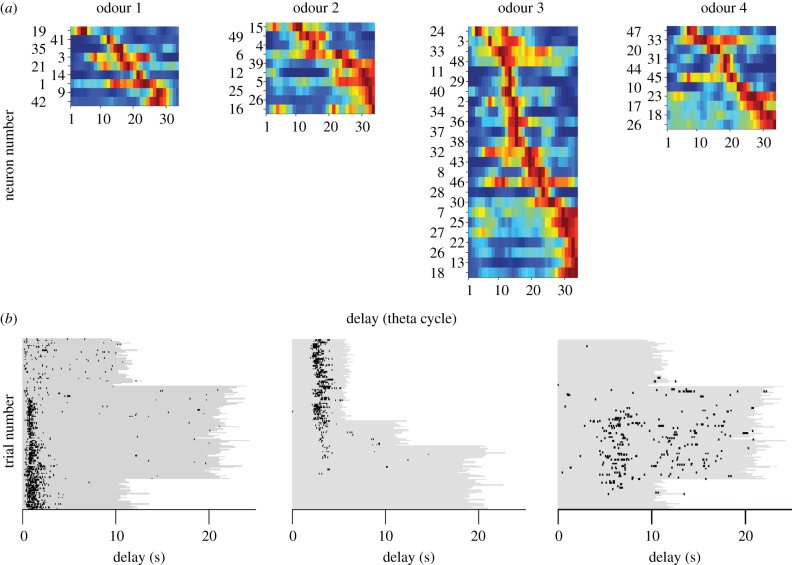
More recently, in a series of experiments MacDonald et al. [107,108] explored the nature of the temporal representation in the HPC for memories of distinct event sequences. In these experiments, freely moving [107] or immobilized [108] rats learned to distinguish among different event sequences, then tested while monitoring the activity of large ensembles of hippocampal CA1 neurons. Here, the sequences were composed of two discrete events, such as the presentation of distinct odours, separated by an ‘empty’ constant delay period. Three general conclusions can be drawn from these experiments. Hippocampal neurons signalled either the first or second event in the sequence, but the most striking finding was that many cells activated during the constant delay period in between the events. Importantly, the neurons selectively activated at different moments and were therefore called ‘time cells’ (figure 4a).3 To better illustrate the temporal signature of these cells, figure 4b plots normalized firing rates from an ensemble of cells recorded simultaneously during the delay from one experiment. It is readily apparent that the cells activated in series, and the overlap among their firing fields bridged the entire delay that separated the two events in the sequence. Of note is that the spread of a firing field is larger for time cells whose peak time of activity occurs later in the delay. This feature is reminiscent of peak interval response timing data discussed in §5 (e.g. figure 2a), and suggests that time cells encode specific durations in relation to the first event in the sequence memory. A second general conclusion is that largely separate time cell ensembles can activate to represent a distinct sequence memory, and the sequence of activity is related to correctly remembered event sequences (figure 5a). Finally, when the delay period is lengthened, the majority of time cells respond by activating in a qualitatively different manner (figure 5b). Some time cells selectively activate at different moments, whereas others stop firing altogether. Some cells that were previously silent begin to selectively fire at moments during the delay. This finding is consistent with the idea that a sequence memory includes information about the duration between its component events, because altering the duration entailed a reorganization of the memory represented in the hippocampal network.
Figure 4.
Time cells selectively encode different moments during the constant delay period between a sequence of events. (a) Each panel (five in total) shows data recorded from a single time cell during the delay period. Each panel includes a raster plot (top: grey-shaded area) illustrating action potentials (black ticks) recorded from the time cell during the delay period and referenced to the end of the first event in the sequence. Each row of the raster plot indicates a single trial (i.e. the presentation of one event sequence). Below the raster plot is trial-averaged activity of the time cell. (b) A heat plot showing activity from a population of hippocampal time cells recorded at the same time that activated in sequence during the delay. Each row shows trial-averaged normalized activity (100 ms bins; red = more activity) for one single neuron across the delay in the recording session. The rows are sorted by increasing latency until maximum activity.
Figure 5.
