Abstract
The jump–yip display of black-tailed prairie dogs (Cynomys ludovicianus) is contagious, spreading through a prairie dog town as ‘the wave’ through a stadium. Because contagious communication in primates serves to assess conspecific social awareness, we investigated whether instigators of jump–yip bouts adjusted their behaviour relative to the response of conspecifics recruited to display bouts. Increased responsiveness of neighbouring town members resulted in bout initiators devoting a significantly greater proportion of time to active foraging. Contagious jump–yips thus function to assess neighbours’ alertness, soliciting social information to assess effective conspecific group size in real time and reveal active probing of conspecific awareness consistent with theory of mind in these group-living rodents.
Keywords: contagious communication, group-size effect, public information, theory of mind, foraging–vigilance trade-off, prairie dogs
1. Introduction
Members of group-living species rely upon collective vigilance to detect predators [1], occasionally synchronizing vigilance so as to enhance the detection of potential threats [2,3]. As the number of individuals residing within a group increases, each individual can devote less time to vigilance and more time to other activities, for example foraging [1,4,5], owing, in part, to the cumulative effect of each individual's finite probability of detecting presumptive threats [6–8]. Where such group-size effects are dependent upon collective detection, however, individual group members must assess the size of the group in which they reside [9], and perhaps more importantly, the vigilance of fellow group members [10,11] to tailor their behaviour to the situation at hand.
Black-tailed prairie dogs (Cynomys ludovicianus) are group-living, semi-fossorial rodents that are subject to intense predation by terrestrial and avian predators [12,13]. Classic group-size effects, with diminishing individual allotment of time to vigilance and increasing time devoted to foraging with increasing group size, have been documented observationally [14] and experimentally [15] among black-tailed prairie dogs, though the mechanism via which group size is assessed is unknown. Just as synchrony in the activity of group members promotes social cohesion [16,17] through social facilitation among individuals [18], fine-tuning one's foraging–vigilance trade-off relative to the vigilance of other group members would prove adaptive [11].
We investigated contagious, multimodal ‘jump–yip’ displays of black-tailed prairie dogs as a potential means of adjusting the instigating individual's foraging–vigilance trade-off relative to the responsiveness, and hence vigilance, of neighbouring group members. In instigating a jump–yip bout, an individual raises its anterior torso above the ground, achieving at least an erect posture, though sometimes propelling itself from the ground. This postural change is coupled with the extension of the signaller's forelimbs and the emission of a vocalization that can be described phonetically as ‘wee-oo’ [19] as the anterior torso is raised and then lowered, resulting in a multimodal display lasting around a second ([20]; see the electronic supplementary material, movie S1). Unlike other prairie dog vocalizations or displays, the jump–yip is typically contagious, spreading from the initiator through neighbouring colony members as ‘the wave’ through a stadium ([20]; see the electronic supplementary material, movie S2). To date, the function of the jump–yip has proved elusive. Initial accounts were purely descriptive [21,22], though subsequent researchers have suggested that the jump–yip serves as a territorial call [12], an ‘all-clear’ [13,23] or ‘end-of-danger’ signal [12], a means of promoting social bonding within the group (‘contact group cohesion’; [19]), an indicator that the signaller is less likely to show escape behaviour in the face of threat, promoting temporarily heightened vigilance among conspecifics [20], or as Owings & Coss [24] suggested for various animal signals, a means of managing conspecifics. Here, such management would presumably promote reliance on conspecifics for predator detection, thereby allowing the signaller to devote time to activities other than watchfulness.
The acquisition of information from conspecifics proves adaptive in contexts including foraging [25], habitat choice [26], mate choice [27], parenting [28], tool use [29], and both anti-brood parasite [30] and anti-predator responses [31]. Although social learning and public information use are often considered tacitly different concepts [32–34], the effective utilization of socially available information requires that individuals assess the veracity of the information they receive [35–37], and sample available information on an ongoing basis to ensure that information is up-to-date [38]. Such assessment is of particular importance where ignoring information proves costly [39], as would be the case where animals are faced with the risk of predation [8].
