Abstract
The ‘diversity–stability hypothesis’, in which higher species diversity within biological communities buffers the risk of ecological collapse, is now generally accepted. However, empirical evidence for a relationship between β-diversity (spatial turnover in community structure) and temporal stability in community structure remains equivocal, despite important implications for theoretical ecology and conservation biology. Here, we report strong β-diversity–stability relationships across a broad sample of fish taxa on Australia's Great Barrier Reef. These relationships were robust to random sampling error and spatial and environmental factors, such as latitude, reef size and isolation. While β-diversity was positively associated with temporal stability at the community level, the relationship was negative for some taxa, for example surgeonfishes (Acanthuridae), one of the most abundant reef fish families. This demonstrates that the β-diversity–stability relationship should not be indiscriminately assumed for all taxa, but that a species’ risk of extirpation in response to disturbance is likely to be taxon specific and trait based. By combining predictions of spatial and temporal turnover across the study area with observations in marine-protected areas, we conclude that protection alone does not necessarily confer temporal stability and that taxon-specific considerations will improve the outcome of conservation efforts.
Keywords: β-diversity, Bray–Curtis, disturbance, long-term monitoring, mantel, time series
1. Introduction
The diversity–stability hypothesis states that greater species diversity within biological communities will reduce the risk of ecological collapse. This has been widely debated since its inception in the middle of the last century [1]. Although initially challenged by evidence for reduced stability in species-rich communities [2], the diversity–stability hypothesis has become a widely used rule of thumb and assumption in ecology [3,4] and conservation planning [5]. A range of ecological mechanisms are likely to contribute to the manifestation of this relationship, including: (i) weaker biotic interactions, such as competition and predation in species-rich communities, promoting species persistence [4,6]; (ii) different responses of species to environmental fluctuations, promoting coexistence between species as a result of greater niche partitioning [4,7]; and (iii) greater functional redundancy in species-rich communities, whereby multiple species in each functional group help to maintain that ecological function and thereby contribute to overall community stability [8]. Alternatively, diversity–stability relationships might arise as a sampling artefact, where species present at a site are detected in some but not all instances [9], spuriously inflating estimates of temporal turnover in community structure.
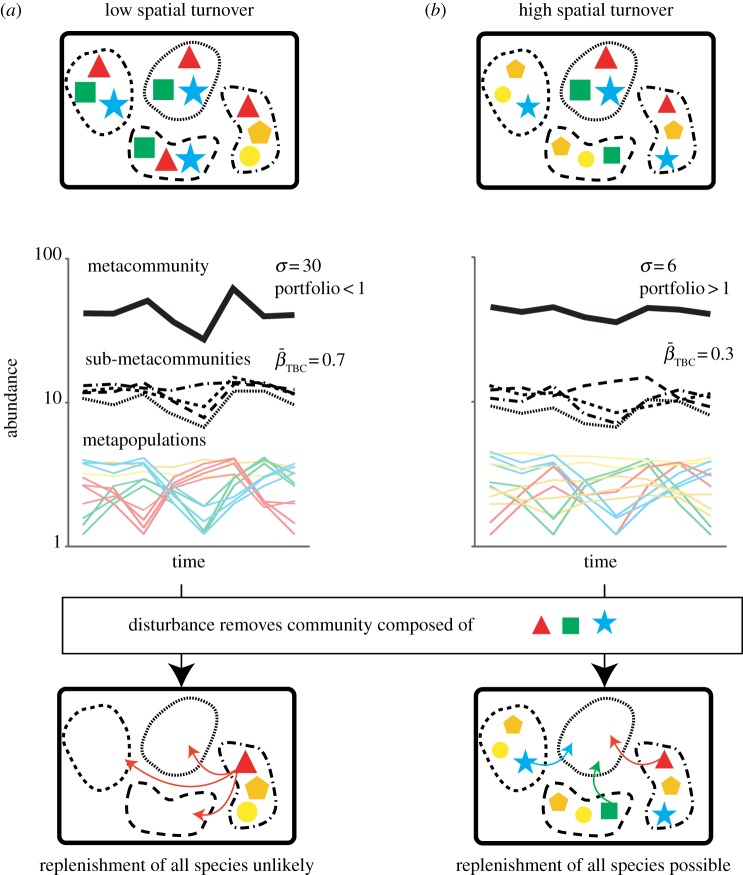
Higher stability strengthens a community's ability to resist disturbance and invasion, and/or recover rapidly from a perturbation [3,4]. Multivariate approaches that quantify temporal dissimilarity in community structure provide a useful way to define stability by documenting changes in community structure resulting from extinction and colonization [10]. However to date, studies investigating the diversity–stability relationship have focused mainly on α-diversity (i.e. local species richness), while its counterpart—the relationship between β-diversity (spatial turnover in community structure) and stability—remains relatively overlooked [11]. β-diversity–stability relationships should be expected within metacommunities, where networks of communities are linked by migration and dispersal [12] and can extend over regional scales (more than 1000 km; figure 1). Under such conditions, structurally diverse communities are likely to display a range of responses to environmental fluctuations, thus generating asynchronous abundance dynamics among the component communities and thereby stabilizing the metacommunity as a whole. This dynamic underpins the portfolio effect in ecological communities [7,13] and metapopulations [14] and is, in theory, also applicable to metacommunity dynamics. It follows that high β-diversity should increase the odds that at least some communities will resist disturbances, such as invasions, diseases or extreme climate events, better than others, and thereby act as refuges from which neighbouring patches can be replenished [11]. Therefore, the risk of local extinctions should decrease, resulting in more stable community structure, and thus decreased temporal turnover. Although such predictions can be tested using simulations (figure 1), empirical tests are still largely lacking.
Figure 1.
