Abstract
Background.
There is growing interest in the primary care management of patients with chronic non-cancer pain (CNCP) who are prescribed long-term opioid therapy.
Objective.
The aim of this study was to examine the care management practices and medical utilization of patients prescribed high doses of opioids relative to patients prescribed traditional doses of opioids.
Methods.
We conducted a retrospective cohort study of veterans who had CNCP in 2008 and reviewed medical care for the prior 2 years. Patients with CNCP who were prescribed high-dose opioid therapy (≥180mg morphine equivalent per day for 90+ consecutive days; n = 60) were compared with patients prescribed traditional dose opioid therapy (5–179mg morphine equivalent per day for 90+ consecutive days; n = 60).
Results.
Patients in the high-dose group had several aspects of documented care that differed from patients in the traditional dose group, including more medical visits, attempting an opioid taper, receiving a urine drug screen and developing a pain goal. The majority of variables that were assessed did not differ between groups, including documented assessments of functional status or co-morbid psychopathology, opioid rotation, discussion of treatment side effects, non-pharmacological treatments or collaboration with mental health or pain specialists.
Conclusions.
Further work is needed to identify mechanisms for optimizing care management for patients with CNCP who are prescribed high doses of opioid medications.
Keywords. Chronic pain, clinical treatment guidelines, high-dose opioids, opioids, primary care.
Introduction
With the increased use of opioids to treat chronic non-cancer pain (CNCP) and limited data on the long-term effectiveness of opioids,1 there is growing interest in the development of clinical guidelines to manage pain. Recent guidelines have been published, which provide recommendations for the assessment and treatment of CNCP, including management with chronic opioid therapy (COT).2,3 Guideline criteria typically include recommendations for comprehensive assessment of biopsychosocial functioning, pain intensity, pain-related function, adverse effects and evaluation of aberrant behaviours and concurrent substance use.
Recent studies using administrative data have shown that opioid treatment guidelines are not routinely incorporated into clinical practice.4,5 Additional studies involving extensive review of medical record data found that guideline-recommended opioid management practices were infrequently utilized6 and additional training combined with clinician feedback did not increase provision of documented guideline-concordant pain care.7 Adherence to aspects of opioid treatment guidelines (e.g. risk monitoring) may be somewhat improved for patients at higher risk, such as those with a substance use disorder.4
The issue of high-dose opioid therapy is receiving increased attention. High-dose opioid therapy occurs in 2–3% of patients with CNCP or low back pain and among 8% of patients prescribed COT. High-dose opioid therapy was also characterized by high rates of co-morbid medical, psychiatric and substance use disorders.8,9 The dose of opioids is a major contributing factor in adverse events such as fractures, emergency room visits, poorer treatment outcomes and overdose.10–15 Limited research has been conducted examining correlates of care and treatment outcomes among patients prescribed high-dose opioid therapy. Additional data are needed on patterns of care and treatment approaches for patients prescribed high-dose opioid therapy, in order to reduce potential adverse effects and provide optimal treatment.
Given the risks associated with prescribing high-dose opioid therapy, these patients may benefit from more intensive treatment monitoring and/or management, which are more closely aligned with treatment guidelines. The purpose of this study was to evaluate treatment practices for CNCP among patients prescribed very high doses of opioids (defined as receiving daily opioid doses ≥180mg morphine equivalent, for 90+ days) and to evaluate if patients who are prescribed high doses of opioid medications receive different care than patients prescribed traditional doses of opioids.
Methods
Data were collected from the electronic medical records of patients receiving care at a Veterans Affairs (VA) Medical Center. The Institutional Review Board of the local VA Medical Center provided approval for this study.
