Abstract
Communication is a characteristic of life, but its reliability and basic definition are hotly debated. Theory predicts that trade among mutualists requires high reliability. Here, we show that moderate reliability already allows mutualists to optimize their rewards. The colours of Mediterranean fleshy-fruits indicate lipid rewards (but not other nutrients) to avian seed dispersers on regional and local scales. On the regional scale, fruits with high lipid content were significantly darker and less chromatic than congeners with lower lipid content. On the local scale, two warbler species (Sylvia atricapilla and Sylvia borin) selected fruit colours that were less chromatic, and thereby maximized their intake of lipids—a critical resource during migration and wintering. Crucially, birds were able to maximize lipid rewards with moderate reliability from visual fruit colours (r2 = 0.44–0.60). We suggest that mutualisms require only that any association between the quality and sensory aspects of signallers is learned through multiple, repeated interactions. Because these conditions are often fulfilled, also in social communication systems, we contend that selection on reliability is less intense than hitherto assumed. This may contribute to explaining the extraordinary diversity of signals, including that of plant reproductive displays.
Keywords: communication, mutualism, seed dispersal, honest signalling, signal theory, sensory ecology
1. Introduction
Communication is a key feature of life, structuring the interactions among partners [1,2]. This explains why rapidly evolving communication systems can drive adaptive radiations and outpace ecological divergence in other traits [3,4]. However, the conceptualization of communication is contentious and hotly debated. In particular, some researchers view communication as a process that evolved to facilitate information transfer among signallers and perceivers [2,5], whereas others view communication as a process of manipulating rather than as a process of informing others [6,7]. While information transfer requires reliability, this is not required for manipulation. The key issues are thus how reliable communication is and how reliability (or lack thereof) contributes to structuring the interactions among partners.
If the interests of signallers and perceivers diverge (which they commonly do), reliability, a tight covariance between signal and quality, requires costs associated with signalling or with cheating [8–10]. Notably, however, many ecological interactions such as mutualisms appear to lack the costs of signalling or cheating that enforce reliability [11]. Yet, recent studies on visual communication among fleshy-fruited plants and seed dispersers concluded that fruit colours reliably indicate fruit nutritional rewards in distinct communities [12,13]. Because only one study on pollination of Turnera ulmifolia demonstrated selection on the covariance between sensory traits and plant rewards [14], the covariance between fruit colours and nutrients is probably best explicable as by-product information originating from pleiotropy or similar biochemical pathways of nutrients and pigments [12,13]. As such, it is unclear whether animals commonly select the reliability of plant–animal communication.
Here, we investigate the role of reliable communication in structuring seed dispersal in a Mediterranean community. We analysed a 2 year dataset of fruit consumption of two avian seed dispersers: blackcaps (Sylvia atricapilla) and garden warblers (Syliva borin), in Hato Ratón, SW Spain [15,16]. Importantly, Jordano [16] has already determined that both bird species select fruits non-randomly and primarily to maximize lipid intake, because lipids provide the energy essential for long-distance migration and overwintering in southern Spain. Here, we do not repeat that analysis, but rather we aimed to explore whether birds select fruit colours indicating lipid contents.
We derived three predictions from the hypothesis that reliability structures seed dispersal. (i) We expected fruit colour to be reliable (i.e. consistently associated with nutrient rewards) both on a local and regional scale, the latter representing the evolutionary setting that presumably has shaped consumers’ responses. (ii) We expected the nutritional rewards of foraging bouts to be consistently associated with specific fruit colour combinations (figure 1), and (iii) the nutritional intake of consumers responding to reliable signals in sequential foraging decisions (i.e. meals) should be above the intake of a random forager.
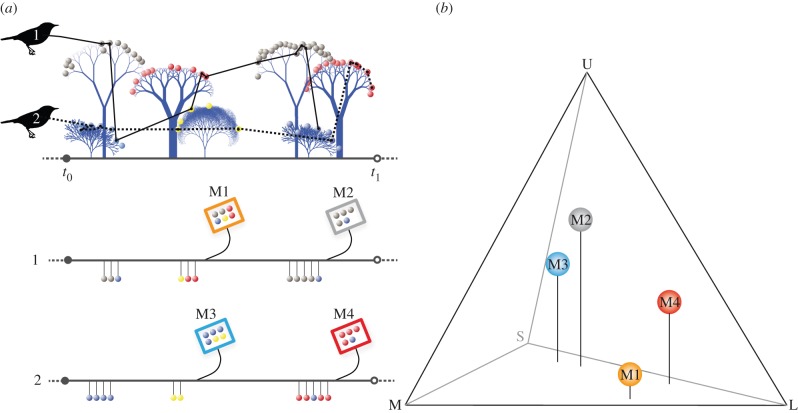
Figure 1.