Comparison of the β-diversity–stability relationship for two metacommunities with: (a) low spatial turnover (mean spatial Bray–Curtis dissimilarity βSBC = 0.4), and (b) high spatial turnover (mean βSBC = 0.6) and constant local (α = 3) and regional (γ = 5) species richness. Within each metacommunity (thick solid lines), four sub-metacommunities (dotted lines) are each composed of three species (coloured symbols). Assumptions underlying the generation of abundance time series are detailed in the electronic supplementary material. Total abundances were calculated at the sub-metacommunity and metacommunity levels over time, along with indices of temporal turnover (βTBC, mean temporal Bray–Curtis dissimilarity) at the sub-metacommunity level, and temporal variance of total abundance (σ) and the mean–variance portfolio effect (portfolio; [14]; see the electronic supplementary material) at the metacommunity level.
The few studies investigating relationships between β-diversity and community stability have rarely considered their many potential conservation applications. Likewise, many conservation-planning algorithms maximize β-diversity indirectly by prioritizing sites with the greatest species complementarity [15]. Such prioritization assumes selected habitats not only include the most species in a given region, but also that such selection promotes the long-term persistence of those species [16] by minimizing their temporal variance in abundances [17]. However, this stability assumption (i.e. local extinctions within high β-diversity areas can be rescued by propagules from neighbouring habitats) has rarely been addressed explicitly in systematic reserve-selection algorithms [16] and although supported by theory [6,18], remains untested empirically.
Any relationship between spatial and temporal turnover in community structure is likely to be scale-dependent and partly driven by stochastic sampling effects [9,19], particularly at fine spatio-temporal scales [11]. For example, both apparent spatial and temporal turnover can be spuriously inflated by repeatedly sampling individual species within the community. Estimates of β-diversity also depend to some extent on local species richness (i.e. α-diversity) and, for instance, tend to be greater between samples with contrasting α-diversity. The relationship between spatial and temporal turnover can also be influenced by the life histories, and phylogenetic and trophic relationships of the species under consideration as well as environmental heterogeneity, habitat isolation, patch size, latitude and the spatial scale considered [11,20]. Clearly, such artefacts need to be controlled in any analysis of β-diversity–stability [21,22].
Although the few previous studies of β-diversity–stability relationships have reported contrasting results (including positive relationships, e.g. [9]), we are unaware of any studies that have investigated interspecific, life-history-related variation in this relationship while simultaneously controlling for potential stochastic and deterministic modifiers. Mechanistically, these species-specific traits and environmental conditions have the potential to affect relationships between β-diversity and community stability through mechanisms of dispersal, community succession and environmental forcing. Adequate testing of these relationships has been hindered by a lack of broad-scale datasets linked to time series of species’ abundances, comprehensive descriptions of life histories, and associated environmental variables and spatial relationships.
Here, we explore β-diversity–stability relationships in coral reef fish communities and their covariates at a regional (more than 1000 km) scale, using one of the most spatially and temporally extensive datasets on coral reef communities that encompasses 16 years of data and samples covering more than 2300 km of reef stretch. We adopt the multivariate, directional and ‘horizontal’ definition of β-diversity [21], a distance-based metric that measures the rate of change in community structure along a spatial or environmental gradient [21] and is therefore more appropriate for analysing rates of species turnover in space and time than the classic ‘hierarchical’ approach where β-diversity is expressed as the ratio, or alternatively, as the difference, of α- and γ-diversity [11,21]. Similarly, we consider stability at the community level as the inverse of temporal turnover in community structure (i.e. species abundances). We also consider stabilization of these fish metacommunities resulting from ecological portfolio effects at the metapopulation level [14], which we aggregate across species. We consider the influence of both spatial and environmental covariates (latitude, reef area and isolation, α- and γ-diversity) and life-history traits (taxonomic family, diet, body size and trophic level) on these relationships, and compare them to null expectations arising from random sampling and the potential influence of α- and γ-diversity on our estimates of β-diversity. Specifically, we (i) test for β-diversity–stability relationships in coral reef fish communities using estimators of spatial and temporal turnover; (ii) investigate and rank deterministic covariates (spatial scale, environmental conditions and life-history traits) and stochastic variables with respect to their effect on these relationships; (iii) derive spatially explicit predictions of turnover patterns based on these relationships; and (iv) using the zoning of Australia's Great Barrier Reef as an example, explore how failure to account for these covariates could lead to unexpected and potentially sub-optimal conservation outcomes.
2. Material and methods
(a). Study reefs and data collection
Australia's Great Barrier Reef consists of more than 2900 reefs extending over 2300 km between 9° and 24° S latitude (see the electronic supplementary material, figure S1). Between 1994 and 2011, reef communities of the Great Barrier Reef have been monitored yearly by the Australian Institute of Marine Science's Long-Term Monitoring Program [23]. As part of this programme, fish communities have been surveyed on 46 reefs in six latitudinal sectors (Cooktown-Lizard Island, Cairns, Townsville, Whitsunday, Swains and Capricorn-Bunker; electronic supplementary material, figure S1). In each sector, at least three reefs were sampled in each of three shelf positions (i.e. inner, middle and outer) except the Swains and Capricorn-Bunker sectors that only comprised inner and mid-shelf reefs, and inner reefs, respectively. We defined a region as the combination of each latitudinal sector by cross-shelf location (n = 15). Abundances of 214 fish species in 10 families (representing the most common and identifiable fishes [23]) were estimated each year within a total of 15 transects per reef in each region (see the electronic supplementary material).
(b). Indices of spatial and temporal turnover
We used Bray–Curtis (spatial) dissimilarity based on abundance data βSBC [24,25] and Simpson dissimilarity βSIM based on incidence data [26] as indices of spatial turnover. The main advantages of βSBC is that it excludes joint absences (i.e. it does not assume that two sites are more similar if they both lack particular species) [21,25] and in contrast to other metrics, accounts for differences between abundant species as much as differences between rare ones [25]. We also chose βSIM because it is independent of richness gradients, and thus represents the true turnover (as opposed to nestedness) between communities (see the electronic supplementary material for equations, detailed description and properties of these indices). Indices of temporal turnover included the Bray–Curtis (temporal) dissimilarity with abundance data βTBC, and Mantel's multivariate abundance autocorrelation coefficient at lag = 1 year, βTMA, with values close to 0 associated with stochastic year-to-year variation in community structure.