Inclusion/exclusion criteria
In order to identify patients with chronic pain, we reviewed pain numeric rating scores (NRS) documented in the electronic medical record. Pain screening is routinely done in the VA system as part of the ‘Pain as the 5th Vital Sign’ initiative. NRS are rated on an 11-point Likert scale from 0 (no pain) to 10 (worst pain imaginable). Patients were indicated as having CNCP if they had pain NRS ≥4 recorded in three separate months within the year 2008. This methodology is consistent with prior research, which suggests that pain NRS greater than or equal to 4 are indicative of moderate to severe pain and pain lasting longer than 3 months is indicative of chronic pain. Patients who received any medical care at the VA Medical Center during the 2008 calendar year were potentially eligible for inclusion. Patients were excluded if they had any visits to a VA opioid substitution program during 2008, had a cancer diagnosis in the prior 6 years, had surgery within the past 6 months or died in 2008. Among this group of patients with CNCP, we identified patients who were prescribed COT, which was defined as receiving an opioid prescription for ≥90 consecutive days.
Of the patients who were eligible for inclusion, we randomly selected 60 subjects from two groups: CNCP patients prescribed high doses of opioid mediations (defined as receiving ≥180mg morphine equivalent per day) for ≥90 consecutive days and CNCP patients prescribed traditional doses of opioid medications (defined as 5–179mg of morphine equivalent per day) for ≥90 consecutive days. Opioid doses were converted to average daily dose in morphine equivalents based on prior research.8
Procedure
An index date was calculated for each patient, which was the earliest date in 2008 of a 90-consecutive-day episode of opioid use. All clinical notes in the electronic medical record data were reviewed by a trained research assistant for the 2 years prior to the index date. The purpose was to examine the medical care of patients with CNCP to evaluate the care they received.
A chart review tool was created for the medical record review to examine the management of patients who were prescribed COT. The initial tool was based on recent work in which we developed a chart review tool to assess clinicians’ adherence to pain treatment guidelines.7 This tool underwent revision and expansion. The final chart review tool required each coder to address the presence of 15 behaviours from the providers’ documentation of patient care. In addition, a coding manual was created, which provided operational definitions and examples of documentation from each category. Table 1 provides a summary of the behaviours that were evaluated in the medical records. Behaviours that were examined include the following: if primary medication provider (PMP) assessed patient’s functional status, as well as assessing for depression, anxiety, substance use, psychosocial stressors, and aberrant behaviours; if PMP consulted with mental health provider or pain specialist; if PMP developed a pain goal or discussed side effects of medication with patient; if PMP tapered or rotated opioid medication; if PMP prescribed a non-opioid medication or recommended a non-pharmacological intervention for pain management; and if the patient participated in a non-pharmacological approach to pain management. [Note that we are referring to PMP instead of the primary care provider (PCP), as we are referring to the clinician who was principally responsible for prescribing opioids to a given patient. In most instances, this was the PCP.] We did not assess whether the PMP evaluated pain intensity, as this is now a common practice in the VA.
Table 1.
Operational definitions
| Functional status | Notation addressing the impact pain has on ability to function physically, socially or occupationally and also may include activities of daily living or any medical record documentation of patient walking or exercising |
| Depression | Notation addressing depressive symptoms by discussion of depressed mood, documenting depressive symptoms or formal assessment of depression |
| Anxiety | Notation addressing anxiety or fear as it is related to a pain condition by documenting anxiety symptoms or formal assessment of anxiety |
| Substance use/abuse | Notation indicating evaluation of current substance use and document non-substance use or indicating that patient participates in a substance abuse treatment program |
| Psychosocial stressors | General discussion of life stressors, problems at work, at home or in relationships |
| Prescribed non-opioid medications | Evidenced by prescription from provider or provider’s recommendation of patient to use non-opioid pain medications (e.g. capsaicin cream, NSAIDs) |
| Rotated opioid medication | Evidenced by change to a new pain medication in hopes of improved pain control or reduction in side effects |
| Tapered the dose of opioid medication | Evidenced by an explicit statement that the provider is titrating the dose of the patient’s pain medication or marked reduction in opioid dose with appropriate documentation |
| Administered urine drug screen | Documentation by clinician of results of a urine drug screen that had been performed at the time of the visit or within 30 days prior to appointment, or review of laboratory data indicating urine drug screen was administered |
| Discussed side effects of opioid prescriptions | Notation addressing common side effects of opioid pain medications or indication that patient and provider discussed potential side effects |
| Considered a non-pharmacological approach | Provider documented consideration, discussion or patient education about self-management or non-pharmacological pain management treatments |
| Patient participated in non-pharmacological approach | Documentation in medical record that patient engaged in a self-management or non-pharmacological pain management treatment |
| Collaboration with mental health provider | Notation identifying mental health care in the clinical note, designating mental health provider as a co-signer of the note or identifying a mental health goal in the note |
| Consulted with a pain specialist | Notation identifying recommendation, referral or current consultation with pain specialist, pain treatment program, surgeons or other provider (e.g. anaesthesiologist, physiatrist and rheumatologist), specifically for the treatment of pain |
| Developed a pain goal | Notation addressing work towards a goal related to chronic pain by creating specific physical, social or occupational activities, medication dosage or reduction in pain score that the patient would like to integrate into the treatment plan (i.e. ‘When asked about goals, patient wants to be able to do his mother-in-law’s yard-work without having to stop due to pain) |
NSAIDs, non-steroidal anti-inflammatory drugs.