We hypothesized that the instigation of a jump–yip bout serves to probe neighbouring individuals for feedback regarding their current vigilance state. If that were the case, we predicted that subsequent time devoted to personal vigilance by bout instigators would be directly proportional to the latency of the first conspecific to respond to the instigator's jump–yip (greater delay in response indicating reduced alertness of neighbours), and inversely proportional to the number of conspecifics joining a jump–yip bout (a smaller number indicating reduced collective vigilance) as well as the overall duration of the bout (reduced length of contagion indicative of lesser collective vigilance). Given the well-documented trade-off between foraging and vigilance in black-tailed prairie dogs [13–15], we also predicted that the allocation of time to foraging by bout instigators would be inversely correlated with the latency of the first conspecific to respond in kind with a jump–yip and positively correlated with the number of individuals recruiting to, and the overall duration of jump–yip bouts.
2. Material and methods
To ascertain whether instigator vigilance and foraging behaviour were affected by subsequent jump–yip bout properties (latency to first response, number of individuals recruited and bout duration), we video recorded a total of 173 jump–yip bouts during November 2003 and from May through September 2004 within 16 distinct prairie dog towns spread across six populations, including 14 naturally occurring towns in South and North Dakota, USA, and two introduced towns in Winnipeg, Manitoba, Canada (see R.L. Senkiw M.Sc. thesis for further details; downloadable at: http://mspace.lib.umanitoba.ca/bitstream/1993/2842/1/MSc%20Thesis.pdf). To ensure our results were unconfounded, we eliminated bouts interrupted by the appearance of predators, prairie dog vocalizations other than those constituting part of the jump–yip bout, humans or vehicles, and those where the behaviour of the bout instigator could not be distinguished because it was out of frame or out of focus in the video recording, or where any ambiguity existed regarding the identity of the bout initiator. Thus, our final dataset included data from 27 independent bout initiators from 14 distinct towns (one to three bouts per town) among the six populations, for which we quantified the proportion of time the bout instigator engaged in vigilance (with its head above the horizontal plane) and the proportion of time spent foraging (head below the horizontal plane, grazing or chewing) in the 1 min subsequent to the second syllable of the bout instigator's ‘wee-oo’ call. Postures in which the head was below the horizontal plane but where neither active grazing nor chewing occurred were not scored as vigilance or foraging. Limiting estimation of vigilance and foraging to only 1 min maximized the likelihood that the bout instigator's behaviour was attributable solely to properties of the current jump–yip bout. Bouts were considered independent of each other if at least 4 s elapsed without an individual manifesting a jump–yip, based on the 5 s criterion Smith et al. [20] employed to delineate unique bouts, and on an obvious discontinuity in the distribution of individuals recruiting to bouts after 4 s in our larger 173-bout sample of videotaped jump–yip bouts. For each bout, we recorded the latency (s) for the first individual to respond with a jump–yip to the bout instigator, the number of jump–yip responses within each bout and the overall duration of each bout (s). We applied linear regressions to test for relationships between those three independent variables, and the proportions of time the bout instigator devoted to either vigilance or foraging in the post-bout period, considering those statistically significant where p < 0.05.