(a) Hippocampal time cells represent largely distinct memories of event sequences. Each panel depicts a heat plot illustrating normalized trial-averaged activity from a population of time cells during the constant delay as in figure 4b. In this experiment, rats were trained to distinguish four different two-event sequences that differed according to which event (odour) started the sequence. On the top of each heat plot is a label (odour 1–4) that indicates the specific odour that started each event sequence. The rows are sorted by increasing latency until maximum activity, and activity is referenced to the beginning of the delay. Note that the time during the delay period has been transformed to theta cycle time because cells were largely phase locked to this conspicuous hippocampal rhythm (see [102] for details). There are 49 neurons in total, and they were recorded at the same time from a single rat. (b) When the duration of the delay period is extended, the majority of time cells responded by activating in a qualitatively different manner. The three panels show a raster plot indicating time cell action potentials (black ticks) during the recording session. The delay period is indicated with grey shading, and trials are ordered as they occurred during the recording session.
These findings suggest that duration is encoded incidentally in service of remembering non-spatial event sequences. The results parallel those reported from other neurophysiological studies in rats, monkeys and humans that demonstrate a rich representation of time in the HPC [109–112]. Moreover, the results are consistent with classic computational modelling of HPC function that predicts the existence of ‘context’ cells in the HPC that collectively activate in sequence to bridge delays between to-be-remembered items in memory [113,114]. Altogether, the data strongly suggest that the HPC supports the temporal organization of episodic memory by providing a scaffold onto which relevant events are placed. This organizational function supports a memory for ‘when’ events A and B occurred insofar that we can remember, for example, that B came after A. However, this representation also contains information about duration. In other words, the hippocampal representation of a sequence can preserve an ordinal relationship between events as well as how much time passed between events.
9. Conclusion
Our efforts towards understanding how the HPC encodes duration memory have been largely based upon prospective timing. In prospective timing, time is in the foreground, because humans and animals actively monitor duration to inform their current estimate of how much time has passed. The available evidence suggests that the HPC is involved with prospective duration memory, but extrahippocampal structures appear to be more critical. In retrospective timing, time is in the background and incidentally encoded in support of organizing sequential event representations in memory—a process for which the HPC is necessary. The duration information is accessible upon remembering the event sequences, and may be embodied in the sequential firing of time cells that encode a rich temporal representation of distinct event sequences.
Given that duration coding is a ubiquitous property of neural circuits, there needs to be a greater emphasis on understanding the many kinds of memory representations that are deployed to make duration estimates and how their similarities and differences map onto their underlying neural circuitry. To distinguish between the kind of memory deployed in prospective and retrospective timing, I borrow an analogy that Jacoby et al. [115] made while noting that memories are often expressed to accomplish a task in the present without remembering the learning experiences. They compared the distinction between remembering or not remembering with the distinction between using a hammer to pound a nail or attending to the hammer as an object. Just as we can treat a hammer differently despite it being the same object, so too can the same be said for how we can treat memory. Keeping with their analogy, in prospective paradigms, duration memory is treated as a tool to enhance performance in the present. The circuits consistently engaged in prospective timing overlap with regions commonly implicated in the learning and expression of procedural memories [116]. In this way, prospective timing is inextricably connected to the process by which we optimally organize specific behaviours and other cognitive resources in real time (e.g. attention), and this process operates capably without the HPC. Conversely, in retrospective paradigms, we remember an experience to judge its duration, and as such our attention is on the past. The HPC and surrounding areas in the MTL would be expected to be critical for this latter process, but not necessarily the former one. The challenge now is to understand how remembered duration is encoded in the HPC and the MTL, and how this representation differs from and contributes to prospective timing mediated by other neural circuits in service of consciousness and cognition.
Acknowledgements
I thank Howard Eichenbaum for his support over the years. I also apologize to the many colleagues whose work I could not discuss in more detail or cite on account of space limitations.
Endnotes
As noted in §1, context refers to a changing constellation of elements that provide a background in which events occur.
It is notable that the tasks used here do not promote the development of any obvious response timing. Response timing in trace fear conditioning is not a common finding across studies.
In freely moving animals, a detailed statistical analysis of the firing patterns of neurons revealed that the timing signal was independent of many other variables for which the HPC is known to signal, such as the rat's spatial location [107]. In the other experiment, the rats were head-fixed and did not move during the delay throughout testing [108].