Preliminary training of medical record reviewers occurred in a half-day workshop. Initially, two raters independently coded the medical records from the same 10 patients. The two raters met with the study principal investigator to discuss discrepancies and come to agreement. Additional charts were reviewed, and once agreement was greater than 80% on all variables, the raters each coded charts separately. In order to prevent drift and ensure high inter-rater reliability, charts were randomly selected for joint review on an ongoing basis, which resulted in 37.5% of all charts reviewed by two coders. A total of 120 patient records were reviewed (60 patients in the high-dose group and 60 patients in the traditional dose group).
Demographic, pharmacy, diagnostic and medical utilization data
Demographic and clinical data were extracted from the Veterans Integrated Service Network-20 Data Warehouse. The Data Warehouse contains data from the main clinical software packages of the regional VA health care facilities and two national VA databases. Demographic data included age, gender, race, marital status and VA service-connected disability status. Pharmacy data were reviewed to extract information on prescriptions of current opioid analgesics at the time of each patient’s index date. Pain and psychiatric diagnoses were obtained using the International Classification of Diseases, Ninth Revision, Clinical Modification codes listed in medical encounter records for the 2 years prior to the index date. VA medical service utilization data over the 2 years prior to the index date were assessed for visits to the emergency room, physical therapy, orthopaedics, individual mental health treatment, group mental health treatment and specialty substance abuse treatment.
Statistical analyses
The groups were compared in order to examine if differences existed between patients in the high-dose group and the traditional dose group in the care they received. Categorical variables were analyzed using chi-square tests and continuous variables were analyzed using t-tests. With a total sample size of 120, using an alpha of 0.05, we had power greater than 80% to detect an effect size of 0.30.16 This effect size is comparable to a small to moderate clinically significant effect. Multivariate logistic regression analyses were conducted to determine if differences identified in bivariate comparisons remained significant after controlling for potential covariates. These analyses controlled for age, pain intensity and diagnosis of co-morbid major depressive disorder. The primary independent variable of interest was opioid group (high-dose group or traditional dose group).
Results
Demographic and clinical characteristics
There were no significant differences found between the high-dose and the traditional dose groups in the areas of age, gender, race, marital status or per cent of patients who are VA service connected (Table 2). Patients from the high-dose group had an average daily opioid dose of 398.7mg (SD = 722.2) morphine equivalent (range = 180–5796.4; median = 268.3). Patients from the traditional dose group had an average daily opioid dose of 23.9mg (SD = 15.5) morphine equivalent (range = 5–72.1; median = 20.3). Numeric pain intensity scores and pain diagnoses did not differ between the two groups. Rates of psychiatric and substance use disorders did not significantly differ between groups, with the exception of bipolar disorder, which occurred more often among patients in the traditional dose group (0.0% versus 8.3%, P = 0.022).
Table 2.