3. Results
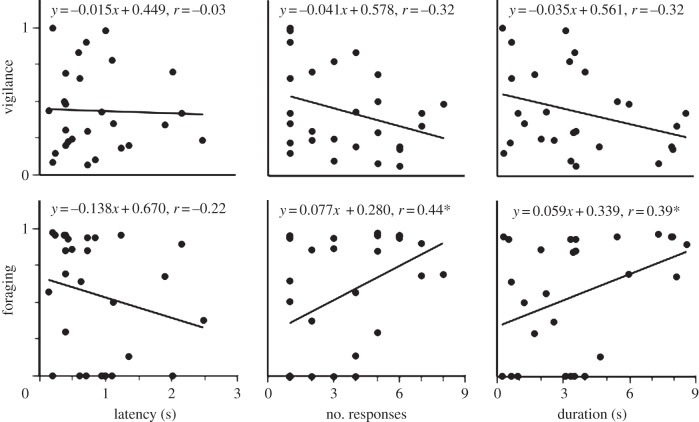
The proportion of time devoted to vigilance by bout initiators was unaffected by the latency for the initial respondent to recruit to a contagious jump–yip bout (F1,25 = 0.03, p = 0.87; figure 1). Further, although time allocated to vigilance tended to decrease with both an increasing number of respondents and increasing bout duration, those declines fell short of statistical significance (F1,25 = 2.86, p = 0.10 and F1,25 = 2.89, p = 0.10, respectively; figure 1). Similarly, although there was a trend toward decreasing time devoted to foraging with increasing latency of conspecifics to respond to the initial propagation of a jump–yip, that change was not statistically significant (F1,25 = 1.31, p = 0.26; figure 1). Statistically significant relationships were detected for the proportionate allocation of time to active foraging, relative to both the number of respondents recruiting to a given jump–yip bout (F1,25 = 5.87, p = 0.02) and the overall duration of contagious display bouts (F1,25 = 4.50, p = 0.04). Bout instigators increased time allocated to foraging with both an increasing number of individuals recruiting to a jump–yip bout and increasing bout duration (figure 1). That said, bout duration was positively correlated with the number of respondents (r = 0.82), though neither bout duration nor the number of respondents recruiting to a jump–yip bout was well correlated with latency to response (r = 0.32 and r = 0.09, respectively). Significant differences or pronounced trends for bout initiators to alter their allocation of time to foraging and vigilance in accord with our predictions exist for five of the six relationships examined, which in itself, should occur rarely by chance alone (binomial p = 0.11).
Figure 1.
Proportionate allocation of black-tailed prairie dog jump–yip bout instigator time to foraging and vigilance relative to the latency (s) of the first respondent to contribute to a contagious bout of calling, the number of respondents recruiting to the bout and the overall duration (s) of the jump–yip bout evoked (*p < 0.05).
4. Discussion
Instigators of jump–yip bouts increased their proportionate allocation of time to foraging as the responsiveness of conspecifics increased. The absence of any relationship between bout instigator time allocation to vigilance and latency of initial conspecific response is not attributable to bout instigators consistently manifesting maximal vigilance, in that the proportion of time devoted to vigilance varied considerably across the range of response latencies observed. Moreover, bout instigators are undoubtedly constrained to devote time to vigilance so as to assess bout response characteristics prior to adjusting their foraging–vigilance trade-off accordingly. The effect of conspecific responsiveness on the proportion of time devoted to foraging is not attributable to variation in group size proper, in that treating aboveground group size as a covariate did not alter our findings. Jump–yip displays thus function to promote the accrual of information regarding collective vigilance within the group, clarifying earlier speculation regarding the function of these displays.
While conspecific responsiveness to the instigation of a jump–yip bout significantly affected the time bout initiators allocated to foraging, only an inconsequential change was detected in instigator personal vigilance with variation in conspecific response. How accurately our simple postural assay of the head being held above the horizontal plane reflects an individual's state of vigilance remains an open question, as does consideration of any potential cost associated with the use of this socially acquired information [40].
Our results are consistent with the assertion by Owings & Coss [24] that tonic communication functions to manage conspecifics, in this case providing up-to-date, context-specific information on the vigilance of conspecific group members. Among group-living species, accurate decision-making is facilitated via the use of social information [41,42], particularly where certain group members are less well informed than others [43]. Conspecific neighbours constitute a particularly important source of information where animals are subject to predation risk [44], and reliance on that information may, in and of itself, prompt social contagion [45]. In mimicking the evasive behaviour of neighbours, group-living insects avoid unseen predators (the ‘Trafalgar effect’; [46]). Similarly, schooling fish avoid previously un-encountered predators by mimicking their neighbours’ behaviour [47].