Funding statement
This work was supported by National Institute of Mental Health grant no. MH095297.
References
- 1.Tulving E. 1983. Elements of episodic memory. New York, NY: Oxford University Press. [Google Scholar]
- 2.Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. 1997. Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277, 376–380. ( 10.1126/science.277.5324.376) [DOI] [PubMed] [Google Scholar]
- 3.Tulving E, Markowitsch HJ. 1998. Episodic and declarative memory: role of the hippocampus. Hippocampus 8, 198–204. () [DOI] [PubMed] [Google Scholar]
- 4.Steinvorth S, Levine B, Corkin S. 2005. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M, W.R. Neuropsychologia 43, 479–496. ( 10.1016/j.neuropsychologia.2005.01.001) [DOI] [PubMed] [Google Scholar]
- 5.Bartsch T, Döhring J, Rohr A, Jansen O, Deuschl G. 2011. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc. Natl Acad. Sci. USA 108, 17 562–17 567. ( 10.1073/pnas.1110266108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire EA. 2001. Neuroimaging studies of autobiographical event memory. Phil. Trans. R. Soc. B 356, 1441–1451. ( 10.1098/rstb.2001.0944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St. Jacques PL, Rubin DC, LaBar K, Cabeza R. 2008. The short and long of it: neural correlates of temporal-order memory for autobiographical events. J. Cogn. Neurosci. 20, 1327–1341. ( 10.1162/jocn.2008.20091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayes AR, Isaac CL, Holdstock JS, Hunkin NM, Montaldi D, Downes JJ, Macdonald C, Cezayirli E, Roberts JN. 2001. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn. Neuropsychol. 18, 97–123. [DOI] [PubMed] [Google Scholar]
- 9.Fortin NJ, Agster KL, Eichenbaum HB. 2002. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 5, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staresina BP, Davachi L. 2009. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron 63, 267–276. ( 10.1016/j.neuron.2009.06.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schendan HE, Searl MM, Melrose RJ, Stern CE. 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37, 1013–1025. ( 10.1016/S0896-6273(03)00123-5) [DOI] [PubMed] [Google Scholar]
- 12.Tubridy S, Davachi L. 2011. Medial temporal lobe contributions to episodic sequence encoding. Cereb. Cortex 21, 272–280. ( 10.1093/cercor/bhq092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins LJ, Ranaganath C. 2010. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J. Neurosci. 30, 15 558–15 565. ( 10.1523/JNEUROSCI.1337-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olton DS. 1985. The temporal context of spatial memory. Phil. Trans. R. Soc. Lond. B 308, 79–86. ( 10.1098/rstb.1985.0011) [DOI] [Google Scholar]
- 15.Fraisse P. 1984. Perception and estimation of time. Psychol. Rev. 35, 1–36. ( 10.1146/annurev.ps.35.020184.000245) [DOI] [PubMed] [Google Scholar]
- 16.Block RA. 1990. Models of psychological time. In Cognitive models of psychological time (ed. Block RA.), pp. 1–35. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 17.Kahana MJ, Howard MW, Polyn SM. 2008. Associative retrieval processes in episodic memory. In Learning and memory: a comprehensive reference (ed. Byrne J.), pp. 467–490. Oxford, UK: Academic Press. [Google Scholar]
- 18.Brown GD, Neath I, Chater N. 2007. A temporal ratio model of memory. Psychol. Rev. 114, 539–576. ( 10.1037/0033-295X.114.3.539) [DOI] [PubMed] [Google Scholar]
- 19.Mauk MD, Buonomano DV. 2004. The neural basis of temporal processing. Ann. Rev. Neurosci. 27, 307–340. ( 10.1146/annurev.neuro.27.070203.144247) [DOI] [PubMed] [Google Scholar]
- 20.Buhusi CV, Meck WH. 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. ( 10.1038/nrn1764) [DOI] [PubMed] [Google Scholar]