Comparison of demographic characteristics and clinical diagnoses
| High-dose group (n = 60) | Traditional dose group (n = 60) | P value | |
|---|---|---|---|
| Age | 57.3 (9.8) | 57.2 (13.01) | 0.986 |
| Male | 95.0% (57) | 88.3% (53) | 0.186 |
| Race | |||
| Caucasian | 70.0% (42) | 60.0% (36) | 0.251 |
| Not reported | 25.0% (15) | 38.3% (23) | 0.116 |
| Other | 5.0% (3) | 1.7% (1) | 0.309 |
| Marital status | |||
| Married | 51.7% (31) | 58.3% (35) | 0.463 |
| Separated or divorced | 33.3% (20) | 31.7% (19) | 0.845 |
| Single | 11.7% (7) | 5.0% (3) | 0.186 |
| Widowed | 3.3% (2) | 5.0% (3) | 0.648 |
| VA service connected | 98.3% (59) | 98.3% (59) | 1.00 |
| Average daily dose in morphine equivalent | 398.7mg (722.2) | 23.9mg (15.5) | <0.001 |
| Median daily opioid dose | 268.3 mg | 20.3 mg | – |
| Pain intensity | 6.6 (1.7) | 6.5 (1.2) | 0.527 |
| Pain diagnoses | |||
| Neck or joint pain | 73.3% (44) | 76.7% (46) | 0.673 |
| Low back pain | 76.7% (46) | 63.3% (38) | 0.111 |
| Arthritis | 70.0% (42) | 56.7% (34) | 0.130 |
| Neuropathy | 16.7% (10) | 21.7% (13) | 0.487 |
| Migraine | 11.7% (7) | 15.0% (9) | 0.591 |
| Fibromyalgia | 11.7% (7) | 8.3% (5) | 0.543 |
| Psychiatric diagnoses | |||
| Depression | 61.7% (37) | 58.3% (35) | 0.709 |
| Dysthymia | 18.3% (11) | 15.0% (9) | 0.624 |
| Bipolar disorder | 0.0% (0) | 8.3% (5) | 0.022 |
| PTSD | 41.7% (25) | 31.7% (19) | 0.256 |
| Panic disorder | 10.0% (6) | 8.3% (5) | 0.752 |
| Other anxiety disorders | 20.0% (12) | 20.0% (12) | 1.000 |
| Schizophrenia | 0.0% (0) | 5.0% (3) | 0.079 |
| Substance use disorder | 30.0% (18) | 25.0% (15) | 0.540 |
| Tobacco use | 38.3% (23) | 35.0% (21) | 0.705 |
PTSD, post-traumatic stress disorder. Column values indicate % (n) for categorical variables or mean ± SD for continuous variables.
Clinician assessments and activities
Table 3 displays the documented assessment and treatment activities patients received from their PMP in the 2 years prior to the index date. Patients in the high-dose group averaged more visits to their PMP over the course of 2 years (M = 6.9 visits for the high-dose group versus M = 5.3 visits for the traditional dose group, P = 0.022). The two groups did not differ in likelihood of assessment for depression, anxiety or substance use by their PMPs.
Table 3.
Comparison of documented PMP assessments and activities over 2 years
| High-dose group (n = 60) | Traditional dose group (n = 60) | P value | |
|---|---|---|---|
| Average number of visits to PMP | 6.9 (4.9) | 5.3 (2.7) | 0.022 |
| Assessed for | |||
| Functional status | 80.0% (48) | 78.3% (47) | 0.822 |
| Depression | 91.7% (55) | 95.0% (57) | 0.464 |
| Anxiety | 88.3% (53) | 90.0% (54) | 0.769 |
| Substance use/abuse | 88.3% (53) | 95.0% (57) | 0.186 |
| Psychosocial stressors | 65.0% (39) | 63.3% (38) | 0.849 |
| Activities conducted | |||
| Prescribed non-opioid medication for pain | 45.0% (27) | 65.0% (39) | 0.028 |
| Rotated opioid prescription | 23.0% (14) | 18.3% (11) | 0.500 |
| Tapered opioid prescription dose | 28.3% (17) | 11.7% (7) | 0.022 |
| Administered urine drug screen | 45.0% (27) | 20.0% (12) | 0.003 |
| Discussed side effects of opioid prescription | 41.7% (25) | 51.7% (31) | 0.272 |
| Considered a non-pharmacological approach | 71.7% (43) | 67.0% (40) | 0.553 |
| Patient participated in non-pharmacological approach | 50.0% (30) | 50.0% (30) | 1.000 |
| Collaborated with mental health provider | 38.3% (23) | 40.0% (24) | 0.852 |
| Consulted with a pain specialist | 31.7% (19) | 23.3% (14) | 0.307 |
| Developed a pain goal | 48.3% (29) | 20.0% (12) | 0.001 |
Column values indicate % (n) for categorical variables or mean ± standard deviation for continuous variables.