Based on both previous empirical findings and an individual-based model, Beauchamp et al. [11] argued that selection would favour monitoring and copying the vigilance of neighbours, resulting in collective waves of vigilance that would facilitate collective detection of predators. Given the coevolutionary nature of predator–prey relationships, however, predators might be expected to cue-in on, and coordinate their attacks relative to lulls in such waves. Our empirical findings for black-tailed prairie dogs reveal collective waves of coordinated behaviour, presumably optimizing foraging efficiency of individuals reciprocally sharing information regarding collective vigilance. Participants within a bout thus likely benefit via reciprocal altruism [48] among resident town members, with any risk of enhanced detection by presumptive predators owing to the production of this conspicuous display being shared among signallers. Such broadly subscribed patterns of display behaviour may also serve as a potent pronouncement of vigilance [49], thereby reducing predation risk among town members in general.
The wave-like spread of jump–yip displays through towns of black-tailed prairie dogs is consistent with literature implicating the use of public information in social evolution [33,34] and contagious displays in particular, in the evaluation of conspecific social awareness [50]. Yawning in humans and other primates [51,52] and laughter among humans [53] provide familiar examples of contagious displays. Platek et al. [51], among others, have reported that such behaviours are associated with self-processing and empathy in humans. Indeed, emotional contagion among humans has been considered at least an important precursor to more advanced ‘Theory of mind’ abilities [54], wherein contagion represents the first step toward respondent awareness of the emotional state of the instigator by invoking that same state in the respondent [55]. Emotional contagion in humans, however, transcends superficial motor mimicry and represents a means of sharing affect of considerable evolutionary antiquity [56]. Although the existence of a relationship between empathy and social contagion has long been recognized for non-human primates [57], it has more recently been reported for dogs [58]. Applied to our findings, it is evident that in responding to the emergent display properties of neighbours recruited to a jump–yip bout, black-tailed prairie dogs manifest at least a rudimentary awareness of the state of conspecific group members. As Barrett et al. [59] aptly point out, communication and mind are intimately intertwined, in that the ‘mind’ is a form of social participation or process, rather than an entity unto itself, ultimately serving to rationalize the organism's social environment. In this light, it is not surprising that these highly social animals have evolved coordinated social behaviour and commensurate cognitive abilities [60] promoting their success in the face of intense predation pressure. Further study is required to elucidate additional nuances of the mechanism, along with the ultimate implications of this newly discovered, adaptive social contagion in prairie dogs.
Acknowledgements
We thank Drs Daniela Campobello, Maurizio Sarà, Debbie Kelly and two anonymous reviewers, along with Associate Editor Dr Nadia Aubin-Horth for insightful comments on an earlier draft of this manuscript. We also thank Dr Susan Cosens for input during the course of R.S.'s thesis work, and Dr Bob Wrigley (Assiniboine Park Zoo), Ken Cudmore (Fort Whyte Centre), Penny Knuckles and Michael Oehler (Theodore Roosevelt National Park), Terry Lincoln (Dakota Zoo), Dan Miller (Bramble Park Zoo) and Dan Foster (Wind Cave National Park) for facilitating access to the prairie dogs.
All research adhered to the guidelines of the Canadian Council on Animal Care as detailed under Protocol 03-034 approved by the University of Manitoba's Fort Garry Campus Protocol Management and Review Committee, and under US National Park Service Scientific Research Permit THRO-2003-SCI-0011.
Data accessibility
Bout response characteristic and bout initiator behavioural data file: Dryad doi:10.5061/dryad.g76t8.
Funding statement
Funding for this research was provided by a University of Manitoba Graduate Fellowship and a Sigma Xi Grant-in-Aid of Research to R.S., and by the Natural Sciences and Engineering Research Council of Canada in the form of a Postgraduate Scholarship to R.S. and Discovery grant (no. 154271) funding to J.H.