The trade of resources characterizing mutualistic interactions leads to multiple, repeated interactions among individual producers and consumers. For example, birds use visual information to decide which fruits to consume. Two individual birds combine different fruit species in their meals during a short feeding bout (t0 − t1), along their foraging sequence, in which they visited different fruiting plants. M1–M4 indicate the composition of four meals, i.e. the number of fruits consumed and their species identity (a). For illustrative purposes, M1 and M2 represent two foraging bouts of the same individual. In our analyses, we considered only a single meal for each individual bird. The colours that birds see can be represented within a three-dimensional chromaticity diagram characterized by the coordinates {x, y, z} (b). The locations of different meals (a) in the visual space (b) are defined by the coordinates of the fruit species that each meal includes, weighted by the number of fruits from each species. In other words, the visual profile of each meal is scaled given the relative contribution of fruit species within that meal. (Online version in colour.)
2. Material and methods
(a). Bird diet
Blackcaps and garden warblers are highly frugivorous during migration and overwintering, when more than 90% of their diet consists of fruits [15]. The frugivorous diet of blackcaps and garden warblers was obtained from September to November 1981 and 1982 from Hato Ratón, Doñana National Park, southern Iberian Peninsula [16]. It is known that both species mainly forage on fruits to maximize lipid intake at Hato Ratón [16], a critical resource to fuel migration and overwintering. All birds were mist-netted, ringed and released. Based upon ring numbers, we selected only the meal from the first time each individual was caught for further analyses. We estimated the nutritional intake resulting from a total of 1726 consumed fruits identified in 377 and 134 faecal samples of blackcaps and garden warblers, respectively. These samples were obtained from the faeces of birds and from flushing their digestive tract with 1% sodium chloride water solution [15,16]. The number of consumed fruits was estimated from seeds and the proportion of remaining fruit skin after microhistological analysis [15,16]. The amount of fruits per species was visually estimated to the nearest 5% of an entire fruit, ranging from 0.05 to 11 fruits per sample.
Our samples reveal the short-term sequence of fruits ingested by freely foraging warblers (i.e. meals). Meals reflect fruit choice within less than an hour before birds were captured owing to their very short retention times of ingesta, on average less than 45 min [15–17]. We envision these samples as instantaneous ‘probes’ into the feeding sequence of individual foraging birds (figure 1a). Because the majority of faeces contained seeds or fruit remains from more than one plant species, any relationship between the nutritional intake of birds and the colour of consumed fruits is not a simple consequence of the traits of a single fruit species, but a consequence of birds mixing different fruits. We estimated the total nutritional intake per meal as the sum of the product of the number of consumed fruits across all fruit species in a meal, the amount of fruit pulp per fruit and the percentage of nutrients relative to the dry fruit mass.
Null models describing random foraging are based on direct counts of fruit production (on fifteen 15 m× 1.5 m plots) and availability during the study period, as well as weekly counts on fruit phenology [15,16].
(b). Fruit traits
The information about pulp nutrients and fruit colours used in this study was obtained from the western Mediterranean fleshy-fruit dataset [13]. Briefly, the contents of crude lipids in fruit pulp (percentage of pulp dry mass) were analysed by Soxhlet extraction. Protein contents are based upon nitrogen extraction with Kjeldahl using the conversion factor of 6.25. Non-structural carbohydrates were estimated as difference between dry fruit mass and the contents of lipids, protein and fibre.
Fruit traits are at least partly determined by plant phylogeny. Previously, we have shown that—independent of plant phylogeny—fruit colour is associated with their lipid contents, and fruit brightness is associated with their contents of non-structural carbohydrates across 111 species of fleshy-fruited plants in southern Spain [13]. More specifically, phylogenetically independent contrasts of lipid and sugar contents correlated most closely with hue and brightness, whereas morphological and other nutritional traits (e.g. protein contents) did not correlate with visual traits. The pervasive association between nutritional rewards and visual traits indicates that fruit colour could qualify as a reliable signal of rewards. Fruit lipids are hydrophobic and thus negatively correlated with the contents of hydrophilic carbohydrates in this dataset (Pearson correlation r = −0.47, d.f. = 103, p < 0.0001). The contents of lipids and carbohydrates are unrelated to the protein contents of fruits (Pearson correlation r < 0.10, d.f. = 108, p > 0.27).
Our previous analysis does not resolve, however, whether seed dispersers can perceive the association between fruit colour and nutritional rewards as it was done based on human colour vision. In addition, our analysis was based on a tree of species that occur within different communities or that fruit at different times of the year. Thus, it remains unclear whether reliability occurs regionally and locally and whether frugivorous animals could select for it. Additionally, the tree used does not resolve intrageneric relationships. We therefore assessed whether the covariance between nutritional rewards and fruit colour also persists if (i) analysed within the avian colour space and (ii) whether it is consistently found within genera across the entire tree of 111 species.
We thus selected 46 congeneric species belonging to 23 genera that are broadly distributed throughout the phylogeny of Mediterranean species [13] (see electronic supplementary material, table S1). For each genus, we selected the species with the highest and lowest content of each main nutrient (lipid, sugar, protein). These fruits encompass a broad variation in colour and brightness, ranging from white to black, and nutritional contents (e.g. mean lipid content 7% of dry fruit-pulp mass, ranging between 0.8% and 58%) [13]. Using paired t-tests on log-transformed variables, we then analysed whether the colour variables differed between the groups of relatively high and relatively low nutritional contents, respectively. These tests thus show whether fruit colour transmits reliable information on the nutritional quality of fruits, i.e. whether there is a consistent trend, independent of phylogenetic affinity, for visual parameters (x-, y-, z-coordinates and the excitation of the double cone) to differ in relation to the nutrient content of the pulp.