PMPs were significantly more likely to develop a pain goal with the patients from the high-dose group (48.3% versus 20.0%, P = 0.001). Pain goals typically consisted of the provider assisting in creating a goal based on the patients’ interest in engaging in a behaviour or activity that was realistic for the patient to accomplish. The high-dose group was less likely to be prescribed a non-opioid medication for pain (45.0% versus 65.0%, P = 0.028). Patients in the high-dose group were more likely to have attempted an opioid taper (28.3% versus 11.7%, P = 0.022) and to have been administered urine drug screens (45.0% versus 20.0%, P = 0.003) in the prior 2 years than patients in the traditional dose group.
Logistic regression analyses were conducted to evaluate whether the bivariate relationships identified as significant remained, after controlling for demographic and clinical factors. In these analyses, patients in the high-dose group were more likely to have received an opioid taper [odds ratio (OR) = 3.50, 95% confidence interval (CI) = 1.27–9.66] and to have developed a pain goal with their provider (OR = 3.93, 95% CI = 1.71–9.01). The overall model was also significant for collaboration with a mental health provider; however, the variable opioid prescription status (high dose versus traditional dose) was not statistically significant, and the only statistically significant variable in the analysis examining collaboration with a mental health provider was having a current diagnosis of major depressive disorder (OR = 3.45, 95% CI = 1.46–8.13).
Medical utilization
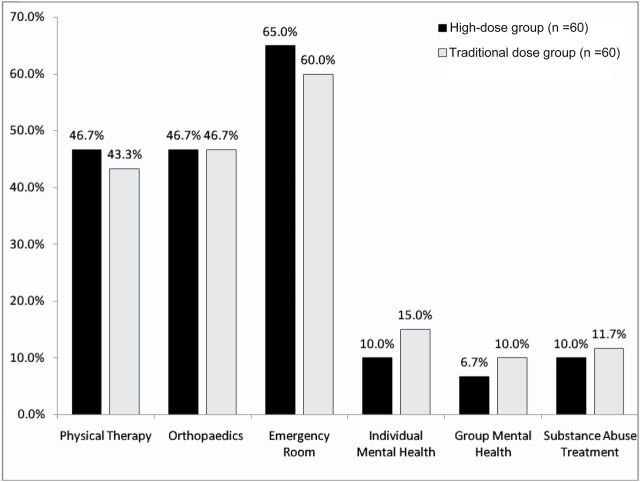
There were no significant differences between the two groups in visits to physical therapy, orthopaedics or the emergency room in the 2 years prior to the index date (Fig. 1). Among the subset of patients with documented diagnoses of major depression or dysthymic disorder, there was a significant difference between the two opioid groups in rates of treatment with antidepressant medications (28.3% versus 11.7%, P = 0.022). Among patients with a documented anxiety disorder, those in the high-dose group were more likely to receive a co-prescription for benzodiazepine medications (78.1% versus 48.0%, P = 0.018). There were no significant differences between groups in receipt of individual or group mental health therapy, or participation in substance abuse treatment.
Figure 1.