References
- 1.Elgar M. 1989. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. 64, 13–33 (doi:10.1111/j.1469-185X.1989.tb00636.x) [DOI] [PubMed] [Google Scholar]
- 2.Pays O, Renaud PC, Loisel P, Petit M, Gerard J, Jarman PJ. 2007. Prey synchronize their vigilant behavior with other group members. Proc. R. Soc. B 274, 1287–1291 (doi:10.1098/rspb.2006.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend SW, Zöttl M, Manser M. 2011. All clear? Meerkats attend to contextual information in close calls to coordinate vigilance. Behav. Ecol. Sociobiol. 65, 1927–1934 (doi:10.1007/s00265-011-1202-6) [Google Scholar]
- 4.Quenette P-Y. 1990. Functions of vigilance behaviour in mammals: a review. Acta Oecol. 11, 801–818 [Google Scholar]
- 5.Roberts G. 1996. Why individual vigilance declines as group size increases. Anim. Behav. 51, 1077–1086 (doi:10.1006/anbe.1996.0109) [Google Scholar]
- 6.Pulliam HR. 1973. On the advantages of flocking. J. Theor. Biol. 38, 419–422 (doi:10.1016/0022-5193(73)90184-7) [DOI] [PubMed] [Google Scholar]
- 7.Lima SL. 1995. Back to the basics of anti-predatory vigilance: the group size effect. Anim. Behav. 49, 11–20 (doi:10.1016/0003-3472(95)80149-9) [Google Scholar]
- 8.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: review and prospectus. Can. J. Zool. 68, 619–640 (doi:10.1139/z90-092) [Google Scholar]
- 9.Elgar MA, Burren PJ, Posen M. 1984. Vigilance and perception of flock size in foraging house sparrows (Passer domesticus L.). Behaviour 90, 215–223 (doi:10.1163/156853984X00146) [Google Scholar]
- 10.Lima SL, Zollner PA. 1996. Anti-predatory vigilance and the limits to collective detection: visual and spatial separation between foragers. Behav. Ecol. Sociobiol. 38, 355–363 (doi:10.1007/s002650050252) [Google Scholar]
- 11.Beauchamp G, Alexander P, Jovani R. 2012. Consistent waves of collective vigilance in groups using public information about predation risk. Behav. Ecol. 23, 368–374 (doi:10.1093/beheco/arr194) [Google Scholar]
- 12.King JA. 1955. Social behavior, social organization, and population dynamics in a black-tailed prairie dog town in the Black Hills of South Dakota. Contrib. Lab. Vert. Biol. 67, 1–123 [Google Scholar]
- 13.Hoogland JL. 1995. The black-tailed prairie dog: social life of a burrowing mammal. Chicago, IL: University of Chicago Press [Google Scholar]
- 14.Hoogland JL. 1979. The effect of colony size on individual alertness of prairie dogs: Sciuridae (Cynomys spp.). Anim. Behav. 27, 394–407 (doi:10.1016/0003-3472(79)90174-X) [Google Scholar]
- 15.Kildaw SD. 1995. The effect of group size manipulations on the foraging behavior of black-tailed prairie dogs. Behav. Ecol. 6, 353–358 (doi:10.1093/beheco/6.4.353) [Google Scholar]
- 16.Focardi S, Pecchiol E. 2005. Social cohesion and foraging decrease with group size in fallow deer (Dama dama). Behav. Ecol. Sociobiol. 59, 84–91 (doi:10.1007/s00265-005-0012-0) [Google Scholar]
- 17.Gautrais J, Michelena P, Sibbald A, Bon R, Deneubourg J-L. 2007. Allelomimetic synchronization in Merino sheep. Anim. Behav. 74, 1443–1454 (doi:10.1016/j.anbehav.2007.02.020) [Google Scholar]
- 18.Clayton DA. 1978. Socially facilitated behavior. Q. Rev. Biol. 53, 373–392 (doi:10.1086/410789) [Google Scholar]
- 19.Waring GH. 1970. Sound communications of black-tailed, white-tailed, and Gunnison's prairie dogs. Am. Midl. Nat. 83, 167–185 (doi:10.2307/2424014) [Google Scholar]
- 20.Smith WJ, Smith SL, DeVilla JG, Oppenheimer EC. 1976. The jump–yip display of the black-tailed prairie dog, Cynomys ludovicianus. Anim. Behav. 24, 609–621 (doi:10.1016/S0003-3472(76)80075-9) [DOI] [PubMed] [Google Scholar]