We calculated spatial turnover indices (βSBC or βSIM) using species abundance (or incidence) matrices for each reef, averaged across all years. In this case, we defined spatial turnover as the mean dissimilarity between each reef and other neighbouring reefs within the same region. We calculated temporal turnover indices (βTBC or βTMA) within each reef by comparing community structure across years (n = 15). Such pairwise dissimilarity indices were required because methods using multiple dissimilarity metrics have only been developed for incidence matrices. These procedures resulted in one value for each spatial and temporal turnover index per reef (n = 46), thereby avoiding any temporal lag effects of disturbances that could affect relationships between spatial and temporal turnover at a yearly time step.
To assess qualitatively the relationship between spatial turnover in fish community structure and temporal stability at a regional scale, we calculated the mean–variance portfolio effect [14] for each species and within each region. Here, the portfolio effect represents the difference between the observed log temporal variance of a metapopulation and that predicted for it given the mean–variance relationship of its individual populations (see the electronic supplementary material); this assumes that the metapopulation acts with the same dynamics as any component subpopulation. The mean–variance portfolio effect, thus measures the increase in stability owing to subpopulation diversity within a metapopulation (portfolio effects > 1 are ‘stabilizing’) [14]. We then plotted the range of portfolio effects against the mean spatial dissimilarity in community structure for reefs of the same region. To explore whether this relationship reflects a gradient in regional (γ) diversity, we also examined spatial and temporal dissimilarity in community structure for reefs of different regions (quantitative assessment at this scale was impossible owing to small sample sizes). We did this by comparing, for each region, spatial dissimilarity (among reefs) and temporal dissimilarity (among years) using non-metric multi-dimensional scaling based on Bray–Curtis dissimilarity (nMDS; e.g. [25]). Regional (γ) diversity was defined as the total number of species within each region. Input matrices were reef-by-species abundance matrices (i.e. abundances averaged over years for each reef) and the year-by-species abundance matrices (i.e. abundances averaged across reefs within a region for each year).
(c). Models
We modelled temporal turnover as a function of spatial turnover using generalized linear mixed-effects models (GLMM) with a binomial error distribution and a logit link function, commonly used for dissimilarity metrics that are comparable to proportions [27]. While pairwise dissimilarity indices have previously been modelled using ordinary least-squares regression [28], a hierarchical (multi-level) framework accounts explicitly for the non-independence resulting from a hierarchical structure in the data [29,30]. We thus coded the region (i.e. spatial cluster of two to five reefs) as a random effect to account for the non-independence of the reefs it encompasses, which results from the way turnover indices were calculated for reefs of a same region [29] (see the electronic supplementary material for further details on GLMM).
We assessed GLMM performance using per cent deviance explained (De) to provide an index of the model's goodness-of-fit [31] and Akaike's information criterion corrected for small sample sizes (AICc) to provide an index of Kullback–Leibler information loss, which we used to assign relative strengths of evidence to the different competing models [32]. Evidence for spatial autocorrelation in GLMM residuals was assessed at five distance classes using Bonferroni correction [25]. We qualified the relationship between spatial and temporal turnover as positive when there was evidence for a positive relationship between them (or negative for a negative relationship), with the effect size expressed as the absolute value of the coefficient of regression, b. This approach, compared with the one based on raw b-values, avoids confusion where exponential decay (negative) functions associated with negative b result in positive relationships. We then assessed the effects of spatial and environmental factors and life-history traits on the relationship between spatial and temporal turnover by refitting each GLMM using the dissimilarity indices calculated on subsets of the sample-by-species matrix (see the electronic supplementary material). We compared mean and 95% confidence intervals (CIs) (calculated as the mean ± 1.96 × standard error, which accounts for different sample sizes) for b to those obtained by fitting the models using the entire sample-by-species matrix.
We assessed the importance of random sampling error by refitting each model after randomly removing, with replacement, one transect per reef and per year for a total of 1000 simulations [20]. We compared the subsequent distribution of b to that obtained under a null model accounting for potential α- and γ-diversity effects, obtained using 1000 draws of species identities (based on their relative probability of occurrence among samples) and individual abundances (based on species-specific abundance distributions across samples) within samples, while holding constant the total number of species in each region and each sample each time (following the method in [22]). Where the distributions of model coefficients that accounted for sampling error and that were generated under a null model differed, we deemed the evidence for a relationship between spatial and temporal turnover robust to any underlying effects of (i) random sampling error and (ii) α- or γ- on β-diversity.
To examine potential geographical and environmental drivers of spatial and temporal turnover in these reef fish communities, we fitted boosted regression trees (BRTs) [33] as a function of distance to the coast, distance to the outer edge of the barrier reef, latitude, annual sea surface temperature (mean, minimum and seasonal range), reef area and isolation (see [20,34] for a detailed description of these covariates) observed at the study reefs, and used the BRT to predict βSBC (spatial turnover) across the entire Great Barrier Reef. We also included the total number of species at each reef (α-diversity) and in each region (γ-diversity) to control for its potential influence on β-diversity [19] (see the electronic supplementary material for further details on BRT). For these types of predictions, BRT is recommended over other modelling approaches for its ability to accommodate nonlinear relationships such as those typically characterizing β-diversity metrics and environmental gradients [27]. We then modelled spatial patterns in βTBC (temporal turnover) as a function of predicted βSBC using the GLMMs in a model-averaging procedure based on their respective AICc weights (wAICc).
Lastly, we tested whether one example of systematic conservation planning, the 2004 Great Barrier Reef Marine Park rezoning scheme [35] prioritized sites with higher spatial turnover and higher temporal stability. This rezoning scheme was designed using Marxan, a widely used software package for marine reserve planning [36], that implements complementarity and spatially explicit annealing algorithms. We compared (two-sample t-test) spatial and temporal turnover rates on protected versus unprotected reefs according to this rezoning scheme, with protected reefs (n = 20) being defined as reefs where fishing was prohibited (i.e. ‘scientific research’, ‘Marine National Park’ or ‘preservation’ zones) and unprotected reefs (n = 26) as those where fishing was permitted (i.e. ‘general use’, ‘habitat protection’ or ‘conservation park’ zones).