Health care utilization in the 2 years prior to each patient’s index date. Note: The two groups did not differ on any medical utilization variables that were assessed (all P values > 0.05)
Discussion
There is increasing concern about the negative side effects of opioid medications. These concerns are heightened for patients prescribed high doses of opioids. We examined the documented clinical management of patients with CNCP who were prescribed daily opioid doses of 180mg morphine equivalent or more, relative to patients prescribed lower opioid doses, to attempt to better understand the clinical care received by this group of patients. In some ways, the types of care did not differ between the two groups. Patients in the high-dose group had documented assessments for functional status, depression, anxiety and psychosocial stressors at rates that did not significantly differ from patients who were prescribed traditional doses of opioids. There were no differences between groups in the use of non-pharmacological approaches for pain management, opioid rotation, documented discussion of medication-related side effects or consultation with pain specialists or mental health providers. Alternatively, patients in the high-dose group had more visits with their PMPs and were more likely to develop a pain goal with their PMPs, receive urine drug screen testing and receive opioid tapers. Additionally, PMPs were less likely to prescribe non-opioid prescriptions for pain to patients in the high-dose group.
Because higher doses of opioids may confer greater risks,10–15 it is incumbent to enhance care for patients with CNCP who are prescribed high doses of opioids. It is encouraging that patients in the high-dose group had some aspects of guideline-concordant care occur at higher rates (such as more frequent visits with their PMPs, discussions regarding pain status and treatment options and receiving urine drug screens). However, patients in the high-dose group were also more likely to receive concurrent prescriptions for benzodiazepines and less likely to receive a non-opioid medication for pain. These prescribing patterns may be problematic as sedative-hypnotic medications may interact with opioids to increase the likelihood of adverse events and non-opioid pain medications are often recommended as one part of a comprehensive pain management program to help enhance pain control. Additionally, there were a high proportion of patients who had documented diagnoses of an alcohol or substance use disorder, but did not participate in substance abuse specialty treatment, while continuing to receive prescriptions for opioid medications. Opioid treatment guidelines uniformly indicate that opioid therapy is contraindicated for patients with a substance use disorder who are not in treatment.2,3
When reviewing data for patients for 2 years prior to their 2008 index date, we found that all patients in the high-dose group had been prescribed high-dose opioids since 2006, or longer. Similarly, all 60 patients who were in the traditional dose group in 2008 were also prescribed traditional doses of opioids in 2006. We did not find evidence of any patient who had new starts of opioids in this time period or had increased or decreased opioid dose to change from one opioid group to another (i.e. no patients were prescribed traditional doses in 2006 and escalated to the high-dose group in 2008). These findings are consistent with other research indicating that once long-term opioid therapy is established, it will often remain ongoing.17 Given that no patients in our group shifted from the high-dose group to traditional dose group, or vice versa, the data indicate that opioid dose also becomes entrenched.
An opioid taper had been attempted by 28% of patients in the high-dose group. Reliable data on reasons for the opioid taper were not available. It was also unclear how successful these opioid tapers were, as all patients in the high-dose group that had undergone an opioid taper remained in the high-dose group. General recommendations exist about methods for tapering an opioid dose, but there is limited research to guide specific recommendations on tapering strategies.2
Across both high- and traditional dose groups, we found substantial numbers of patients who did not have documented consideration of changes to the opioid regimen, discussion of opioid side effects, consideration of or recommendation for non-pharmacological approaches to pain or collaboration with mental health. These findings suggest additional work is needed to improve the receipt of guideline-concordant care across all patients receiving COT. Other studies have identified usual care treatment patterns that diverge from opioid treatment guidelines, including limited use of opioid risk reduction strategies5 and frequent co-prescriptions for opioids with other medications that may have dangerous interactions.18,19 Prior attempts to change care processes or adherence to treatment guidelines for patients with CNCP have had limited success.7,20 Additional research is needed to identify methods for improving adherence to opioid treatment guidelines, for all patients who are prescribed COT.