- 21.Buonomano VD, Laje R. 2010. Population clocks: motor timing with neural dynamics. Trends Cogn. Sci. 14, 520–527. ( 10.1016/j.tics.2010.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James W. 1890. The principles of psychology. New York, NY: H. Holt and Company. [Google Scholar]
- 23.Hicks RE, Miller GW, Kinsbourne M. 1976. Prospective and retrospective judgments of time as a function of amount of information processed. Am. J. Psychol. 89, 719–730. ( 10.2307/1421469) [DOI] [PubMed] [Google Scholar]
- 24.Brown SW, Stubbs DA. 1988. The psychophysics of retrospective and prospective timing. Perception 17, 297–310. ( 10.1068/p170297) [DOI] [PubMed] [Google Scholar]
- 25.Block RA, Zakay D. 1997. Temporal cognition. Curr. Dir. Psychol. Sci. 4, 12–16. [Google Scholar]
- 26.Block RA, Zakay D. 1997. Prospective and retrospective duration judgments: a meta-analytic review. Psychon. Bull. Rev. 4, 184–197. ( 10.3758/BF03209393) [DOI] [PubMed] [Google Scholar]
- 27.Block RA, Hancock PA, Zakay D. 2010. How cognitive load affects duration judgments: a meta-analytic review. Acta Psychol. 134, 330–343. ( 10.1016/j.actpsy.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 28.Bisson N, Tobin S, Grondin S. 2010. An ecological approach to prospective and retrospective timing of long duration: a study involving gamers. PLoS ONE 5, e9271 ( 10.1371/journal.pone.0009271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisson N, Tobin S, Grondin S. 2012. Prospective and retrospective time estimates of children: a comparison based on ecological tasks. PLoS ONE 7, e33049 ( 10.1371/journal.pone.0033049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squire LR, Zola-Morgan S. 1991. The medial temporal lobe system. Science 253, 1380–1386. ( 10.1126/science.1896849) [DOI] [PubMed] [Google Scholar]
- 31.Schacter DL, Tulving E. 1994. What are the memory systems of 1994? In Memory systems (eds Schacter DL, Tulving E.), pp. 1–38. Cambridge, MA: MIT Press. [Google Scholar]
- 32.Eichenbaum H, Cohen NJ. 2001. From conditioning to conscious recollection: memory systems of the brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Lejeune H, Wearden J. 2006. Scalar properties and animal timing-conformity and violations. Q. J. Exp. Psychol. A 59, 1875–1978. ( 10.1080/17470210600784649) [DOI] [PubMed] [Google Scholar]
- 34.Wearden J, Lejeune H. 2006. Scalar properties and human timing–conformity and violations. Q. J. Exp. Psychol. A 61, 569–587. ( 10.1080/17470210701282576) [DOI] [PubMed] [Google Scholar]
- 35.Wearden J. 1991. Do humans possess an internal clock with scalar timing properties. Learn. Motiv. 22, 29–83. ( 10.1016/0023-9690(91)90017-3) [DOI] [Google Scholar]
- 36.Gibbon J. 1977. Scalar expectancy theory and Weber's law in animal timing. Psychol. Rev. 84, 279–325. ( 10.1037/0033-295X.84.3.279) [DOI] [Google Scholar]
- 37.Gibbon J, Church RM, Meck WH. 1984. Scalar timing in memory. Ann. NY Acad. Sci. 423, 52–77. ( 10.1111/j.1749-6632.1984.tb23417.x) [DOI] [PubMed] [Google Scholar]
- 38.Church RM. 2003. A concise introduction to scalar timing theory. In Functional and neural mechanisms of interval timing (ed. Meck WH.), pp. 3–22. Boca Raton, FL: CRC Press. [Google Scholar]
- 39.Gibbon J. 1990. Origins of scalar timing. Learn. Motiv. 22, 3–38. ( 10.1016/0023-9690(91)90015-Z) [DOI] [Google Scholar]
- 40.Fortin C. 1999. Short-term memory in time interval production. Int. J. Psychol. 34, 308–316. ( 10.1080/002075999399611) [DOI] [Google Scholar]
- 41.Lustig C, Matell MS, Meck WH. 2005. Not ‘just’ a coincidence: frontal–striatal interactions in working memory and interval timing. Memory 13, 431–438. [DOI] [PubMed] [Google Scholar]