3. Results
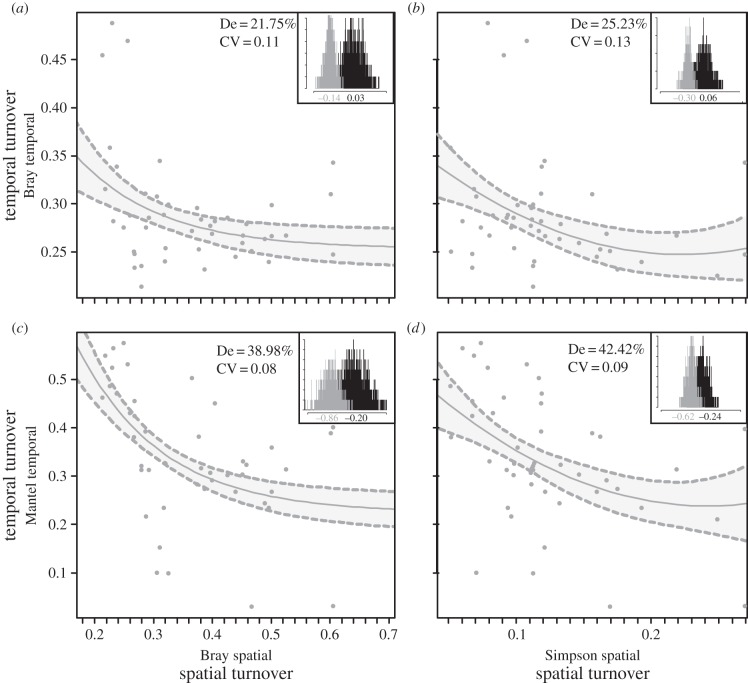
At the community level, and for all combinations of spatial and temporal indices, higher β-diversity (greater spatial turnover) was associated with greater temporal stability in community structure (lower temporal turnover; electronic supplementary material, figure S2). These relationships were robust to random sampling error and to the combined effects of α-diversity and γ-diversity in all cases (p < 0.001; figure 2), with no evidence of spatial autocorrelation in model residuals (p > 0.05). De ranged from 22% to 42%, whereas the cross-validated mean prediction error ranged from 0.08 to 0.13 (figure 2; electronic supplementary material, table S2). Models including α-diversity (111.3 ± 17.1 species; mean ± s.d.) or γ-diversity (140.9 ± 18.4 species) had the lowest support (see the electronic supplementary material, table S3), and therefore were removed from the model set. This finding indicated that α-diversity (local species richness) and γ-diversity (regional species richness) had negligible effect on the relationships between β-diversity and temporal stability in fish community structure. Temporal dissimilarity indices (βTBC and βTMA) were positively correlated (Spearman's ρ = 0.41; p < 0.001; electronic supplementary material, figure S2), indicating that high temporal variation in community structure was associated with high temporal autocorrelation, and therefore that time series were smooth rather than chaotic. Spatial dissimilarity indices (βSBC or βSIM) were also positively correlated (Spearman's ρ = 0.76; p < 0.001; electronic supplementary material, figure S2).
Figure 2.
Relationships between spatial and temporal turnover in reef fish communities. Spatial indices of species turnover (x-axis) include Bray–Curtis (a,c) and Simpson (b,d) dissimilarities. Temporal indices (y-axis) include Bray–Curtis dissimilarity (a,b) and Mantel temporal autocorrelation coefficient with lag = 1 (c,d; see text for details). Regression lines and shaded areas show the mean fitted response and 95% CIs of generalized linear mixed-effects models. Histograms illustrate the distribution of regression coefficients (or effect sizes) that account for random sampling error for a total of 1000 simulations (grey), compared to those based on a null model (black). Values along the x-axis indicate the mean of each distribution. De denotes per cent deviance explained and CV the 10-fold cross-validated mean prediction error.
At the regional scale, species-specific mean–variance portfolio effects generally increased with the spatial dissimilarity in fish community structure (β-diversity) observed within each region, despite a strong threshold effect whereby this relationship reached a plateau after a steep increase at low levels of β-diversity (see the electronic supplementary material, figure S3). Regions characterized by a low among-reef spatial dissimilarity (i.e. closer points on the top panel of the electronic supplementary material, figure S4) had a comparatively high temporal dissimilarity (i.e. points farther apart on the bottom panel of the electronic supplementary material, figure S4) (e.g. outer reefs, in blue), and inversely (e.g. mid-shelf reefs, in green). However, there was no evidence that this ordination reflected any gradient in regional (γ) diversity.