- 21.Seton ET. 1926. The prairie dogs (Cynomys ludovicianus) at Washington Zoo. J. Mamm. 7, 229–230 [Google Scholar]
- 22.Anthony A. 1955. Behavior patterns in a laboratory colony of prairie dogs, Cynomys ludovicianus. J. Mamm. 36, 69–78 (doi:10.2307/1375724) [Google Scholar]
- 23.Smith RE. 1958. Natural history of the prairie dog in Kansas. Lawrence, KS: University of Kansas Press [Google Scholar]
- 24.Owings DH, Coss RG. 2007. Social and antipredator systems: intertwining links in multiple time frames In Rodent societies: ecological and evolutionary perspectives (eds Wolff J, Sherman PW.), pp. 305–316 Chicago, IL: University of Chicago Press [Google Scholar]
- 25.Giraldeau L-A, Caraco T. 2000. Social foraging theory. Princeton, NJ: Princeton University Press [Google Scholar]
- 26.Danchin E, Boulinier T, Massot M. 1998. Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology 79, 2415–2428 (doi:10.2307/176832) [Google Scholar]
- 27.Nordell RR, Valone T. 1998. Mate choice copying as public information. Ecol. Lett. 1, 74–76 (doi:10.1046/j.1461-0248.1998.00025.x) [Google Scholar]
- 28.Forsman JT, Seppänen JT, Nykänen IL. 2012. Observed heterospecific clutch size can affect offspring investment decisions. Biol. Lett. 8, 341–343 (doi:10.1098/rsbl.2011.0970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiten A, Horner V, de Waal FBM. 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740 (doi:10.1038/nature04047) [DOI] [PubMed] [Google Scholar]
- 30.Campobello D, Sealy SG. 2011. Use of social over personal information enhances nest defense against avian brood parasitism. Behav. Ecol. 22, 422–428 (doi:10.1093/beheco/arq225) [Google Scholar]
- 31.Cornell HN, Marzluff JM, Pecoraro S. 2012. Social learning spreads knowledge about dangerous humans among American crows. Proc. R. Soc. B 279, 499–508 (doi:10.1098/rspb.2011.0957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnie KE, Earley RL. 2007. Expanding the scope for social information use. Anim. Behav. 74, 171–181 (doi:10.1016/j.anbehav.2006.12.009) [Google Scholar]
- 33.Danchin E, Giraldeau L-A, Valone TJ, Wagner RH. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 34.Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 35.Cheney DL, Seyfarth RM. 1988. Assessment of meaning and the detection of unreliable signals by vervet monkeys. Anim. Behav. 36, 477–486 (doi:10.1016/S0003-3472(88)80018-6) [Google Scholar]
- 36.Hare JF, Atkins BA. 2001. The squirrel that cried wolf: reliability detection by juvenile Richardson's ground squirrels. Behav. Ecol. Sociobiol. 51, 108–112 (doi:10.1007/s002650100414) [Google Scholar]
- 37.Searcy WA, Nowicki A. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 38.van Bergen Y, Coolen I, Laland KN. 2004. Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962 (doi:10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray MK, Klein BA, Seeley TD. 2012. Honey bees use social information in waggle dances more fully when foraging errors are more costly. Behav. Ecol. 23, 125–131 (doi:10.1093/beheco/arr165) [Google Scholar]
- 40.Giraldeau L-A, Valone TJ, Templeton JJ. 2002. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seppänen J-T, Forsman JT, Mönkkönen M, Thomson RL. 2007. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88, 1622–1633 (doi:10.1890/06-1757.1) [DOI] [PubMed] [Google Scholar]