- 42.Maricq AV, Roberts S, Church RM. 1983. Methamphetamine and time estimation. J. Exp. Psychol. Anim. Behav. Process. 7, 18–30. ( 10.1037/0097-7403.7.1.18) [DOI] [PubMed] [Google Scholar]
- 43.Meck WH. 1983. Selective adjustment of the speed of internal clock and memory processes. J. Exp. Psychol. Anim. Behav. Process. 9, 171–201. ( 10.1037/0097-7403.9.2.171) [DOI] [PubMed] [Google Scholar]
- 44.Meck WH. 1986. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol. Biochem. Behav. 25, 1185–1189. ( 10.1016/0091-3057(86)90109-7) [DOI] [PubMed] [Google Scholar]
- 45.Meck WH, Church RM. 1987. Cholinergic modulation of the content of temporal memory. Behav. Neurosci. 101, 457–464. ( 10.1037/0735-7044.101.4.457) [DOI] [PubMed] [Google Scholar]
- 46.Meck WH. 1996. Neuropharmacology of timing and time perception. Cogn. Brain Res. 3, 227–242. ( 10.1016/0926-6410(96)00009-2) [DOI] [PubMed] [Google Scholar]
- 47.Church RM, Meck WH, Gibbon J. 1994. Application of scalar timing theory to individual trials. J. Exp. Psychol. Anim. Behav. Process. 20, 135–155. ( 10.1037/0097-7403.20.2.135) [DOI] [PubMed] [Google Scholar]
- 48.Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. 1998. Scalar expectancy theory and peak-interval timing in humans. J. Exp. Psychol. Anim. Behav. Process. 24, 15–33. ( 10.1037/0097-7403.24.1.15) [DOI] [PubMed] [Google Scholar]
- 49.Gallistel CR, King A, McDonald R. 2004. Sources of variability and systematic error in mouse timing behavior. J. Exp. Psychol. Anim. Behav. Process. 24, 15–33. [DOI] [PubMed] [Google Scholar]
- 50.Gibbon J. 1992. Ubiquity of scalar timing with a Poisson clock. J. Math. Psychol. 36, 283–293. ( 10.1016/0022-2496(92)90041-5) [DOI] [Google Scholar]
- 51.Richards W. 1973. Time reproductions by H.M. Acta Psychol. 37, 279–282. ( 10.1016/0001-6918(73)90020-6) [DOI] [PubMed] [Google Scholar]
- 52.Cohen NJ, Eichenbaum H. 1993. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press. [Google Scholar]
- 53.Perbal S, Pouthas V, Van der Linden M. 2000. Time estimation and amnesia: a case study. Neurocase 6, 347–356. ( 10.1080/13554790008402782) [DOI] [Google Scholar]
- 54.Van der Linden M. 2003. Semantic memory and amnesia. Implications for theory and rehabilitation. In Memory: basic concepts, disorders and treatment (eds De Deyn PP, Thierry E, D'Hooge R.), pp. 135–147. Leuven, Belgium: Acco. [Google Scholar]
- 55.Williams JM, Medwedeff CH, Haban G. 1989. Memory disorder and subjective time estimation. J. Clin. Exp. Neuropsychol. 11, 713–723. ( 10.1080/01688638908400927) [DOI] [PubMed] [Google Scholar]
- 56.Shaw C, Aggleton JP. 1994. The ability of amnesic subjects to estimate time intervals. Neuropsychologia 32, 857–873. ( 10.1016/0028-3932(94)90023-X) [DOI] [PubMed] [Google Scholar]
- 57.Shaw C, Aggleton JP. 1995. Evidence for the independence of recognition and recency memory in amnesic subjects. Cortex 31, 57–71. ( 10.1016/S0010-9452(13)80105-0) [DOI] [PubMed] [Google Scholar]
- 58.Coull JT, Nobre AC. 2008. Dissociating explicit timing from temporal expectation with fMRI. Curr. Opin. Neurobiol. 18, 137–144. ( 10.1016/j.conb.2008.07.011) [DOI] [PubMed] [Google Scholar]
- 59.Coull JT, Cheng R, Meck WH. 2011. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36, 3–25. ( 10.1038/npp.2010.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P. 2002. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp. Brain Res. 142, 475–485. ( 10.1007/s00221-001-0953-0) [DOI] [PubMed] [Google Scholar]
- 61.Lewis PA, Miall RC. 2006. Remembering the time: a continuous clock. Trends Cogn. Sci. 10, 401–406. ( 10.1016/j.tics.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 62.Penney TB, Vaitilingam L. 2008. Imaging time. In Psychology of time (ed. Grondin S.), pp. 261–294. Bingley, UK: Emerald. [Google Scholar]