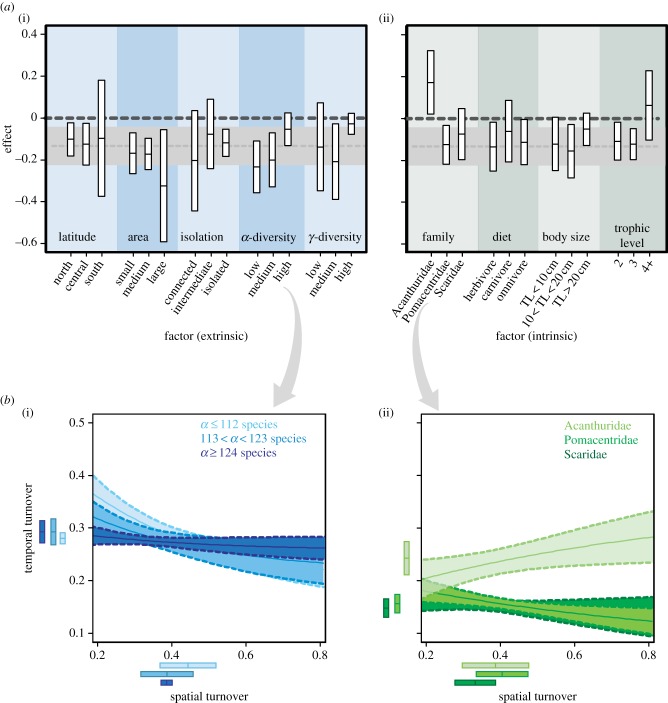
For our analyses of spatial and environmental effects versus those of life-history traits on species turnover rates, we adopted a conservative approach and retained the temporal–spatial turnover relationship that had the lowest goodness-of-fit (βTBC and βSBC; De = 22%; figure 2a) assuming that such effects would be maintained in relationships characterized by a higher goodness-of-fit (results obtained for βTBC and βSIM shown in the electronic supplementary material, figure S5). While most spatial and environmental factors, such as α-diversity or γ-diversity, did not affect the relationship between βTBC and βSBC, the life-history trait ‘family’ strongly influenced this relationship, with the positive relationship between βTBC and βSBC for the Acanthuridae (figure 3; note comparisons are based on the relationship obtained at the community level, indicated by the grey dotted line) contrasting with the negative relationships observed for all other taxonomic families examined. Trophic level > 4 also resulted in a positive relationship between βTBC and βSBC and we observed a similar trend for carnivores and large fish species; however, CIs for this trophic level and for the entire assemblage overlapped (figure 3). The negative relationship between spatial and temporal turnover was also more pronounced for reefs with low α-diversity, as well as for large and connected reefs (figure 3); however as before, the 95% CIs overlapped those obtained for the entire assemblage. Within each factor level, effect-size variance was greater for spatial and environmental factors than for life-history traits (figure 3). The relationship between βTBC and βSIM was influenced by spatial and environmental factors and life-history traits in a similar way, with the relationship being positive not only for Acanthuridae, but also for omnivores, small-bodied species (total length < 10 cm) and lower trophic levels (see the electronic supplementary material, figure S5). With respect to spatial and environmental factors, we found a positive relationship for isolated reefs and reefs in high γ-diversity regions. Variances for each factor level were generally higher for spatial and environmental factors than life-history traits.
Figure 3.
(a) Mean and 95% CIs of the relationship between spatial and temporal turnover (Bray–Curtis) for different spatial and environmental factors and life-history traits. The sign of the effect is positive (or negative) for a positive (or negative) relationship, with the effect size expressed as the absolute value of the regression coefficient (see text for details). TL, total length (cm). For comparison, the light grey dotted line and envelope show the mean and 95% CI, respectively, for the global relationship, depicted in (i). (b) Relationship between spatial and temporal turnover (Bray–Curtis) among levels of increasing α-diversity (i) and for the three most abundant families (ii). Box plots to the left and below each panel plot indicate the means and standard deviations of spatial and temporal turnover indices for three levels of α-diversity and for three taxonomic families (see text for details). Other parameters are identical to figure 2.
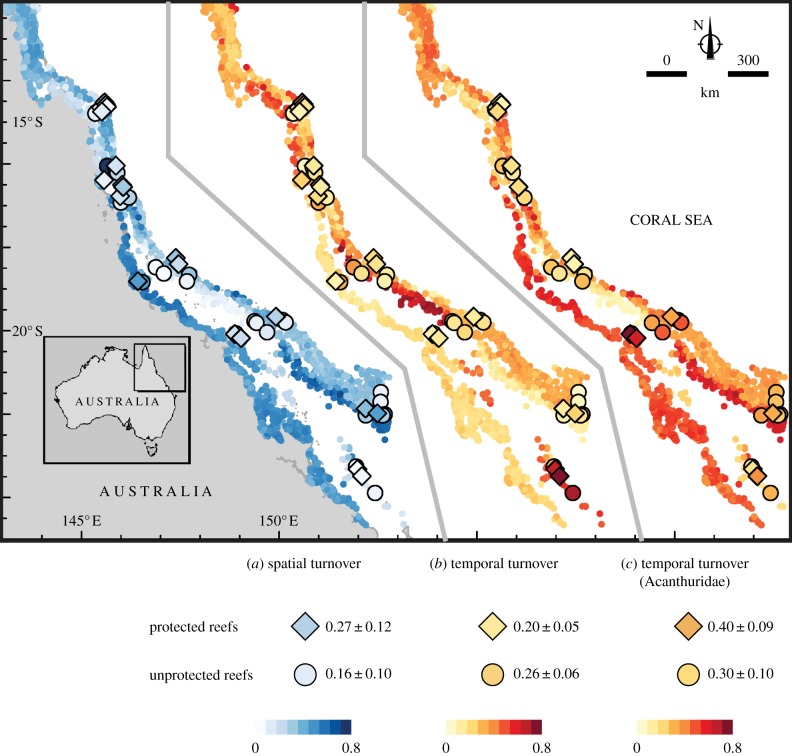
Spatial and temporal patterns of species turnover differed at the community level and for Acanthuridae. For both predicted and observed values, β-diversity (βSBC) was the greatest on mid-shelf, southern reefs (figure 4a), owing mainly to a negative effect of high latitudes and a positive effect of large distances to coast in the BRTs (mostly in the southern Great Barrier Reef; electronic supplementary material, figure S6). By contrast, temporal turnover in fish communities (βTBC) was predicted to be the greatest on southernmost offshore reefs (see also [20]); for example in the Capricorn–Bunker sector, and on central reefs (figure 4b). Applying the same method to the Acanthuridae, β-diversity was strongly correlated with that observed at the community level (Spearman's ρ = 0.52; p < 0.001; electronic supplementary material, figure S7), despite more pronounced cross-shelf gradients. Conversely, predictions of βTBC for the Acanthuridae strongly differed from those at the community level and showed the highest temporal turnover (lowest temporal stability) on coastal reefs, a consequence of the positive relationship between βSBC and βTBC for this family only.
Figure 4.
Observed and predicted (based on BRTs) spatial and temporal turnover of fishes on Australia's Great Barrier Reef: (a) predicted spatial turnover (Bray–Curtis) rates at the community level, (b) predicted temporal turnover (Bray–Curtis) rates at the community level, and (c) predicted temporal turnover (Bray–Curtis) rates for species of the Acanthuridae along and across the Great Barrier Reef, Australia. Filled symbols illustrate observed turnover values for protected reefs (diamonds) versus unprotected reefs (circles) (as per the 2004 Great Barrier Reef Marine Park rezoning scheme [35]) with the mean ± s.d. shown below the map.