The primary limitation of this study is its reliance on documentation provided by the PMP in the patients’ medical charts. Clinicians may vary in accuracy of the documentation of clinical care. The discrepancy between the clinical care provided and medical record documentation could result in an inaccurate depiction of treatment that occurs during a visit. This study was guided by opioid treatment guidelines, which attempt to incorporate the best available evidence to direct clinical practice. However, there are limited empirical data available to inform many aspects of treatment with opioid medications, and guidelines often include recommendations based on expert consensus instead of research findings. Further research is needed to assess the impact of improved adherence to opioid treatment guidelines on clinical outcomes. Another limitation relates to the time frame for viewing medical record data. We reviewed records for 2 years; it may be possible that certain aspects of care occurred prior to the index date, and we did not assess it. It is also possible that clinical care may have occurred at another VA or a non-VA facility, which we were not able to assess. All patients were receiving care at a single VA Medical Center; results may not necessarily generalize to other clinical settings. Finally, care for these patients occurred from 2006 to 2008, and it is unclear how the clinical management of patients with chronic pain who are prescribed opioids has changed in recent years, due to publication of new opioid treatment guidelines2,3 and emerging data reflecting potential benefits and harms associated with opioid medications.
In summary, results of this 2-year retrospective cohort study found that patients who are prescribed high doses of opioids receive some assessment and treatment procedures more frequently than patients who are prescribed traditional doses of opioids, such as frequency of visits, development of a pain treatment goal, opioid tapers and monitoring with urine drug screens. The majority of medical utilization variables and documented treatment procedures that we assessed did not differ between the two groups. In closely reviewing documentation of medical care for patients prescribed long-term opioid therapy for CNCP, we identified multiple aspects of treatment that could be enhanced, in order to be more closely aligned with clinical treatment guidelines. Additional research is needed to test mechanisms for improving adherence with treatment guidelines and to determine if the provision of clinical care in a manner consistent with opioid treatment guidelines enhances the safety or effectiveness of chronic pain treatment.
Declarations
Funding: National Institute on Drug Abuse (K23DA023467 to BJM); Portland Veterans Affairs Medical Center.
Ethical approval: Institutional Review Board of the local VA Medical Center.
Conflict of interest: none.
Acknowledgements
The authors appreciate the assistance of Jonathan Duckart, MPS, for extracting data from the electronic medical record. Dr SG is now affiliated with the Division of Pain Management, Stanford University Medical Center.
References
- 1. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004; 112: 372–80 [DOI] [PubMed] [Google Scholar]
- 2. Chou R, Fanciullo GJ, Fine PG, et al. American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10: 113–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Department of Veterans Affairs and Department of Defense VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain http://www.healthquality.va.gov/Chronic_Opioid_Therapy_COT.asp (accessed on 4 January 2011).
- 4. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med 2011; 26: 965–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med 2011; 26: 958–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med 2011; 12: 740–6 [DOI] [PubMed] [Google Scholar]
- 7. Corson K, Doak MN, Denneson L, et al. Primary care clinician adherence to guidelines for the management of chronic musculoskeletal pain: results from the study of the effectiveness of a collaborative approach to pain. Pain Med 2011; 12: 1490–501 [DOI] [PubMed] [Google Scholar]
- 8. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain 2010; 151: 625–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobus AM, Smith DH, Morasco BJ, et al. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain 2012; 13: 1131–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011; 305: 1315–21 [DOI] [PubMed] [Google Scholar]
- 11. Braden JB, Russo J, Fan MY, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med 2010; 170: 1425–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010; 152: 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171: 686–91 [DOI] [PubMed] [Google Scholar]
- 14. Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am 2009; 91: 919–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med 2010; 25: 310–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen J. Statistical Power Analysis for the Behavioral Sciences 2ndedn. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 17. Martin BC, Fan MY, Edlund MJ, Devries A, Braden JB, Sullivan MD. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med 2011; 26: 1450–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deyo RA, Smith DH, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med 2011; 24: 717–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saunders KW, Von Korff M, Campbell CI, et al. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain 2012; 13: 266–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 2006; 21: 607–12 [DOI] [PMC free article] [PubMed] [Google Scholar]