- 63.Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, Rao SM. 2004. Neural representation of interval encoding and decision making. Cogn. Brain Res. 21, 193–205. ( 10.1016/j.cogbrainres.2004.01.010) [DOI] [PubMed] [Google Scholar]
- 64.Coull JT, Nazarian B, Vidal F. 2008. Timing, storage, and comparison of stimulus duration engage discrete anatomical components of a perceptual timing network. J. Cogn. Neurosci. 20, 2185–2197. ( 10.1162/jocn.2008.20153) [DOI] [PubMed] [Google Scholar]
- 65.Catania AC. 1970. Reinforcement schedules and psychophysical judgments. In Theory of reinforcement schedules (ed. Schoenfeld WN.), pp. 1–42. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- 66.Roberts S. 1981. Isolation of an internal clock. J. Exp. Psychol. Anim. Behav. Process. 7, 242–268. ( 10.1037/0097-7403.7.3.242) [DOI] [PubMed] [Google Scholar]
- 67.Meck WH, Church RM, Olton DS. 1984. Hippocampus, time and memory. Behav. Neurosci. 98, 3–22. ( 10.1037/0735-7044.98.1.3) [DOI] [PubMed] [Google Scholar]
- 68.Olton DS, Meck WH, Church RM. 1987. Separation of hippocampal and amygdaloid involvement in temporal memory dysfunctions. Brain Res. 404, 180–188. ( 10.1016/0006-8993(87)91369-2) [DOI] [PubMed] [Google Scholar]
- 69.Yin B, Meck WH. 2013. Comparison of interval timing behaviour in mice following dorsal or ventral hippocampal lesions with mice having δ-opioid receptor gene deletion. Phil. Trans. R Soc. B 372, 20120466 ( 10.1098/rstb.2012.0466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacDonald CJ, Cheng RK, Meck WH. 2012. Acquisition of ‘Start’ and ‘Stop’ response thresholds in peak-interval timing is differentially sensitive to protein synthesis inhibition in the dorsal and ventral striatum. Front. Integr. Neurosci. 6, 10 ( 10.3389/fnint.2012.00010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinden JD, Rawlins JNP, Gray JA, Jarrard LE. 1986. Selective cytotoxic lesions of the hippocampal formation and DRL performance in rats. Behav. Neurosci. 100, 320–329. ( 10.1037/0735-7044.100.3.320) [DOI] [PubMed] [Google Scholar]
- 72.Young BJ, McNaughton N. 2000. Common firing patterns of hippocampal cells in a differential reinforcement of low rates of response schedule. J. Neurosci. 20, 7043–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staddon JER, Higa J. 1999. Time and memory: towards a pacemaker-free theory of interval timing. J. Exp. Anal. Behav. 71, 215–251. ( 10.1901/jeab.1999.71-215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onodo K, Takahashi E, Sakata S. 2003. Event-related potentials in the frontal cortex, hippocampus, an cerebellum during a temporal discrimination in rats. Brain Res. Cogn. Brain Res. 17, 380–387. ( 10.1016/S0926-6410(03)00139-3) [DOI] [PubMed] [Google Scholar]
- 75.Jackson PA, Kesner RP, Amann K. 1998. Memory for duration: role of hippocampus and medial prefrontal cortex. Neurobiol. Learn. Mem. 70, 328–348. ( 10.1006/nlme.1998.3859) [DOI] [PubMed] [Google Scholar]
- 76.Kyd RJ, Pearce JM, Haselgrove M, Amin E, Aggleton JP. 2007. The effects of hippocampal system lesions on a novel temporal discrimination task for rats. Behav. Brain Res. 187, 159–171. ( 10.1016/j.bbr.2007.09.010) [DOI] [PubMed] [Google Scholar]
- 77.Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. 2001. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus 11, 337–346. ( 10.1002/hipo.1048) [DOI] [PubMed] [Google Scholar]
- 78.Ranganath C, D'Esposito M. 2001. Medial temporal lobe activity is associated with active maintenance of novel information. Neuron 31, 868–873. ( 10.1016/S0896-6273(01)00411-1) [DOI] [PubMed] [Google Scholar]