Confirming greater β-diversity and the assumption of higher temporal stability in protected areas, observed spatial turnover (βSBC) was higher on protected than on unprotected reefs (p = 0.001; figure 4a), whereas we found the opposite trend for temporal turnover (βTBC) at the community level (p = 0.002; figure 4b). Conversely for Acanthuridae, βTBC was higher on protected than on unprotected reefs (p = 0.007; figure 4c).
4. Discussion
Testing β-diversity–stability relationships empirically and understanding the conditions that influence their direction, magnitude and strength are major challenges in community ecology. Based on one of the world's most extensive marine datasets, our analysis and results support theoretical expectations of β-diversity–stability relationships for coral reef fishes over extensive spatial and temporal scales. At the community level, spatial and temporal turnover rates were negatively related, regardless of the turnover metrics used. Furthermore, these β-diversity–stability relationships were robust to random sampling error and underlying patterns of α- or γ-diversity, providing one of the first clear empirical demonstrations of the importance of maintaining high β-diversity to promote community resilience. Model validation at the regional (metacommunity) scale also suggests that structurally diverse communities can stabilize metacommunity dynamics by facilitating different responses to environmental fluctuations at such broad scales. However, the negative relationship between spatial and temporal turnover was not universal; it varied and even switched direction between taxa and to a lesser extent, trophic levels.
The positive relationship we found between spatial and temporal turnover for Acanthuridae probably reflects the way these species respond to environmental change, in particular, their potential role in the prevention (or reversal) of phase shifts from coral to algal-dominated states [37]. Abundant Acanthuridae (e.g. Acanthurus blochi and Naso unicornis) can drive high temporal turnover rates in fish community structure in response to the spatially and temporally varying availability of resources, for example macroalgae [38]. Unlike other herbivorous fishes, such as territorial grazers (Pomacentridae) or scrapers (Scaridae, which tend to be less mobile than Acanthuridae and feed on the epilithic algal matrix), most Acanthuridae species are highly mobile, roving grazers [7] whose local abundances can respond rapidly to local resource fluctuations [39]. Resource fluctuations on Australia's Great Barrier Reef are most likely greater near shore where coral reefs are exposed to moderate to large variation in water quality related to nutrient and sediment runoff [40]. These same reefs had high spatial turnover rates (figure 4), both at the community level and for Acanthuridae alone. Together, these results illustrate the extent to which differential responses of species to environmental fluctuations could be a major driver of variation in the β-diversity–stability relationship. Our findings also support the idea that a species’ proneness to extirpation in response to disturbance can be at least partially trait-based [41,42], and that in the case of Acanthuridae, ecological traits, for example mobility, and the degree of ecological opportunism can influence responses to disturbance, including their magnitude and timing. Acanthuridae was also the only family for which we observed a positive relationship between spatial and temporal turnover, out of the three abundant families for which we tested this relationship; however, an array of often-correlated traits (i.e. trophic level, body size and mobility) seem to affect β-diversity–stability relationships in the same direction (figure 3) suggesting that within fish communities, positive relationships between spatial and temporal turnover might be more frequent than indicated by our analyses.
We show that spatial and environmental covariates, such as latitude, reef area and isolation, and life-history traits, for example body size, had a smaller effect on the β-diversity–stability relationship compared with taxonomic affiliation—with CIs overlapping those observed at the community level, and greater variance in the regression coefficient when spatial and environmental aspects were taken into account. Environmental context can similarly influence spatial and temporal turnover, with for example, both spatial and temporal turnover rates slowing at lower latitudes (at broad spatial scales only) presumably as a consequence of lower environmental heterogeneity [11], or on small and isolated habitat patches owing to a reduced opportunity for colonization from the available species pool [11,43]. Where a variety of processes operate to produce similar effects on spatial and temporal turnover, the overall relationship between both might remain constant, at least with respect to the regression coefficient. That body size had no discernible effect on these relationships is concordant with the idea that allometry might be mostly influential at fine spatial resolutions (i.e. less than 1 km) [11]. Small-bodied species tend to have a higher dispersal potential, resulting in faster colonization and turnover rates at fine spatial scales [11]; however, this idea remains untested for coral reef fishes. Together, these results suggest that similar β-diversity–stability relationships can be expected in different regions, and possibly in different ecosystems, with the direction and magnitude of these relationships varying among taxa in relation to taxon-specific life-history traits. However, given the potential effect of latitude, spatial extent or isolation on both spatial and temporal turnover [11], the widespread occurrence of negative relationships between them, irrespective of the location or ecosystem, requires verification.
Relationships between α-, γ- and β-diversity are likely to be complex [19,21]. For example, where α-diversity differs between samples, estimates of β-diversity automatically increase—irrespective of other mechanisms driving patterns in the latter. Moreover, estimates of β-diversity are also likely to increase in high γ-diversity regions (at constant α-diversity), where a lower proportion of the total available species pool might be represented in each sample, thus decreasing average overlap in species structure among samples. Using a ‘horizontal’ β-diversity measure (sensu [21]) and a null-modelling approach where the total number of species in each region (γ-diversity) and in each sample (α-diversity) are kept constant, it is possible to tease apart their partial effect on β-diversity [21,22]. Adopting such an approach, we showed that: (i) the negative relationship between spatial and temporal turnover was robust to differences in α- and γ-diversity; and that (ii) α- and/or γ-diversity were not the main drivers of spatial and temporal turnover because all distributions of regression coefficients overlapped zero under the null model. There was also no evident γ-diversity gradient in reef ordination based on spatial or temporal dissimilarity in community structure. The effect of α-diversity on spatial or temporal turnover (in isolation) has previously been inconsistent and is probably context specific [19,43]; for example, species richness accounted for virtually all the variation in β-diversity along latitudinal and elevational gradients in woody plant diversity [19], but had no effect on the temporal turnover of a range of aquatic ecosystems ranging from streams to oceans, and from the tropics to near the poles [43]. To our knowledge, no other study so far has assessed the effect of α-diversity on the relationship between spatial and temporal turnover. Here, the only evidence for an effect of α-diversity was a slightly more pronounced negative effect of β-diversity on temporal turnover where α-diversity was low relative to the study region (figure 3), suggesting that the stabilizing effect of β-diversity might be especially strong in species-poor communities. The corollary is that in species-rich communities, other mechanisms, for example asynchronous abundance fluctuations (the portfolio effect; e.g. [7]), might contribute more to the diversity–stability relationship than patterns of β-diversity per se.