- 42.Farrell S. 2011. Social influence benefits the wisdom of individuals in the crowd. Proc. Natl Acad. Sci. USA 108, E625 (doi:10.1073/pnas.1109947108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King AJ, Cowlishaw G. 2007. When to use social information: the advantage of large group size in individual decision making. Biol. Lett. 3, 137–139 (doi:10.1098/rsbl.2007.0017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Juricic E, Beauchamp G, Treminio R, Hoover M. 2011. Making heads turn: association between head movements during vigilance and perceived predation risk in brown-headed cowbird flocks. Anim. Behav. 82, 573–577 (doi:10.1016/j.anbehav.2011.06.014) [Google Scholar]
- 45.Sirot E. 2006. Social information, antipredatory vigilance and flight in bird flocks. Anim. Behav. 72, 373–382 (doi:10.1016/j.anbehav.2005.10.028) [Google Scholar]
- 46.Treherne JE, Foster WA. 1981. Group transmission of predator avoidance behaviour in a marine insect: the Trafalgar affect. Anim. Behav. 29, 911–917 (doi:10.1016/S0003-3472(81)80028-0) [Google Scholar]
- 47.Brown C, Laland KN. 2003. Social learning in fishes: a review. Fish Fish. 4, 280–288 (doi:10.1046/j.1467-2979.2003.00122.x) [Google Scholar]
- 48.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 (doi:10.1086/406755) [Google Scholar]
- 49.Hasson O. 1991. Pursuit-deterrent signals: communication between prey and predators. Trends Ecol. Evol. 6, 325–329 (doi:10.1016/0169-5347(91)90040-5) [DOI] [PubMed] [Google Scholar]
- 50.Hecht EE, Patterson R, Barbey AK. 2012. What can other animals tell us about human social cognition? An evolutionary perspective on reflective and reflexive processing. Front. Hum. Neurosci. 6, 224 (doi:10.3389/fnhum.2012.00224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platek SM, Critton SR, Myers TE, Gallup GG., Jr 2003. Contagious yawning: the role of self-awareness and mental state attribution. Cogn. Brain Res. 17, 223–237 (doi:10.1016/S0926-6410(03)00109-5) [DOI] [PubMed] [Google Scholar]
- 52.Anderson JR, Myowa-Yamakoshi M, Matsuzawa T. 2004. Contagious yawning in chimpanzees. Proc. R. Soc. Lond. B 271, S468–S470 (doi:10.1098/rsbl.2004.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Provine RR. 1996. Laughter. Am. Sci. 84, 38–45 [Google Scholar]
- 54.Gallese V, Keysers C, Rizzolatti G. 2004. A unifying view of the basis of social cognition. Trends Cogn. Sci. 8, 396–403 (doi:10.1016/j.tics.2004.07.002) [DOI] [PubMed] [Google Scholar]
- 55.Preston SD, de Waal FBM. 2002. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–71 (doi:10.1017/S0140525X02000018) [DOI] [PubMed] [Google Scholar]
- 56.Tamietto M, Castelli L, Vighetti S, Perozzo P, Geminiani G, Weiskrantz L, de Gelder B. 2009. Unseen facial and bodily expressions trigger fast emotional reactions. Proc. Natl Acad. Sci. USA 106, 17 661–17 666 (doi:10.1073/pnas.0908994106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brothers L. 1990. The neural basis of primate social communication. Motiv. Emotion 14, 81–91 (doi:10.1007/BF00991637) [Google Scholar]
- 58.Silva K, de Sousa L. 2011. ‘Canis empathicus’: a proposal on dogs' capacity to empathize with humans. Biol. Lett. 7, 489–492 (doi:10.1098/rsbl.2011.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrett L, Henzi SP, Lusseau D. 2012. Taking sociality seriously: the structure of multi-dimensional social networks as a source of information for individuals. Phil. Trans. R. Soc. B 367, 2108–2118 (doi:10.1098/rstb.2012.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slobodchikoff CN, Perla BS, Verdolin JL. 2009. Prairie dogs: communication and community in an animal society. Cambridge, MA: Harvard University Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bout response characteristic and bout initiator behavioural data file: Dryad doi:10.5061/dryad.g76t8.