- 79.Ranganath C. 2010. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus 20, 1263–1290. ( 10.1002/hipo.20852) [DOI] [PubMed] [Google Scholar]
- 80.Yin B, Troger AB. 2011. Exploring the fourth dimension. Front. Integr. Neurosci. 5, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meck WH, Church RM, Church RM. 2013. Hippocampal, time, and memory: a retrospective analysis. Behav. Neurosci. 127, 642–654. ( 10.1037/a0034201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kinsbourne M, Hicks RE. 1990. The extended present: evidence from time estimation by amnesics and normal. In Neuropsychological impairments of short-term memory (eds Vallar G, Shallice T.), pp. 19–29. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 83.Ornstein RE. 1969. On the experience of time. Harmondsworth, UK: Penguin. [Google Scholar]
- 84.Block RA, Reed MA. 1978. Remembered duration: evidence for a contextual-change hypothesis. J. Exp. Psychol. Hum. Learn. Mem. 4, 656–665. ( 10.1037/0278-7393.4.6.656) [DOI] [Google Scholar]
- 85.Poynter WD. 1983. Duration judgment and the segmentation of experience. Mem. Cogn. 11, 77–82. ( 10.3758/BF03197664) [DOI] [PubMed] [Google Scholar]
- 86.Block RA. 1982. Temporal judgments and contextual change. J. Exp. Psychol. Learn. Mem. Cogn. 8, 530–544. ( 10.1037/0278-7393.8.6.530) [DOI] [PubMed] [Google Scholar]
- 87.Block RA. 1985. Contextual coding in memory: studies of remembered duration. In Time, mind, and behavior (eds Michon JA, Jackson JL.), pp. 169–178. Heidelberg, Germany: Springer. [Google Scholar]
- 88.Poynter WD. 1989. Judging the duration of time intervals: a process of remembering segments of experience. In Time and human cognition: a lifespan perspective (eds Levin I, Zakay D.), pp. 305–331. Amsterdam, The Netherlands: North-Holland. [Google Scholar]
- 89.Zakay D, Tsal Y, Moses M, Shahar I. 1994. The role of segmentation in prospective and retrospective time estimation processes. Mem. Cognit. 22, 344–351. ( 10.3758/BF03200861) [DOI] [PubMed] [Google Scholar]
- 90.Hintzman DL, Block RA. 1973. Memory for the spacing of repetitions. J. Exp. Psychol. 99, 70–74. ( 10.1037/h0034761) [DOI] [Google Scholar]
- 91.Hintzman DL, Summers JJ, Block RA. 1975. Spacing judgments as an index of study-phase retrieval. J. Exp. Psychol. Hum. Learn. Mem. 1, 31–40. ( 10.1037/0278-7393.1.1.31) [DOI] [Google Scholar]
- 92.Proctor RW, Ambler BA. 1975. Effects of rehearsal strategy on memory for spacing and frequency. J. Exp. Psychol. Hum. Learn. Mem. 1, 640–647. ( 10.1037/0278-7393.1.5.640) [DOI] [Google Scholar]
- 93.Jacoby LL, Walheim CN. 2013. On the importance of looking back: the role of recursive remindings in recency judgments and cued recall. Mem. Cogn. 41, 625–637. ( 10.3758/s13421-013-0298-5) [DOI] [PubMed] [Google Scholar]
- 94.Hintzman DL. 2011. Research strategy in the study of memory: fads, fallacies, and the search for ‘coordinates of truth.’ Perspect. Psychol. Sci. 6, 253–271. ( 10.1177/1745691611406924) [DOI] [PubMed] [Google Scholar]
- 95.Tzeng OJL, Lee AT, Wetzel CD. 1979. Temporal coding in verbal information processing. J. Exp. Psychol. Hum. Learn. Mem. 5, 52–64. ( 10.1037/0278-7393.5.1.52) [DOI] [Google Scholar]
- 96.Winograd E, Soloway RM. 1985. Reminding as a basis for temporal judgments. J. Exp. Psychol. Learn. Mem. Cogn. 11, 262–271. ( 10.1037/0278-7393.11.2.262) [DOI] [PubMed] [Google Scholar]
- 97.Molet M, Miller RR. In press. Timing: an attribute of associative learning. Behav. Process. ( 10.1016/j.beproc.2013.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cole RP, Barnet RC, Miller RR. 1995. Temporal encoding in trace conditioning. Anim. Learn. Behav. 23, 144–153. ( 10.3758/BF03199929) [DOI] [Google Scholar]