Theory predicts that spatial turnover in community structure can be driven in part by temporal turnover owing to the decreased probability of sampling a given species repeatedly when temporal turnover is high; but this prediction has never been tested directly [9] until now. For fishes on the Great Barrier Reef, the relationship between spatial and temporal turnover was robust to stochastic sampling effects. Nevertheless, accounting for sampling effects increased the variance in the relationship's regression coefficient, indicating non-negligible stochastic effects that should be tested systematically across the range of expected sampling errors. We minimized the effect of failing to detect rare species in temporal surveys by using partial (truncated) species lists that focused on the most common and easily recognizable species. By contrast, surveys of entire species pools that include many rare or cryptic species are likely to be vulnerable to sampling-error effects on spatial turnover in community structure; the rarer a species, the lower its probability of detection during sampling. Thus, with undersampling of rare species, structural dissimilarity between two communities will probably be underestimated [44]. Where both spatial and temporal turnover estimates are potentially biased, we recommend using the dissimilarity index developed by Chao et al. [44], which applies a non-parametric probabilistic approach to Jaccard- and Sorensen-type abundance-based indices, because it accounts for the effects of unobserved rare species.
Our results also demonstrate that marine-protected areas encompassing regions of high β-diversity might not necessarily promote temporal stability in abundance for all taxa. While no management plan can protect all species equally [45], conservation planners may benefit from caution when prioritizing habitat patches for protection using algorithms that maximize β-diversity because increased demographic stability cannot be safely assumed. More investigation is required to test the generality, and explore further, the patterns we have identified here—particularly across other taxa, communities, management scenarios and natural or human-mediated disturbance events. Doing so is becoming increasingly important and challenging as the worldwide degradation of biological communities and ecosystems accelerates [46].
Acknowledgements
We thank all members of the Australian Institute of Marine Science Long-Term Monitoring Program that have contributed to data collection, and S. Connolly, M. Emslie, M. Kulbicki, D. Mouillot, A. MacNeil and L. Thibaut for helpful discussions.
Funding statement
This work was done under the auspices of the Marine Biodiversity Hub, a collaborative partnership supported through funding from the Australian Government's National Environmental Research Program (NERP) (www.nerpmarine.edu.au).
References
- 1.MacArthur R. 1955. Fluctuations of animal populations and a measure of community stability. Ecology 36, 533–536 (doi:10.2307/1929601) [Google Scholar]
- 2.May RM. 1973. Stability and complexity in model ecosystems. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Ives AR, Carpenter SR. 2007. Stability and diversity of ecosystems. Science 317, 58–62 (doi:10.1126/science.1133258) [DOI] [PubMed] [Google Scholar]
- 4.McCann KS. 2000. The diversity–stability debate. Nature 405, 228–233 (doi:10.1038/35012234) [DOI] [PubMed] [Google Scholar]
- 5.Moilanen A, Wilson KA, Possingham H. 2009. Spatial conservation prioritization: quantitative methods and computational tools. Oxford, UK: Oxford University Press [Google Scholar]
- 6.Shurin JB. 2007. How is diversity related to species turnover through time? Oikos 116, 957–965 (doi:10.1111/j.0030-1299.2007.15751.x) [Google Scholar]
- 7.Thibaut LM, Connolly SR, Sweatman HP. 2012. Diversity and stability of herbivorous fishes on coral reefs. Ecology 93, 891–901 (doi:10.1890/11-1753.1) [DOI] [PubMed] [Google Scholar]
- 8.Walker BH. 1992. Biodiversity and ecological redundancy. Conserv. Biol. 6, 18–23 (doi:10.1046/j.1523-1739.1992.610018.x) [Google Scholar]
- 9.Stegen JC, et al. 2012. Stochastic and deterministic drivers of spatial and temporal turnover in breeding bird communities. Glob. Ecol. Biogeogr. 22, 202–212 (doi:10.1111/j.1466-8238.2012.00780.x) [Google Scholar]
- 10.Baeten L, Vangansbeke P, Hermy M, Peterken G, Vanhuyse K, Verheyen K. 2012. Distinguishing between turnover and nestedness in the quantification of biotic homogenization. Biodivers. Conserv. 21, 1399–1409 (doi:10.1007/s10531-012-0251-0) [Google Scholar]
- 11.Soininen J. 2010. Species turnover along abiotic and biotic gradients: patterns in space equal patterns in time? BioScience 60, 433–439 (doi:10.1525/bio.2010.60.6.7) [Google Scholar]
- 12.Leibold MA, et al. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613 (doi:10.1111/j.1461-0248.2004.00608.x) [Google Scholar]
- 13.Tilman D, Wedin D, Knops J. 1996. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379, 718–720 (doi:10.1038/379718a0) [Google Scholar]
- 14.Anderson SC, Cooper AB, Dulvy NK. 2013. Ecological prophets: quantifying metapopulation portfolio effects. Methods Ecol. Evol. 4, 971–981 (doi:10.1111/2041-210X.12093) [Google Scholar]
- 15.Sarkar S. 2006. Ecological diversity and biodiversity as concepts for conservation planning: comments on Ricotta. Acta Biotheor. 54, 133–140 (doi:10.1007/s10441-006-8259-z) [DOI] [PubMed] [Google Scholar]
- 16.Margules CR, Pressey RL. 2000. Systematic conservation planning. Nature 405, 243–253 (doi:10.1038/35012251) [DOI] [PubMed] [Google Scholar]
- 17.Melbourne BA, Hastings A. 2008. Extinction risk depends strongly on factors contributing to stochasticity. Nature 454, 100–103 (doi:10.1038/nature06922) [DOI] [PubMed] [Google Scholar]
- 18.Hanski I, Moilanen A, Gyllenberg M. 1996. Minimum viable metapopulation size. Am. Nat. 147, 527–541 (doi:10.1086/285864) [Google Scholar]
- 19.Kraft N. 2011. Disentangling the drivers of beta diversity along latitudinal and elevational gradients Science 333, 1755 (doi:10.1126/science.334.6061.1348) [DOI] [PubMed] [Google Scholar]
- 20.Mellin C, Huchery C, Caley MJ, Meekan MG, Bradshaw CJA. 2010. Reef size and isolation determine the temporal stability of coral reef fish populations. Ecology 91, 3138–3145 (doi:10.1890/10-0267.1) [DOI] [PubMed] [Google Scholar]
- 21.Anderson MJ, et al. 2011. Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28 (doi:10.1111/j.1461-0248.2010.01552.x) [DOI] [PubMed] [Google Scholar]
- 22.Chase JM. 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328, 1388–1391 (doi:10.1126/science.1187820) [DOI] [PubMed] [Google Scholar]
- 23.Sweatman H, Cheal A, Coleman G, Jonker M, Johns K, Emslie M, Miller I, Osborne K. 2008. Long-term monitoring of the Great Barrier Reef. Status report no. 8. Australian Institute of Marine Science, Townsville, Australia [Google Scholar]
- 24.Bray J, Curtis J. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349 (doi:10.2307/1942268) [Google Scholar]
- 25.Legendre P, Legendre L. 1998. Numerical ecology, 2nd edn Amsterdam, The Netherlands: Elsevier; [In English] [Google Scholar]
- 26.Lennon JJ, Koleff P, Greenwood JJD, Gaston KJ. 2001. The geographical structure of British bird distributions: diversity, spatial turnover and scale. J. Anim. Ecol. 70, 966–979 (doi:10.1046/j.0021-8790.2001.00563.x) [Google Scholar]
- 27.Ferrier S, Manion G, Elith J, Richardson K. 2007. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 13, 252–264 (doi:10.1111/j.1472-4642.2007.00341.x) [Google Scholar]
- 28.Harrison S, Ross SJ, Lawton JH. 1992. Beta-diversity on geographic gradients in Britain. J. Anim. Ecol. 61, 151–158 (doi:10.2307/5518) [Google Scholar]
- 29.Gelman A, Hill J. 2007. Data analysis using regression and multilevel hierarchical models. New York, NY: Cambridge University Press [Google Scholar]
- 30.Qian SS, Cuffney TF, Alameddine I, McMahon G, Reckhow KH. 2010. On the application of multilevel modeling in environmental and ecological studies. Ecology 91, 355–361 (doi:10.1890/09-1043.1) [DOI] [PubMed] [Google Scholar]
- 31.Crawley MJ. 2005. Statistics: an introduction using R. New York, NY: John Wiley & Sons, Ltd [Google Scholar]
- 32.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information theoretic approach, 2nd edn New York, NY: Springer [Google Scholar]
- 33.Elith J, Leathwick JR, Hastie T. 2008. A working guide to boosted trees. J. Anim. Ecol. 77, 802–813 (doi:10.1111/j.1365-2656.2008.01390.x) [DOI] [PubMed] [Google Scholar]
- 34.Mellin C, Bradshaw CJA, Meekan MG, Caley MJ. 2010. Environmental and spatial predictors of species richness and abundance in coral reef fishes. Glob. Ecol. Biogeogr. 19, 212–222 (doi:10.1111/j.1466-8238.2009.00513.x) [Google Scholar]
- 35.Office of the Queensland Parliamentary Counsel. 2012 Marine parks (Great Barrier Reef coast) zoning plan 2004
- 36.Ball IR, Possingham HP, Watts M. 2009. Marxan and relatives: software for spatial conservation prioritisation. In Spatial conservation prioritisation: quantitative methods and computational tools (eds Moilanen A, Wilson KA, Possingham HP.), pp. 185–195 Oxford, UK: Oxford University Press [Google Scholar]
- 37.Cheal A, MacNeil M, Cripps E, Emslie M, Jonker M, Schaffelke B, Sweatman H. 2010. Coral-macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29, 1005–1015 (doi:10.1007/s00338-010-0661-y) [Google Scholar]
- 38.Cheal AJ, Wilson SK, Emslie MJ, Dolman AM, Sweatman H. 2008. Responses of reef fish communities to coral declines on the Great Barrier Reef. Mar. Ecol. 372, 211–223 (doi:10.3354/meps07708) [Google Scholar]
- 39.Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NVC. 2006. Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Change Biol. 12, 2220–2234 (doi:10.1111/j.1365-2486.2006.01252.x) [Google Scholar]
- 40.De'Ath G, Fabricius K. 2010. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 20, 840–850 (doi:10.1890/08-2023.1) [DOI] [PubMed] [Google Scholar]
- 41.Bradshaw CJA, Giam X, Tan H, Brook B, Sodhi N. 2008. Threat or invasive status in legumes is related to opposite extremes of the same ecological and life-history attributes. J. Ecol. 96, 869–883 (doi:10.1111/j.1365-2745.2008.01408.x) [Google Scholar]
- 42.Fordham DA, et al. 2012. Plant extinction risk under climate change: are forecast range shifts alone a good indicator of species vulnerability to global warming? Glob. Change Biol. 18, 1357–1371 (doi:10.1111/j.1365-2486.2011.02614.x) [Google Scholar]
- 43.Korhonen JJ, Soininen J, Hillebrand H. 2010. A quantitative analysis of temporal turnover in aquatic species assemblages across ecosystems. Ecology 91, 508–517 (doi:10.1890/09-0392.1) [DOI] [PubMed] [Google Scholar]
- 44.Chao A, Chazdon RL, Colwell RK, Shen TJ. 2005. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159 (doi:10.1111/j.1461-0248.2004.00707.x) [Google Scholar]
- 45.Wilson KA, McBride MF, Bode M, Possingham HP. 2006. Prioritizing global conservation efforts. Nature 440, 337–340 (doi:10.1038/nature04366) [DOI] [PubMed] [Google Scholar]
- 46.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67 (doi:10.1038/nature11148) [DOI] [PubMed] [Google Scholar]