- 99.Block RA. 2003. Psychological timing without a timer: the roles of attention and memory. In Time and mind II: information-processing perspectives (ed. Helfrich H.), pp. 41–59. Seattle, WA: Hogrefe & Huber. [Google Scholar]
- 100.Shankar KH, Howard MW. 2010. Timing using temporal context. Brain Res. 1365, 3–17. ( 10.1016/j.brainres.2010.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buzsaki G, Moser E. 2013. Memory, navigation, and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138. ( 10.1038/nn.3304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foster DJ, Knierim JJ. 2012. Sequence learning and the role of the hippocampus in rodent navigation. Curr. Opin. Neurobiol. 22, 294–300. ( 10.1016/j.conb.2011.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woodruff-Pak DS, Disterhoft JF. 2008. Where is the trace in trace conditioning? Trends Neurosci. 31, 105–112. ( 10.1016/j.tins.2007.11.006) [DOI] [PubMed] [Google Scholar]
- 104.Clark RE. 2011. Eyeblink conditioning and systems consolidation: an ironic yet powerful pairing. Neurobiol. Learn. Mem. 95, 118–124. ( 10.1016/j.nlm.2010.12.003) [DOI] [PubMed] [Google Scholar]
- 105.McEchron MD, Tseng W, Disterhoft JF. 1998. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J. Neurosci. 23, 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen G, Wang LP, Tsien JZ. 2009. Neural population-level memory traces in the mouse hippocampus. PLoS ONE 4, e8256 ( 10.1371/journal.pone.0008256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. 2011. Hippocampal ‘time cells’ bridge the gap in memory for discontiguous events. Neuron 71, 737–749. ( 10.1016/j.neuron.2011.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacDonald CJ, Carrow S, Place R, Eichenbaum H. 2013. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J. Neurosci. 33, 14 607–14 616. ( 10.1523/JNEUROSCI.1537-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manns JR, Howard MW, Eichenbaum H. 2007. Gradual changes in hippocampal activity support remembering the order of events. Neuron 56, 530–540. ( 10.1016/j.neuron.2007.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. 2008. Internally generated reactivation of single neurons in human hippocampus during free recall. Science 322, 96–101. ( 10.1126/science.1164685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paz R, Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. 2010. A neural substrate in the human hippocampus for linking successive events. Proc. Natl Acad. Sci. USA 107, 6046–6051. ( 10.1073/pnas.0910834107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naya Y, Suzuki WA. 2011. Integrating what and when across the primate medial temporal lobe. Science 333, 773–776. ( 10.1126/science.1206773) [DOI] [PubMed] [Google Scholar]
- 113.Levy WB. 1996. A sequence predicting CA3 is a flexible assocaitor that learns and uses context to solve hippocampal-like tasks. Hippocampus 6, 579–590. () [DOI] [PubMed] [Google Scholar]
- 114.Wallenstein GV, Eichenbaum HB, Hasselmo ME. 1998. The hippocampus as an associator of discontiguous events. Trends Neurosci. 21, 317–323. ( 10.1016/S0166-2236(97)01220-4) [DOI] [PubMed] [Google Scholar]
- 115.Jacoby LL, Kelley CM, Dwyan J. 1989. Memory attributions. In Varieties of memory and consciousness: essays in honour of Endel Tulving (eds Roediger HL, Craik FIM.), pp. 391–422. Hillsdale, NJ: Erlbaum. [Google Scholar]
- 116.Cohen NJ, Squire LR. 1980. Preserved learning and retention of pattern analyzing skill in amnesia: dissociation of knowing how and knowing that. Science 210, 207–209. ( 10.1126/science.7414331) [DOI] [PubMed] [Google Scholar]