(c). Vision modelling
We tested our first prediction on the reliability of fruit colours using models of avian vision. In general, most animals are able to extract information from colours using two different aspects: achromatic and chromatic information on colour. Animals process chromatic and achromatic information by distinct neuronal mechanisms. Variation in brightness is perceived on the scale of black to white; high brightness values indicate white because they are caused by a high reflectance from a surface. Chromatic information results from variation in the saturation and peak wavelengths of the colour spectra; the latter is related to human colour categories (red, green).
Fruit colours were quantified in 2007–2008 by measuring their reflectance spectra with an Ocean Optics USB-2000 spectrometer and a Top Sensor System deuterium–halogen DH-2000 lamp as a standardized light source (DT-MINI-GS-2) and calculated in 5-nm-wide spectral intervals over the range of 300–700 nm [13]. We measured the reflectance of 20 fruits per species and calculated a mean reflectance spectrum for further analyses.
Birds use four cone types for colour vision. To calculate fruit colours according to the visual perception of birds, we modelled the probability of photon capture of each cone by multiplying the mean fruit reflectance spectra from each species with the spectral sensitivities of the cones using the avian eye model developed by Vorobyev & Osorio [18]. We calculated perception of fruit brightness according to the excitation of the avian double cone according to Schaefer et al. [19]. In general, the avian eye model assumes that discrimination is determined by thresholds that are set by noise originating in the cones [18].
We converted cone excitation values for the four avian cone types used for colour vision into the relative cone excitation values by dividing the excitation of each cone by the sum of all cone outputs. Because these values are not independent from each other, we transformed the cone excitation values into the tetrahedral colour space of birds [4,20], where each of the vertices represents the sole excitation of a single cone (figure 1b). The colour space within the tetrahedron is characterized by three Cartesian coordinates {x, y, z} that define the location of each spectrum [4,20]. The origin of the coordinates is the achromatic point where all cones are stimulated equally. Chromaticity, or the strength of a colour signal, is proportional to the Euclidean distance from any point within the tetrahedron to the achromatic point [20]. The x-coordinates range from blue fruits with negative scores to red fruits with positive scores, y-coordinates range from purple fruits (negative scores) to green fruits (positive scores), whereas positive scores on z are indicative of UV reflectance.
In general, the visual system of birds is relatively conservative. The spectral sensitivities of most frugivorous birds including Sylvia warblers are unknown. Similar to most other passerine families, Sylviidae belong to the UVS type of colour vision, where the sensitivity of the short-wavelength cone is biased towards the ultraviolet. We therefore used the well-known UVS spectral sensitivities of blue tits (Cyanistes caeruleus) [21] to model fruit colour perception. However, if spectral sensitivities of the other, VS, type of avian vision are used, the results remain unaltered (e.g. colours are equally associated with lipids: paired t-test, t = 2.67, d.f. = 22, p < 0.05).
(d). Analysing reliability
We tested our first prediction on the reliability of fruit colours on two spatial scales: in the western Mediterranean area (the regional scale) and in the fruits actually consumed by Sylvia warblers at Hato Ratón (the local scale). To test our second and third predictions on how birds respond to fruit colour as a reliable cue of nutritional rewards, we calculated the colour profile of a meal. The colour profile is the sum of the species-specific {x, y, z} coordinates of all fruit species found in a meal weighted by their relative abundance (in %) in that meal. Visual parameters and nutritional intake are thus both compound variables that include the number of fruits in a meal as a weighting factor to estimate the total ‘visual’ and ‘nutritional’ profile of each meal. This means that the colour profile of a meal is independent of the number of fruits eaten in a meal, but dependent on the species composition in that meal. We then analysed the correlation between total intake of lipids, sugars and proteins and the colour profile as well as brightness values across individual faecal samples of warbler species. We found a consistent pattern in both years and therefore included samples from different years in our analyses.
(e). Statistical analyses
On the local scale of Hato Ratón, we analysed the relationship between visual and nutritional fruit traits in multiple regression with permutation test. In this analysis, the variables of each species (nutritional contents, x-, y-, z-coordinates) were weighted by their relative fruit abundance in Hato Ratón (according to the data on fruit availability; see [16]) to account for the fact that seed dispersers encounter some species more frequently than others.
We analysed the relationship between the colour profile and the overall intake of sugars, lipids and proteins in meals using multiple regressions, with nutritional intake as dependent variable and the colour coordinates {x,y,z} and brightness as well as the number of fruits consumed per meal as independent variables. The results remained qualitatively identical if we analysed the residuals of the regression between fruit number and nutritional rewards of meals, showing that the association between nutritional profiles and visual profiles is not a simple autocorrelation caused by the number of fruits eaten.
To analyse whether the two Sylvia warblers choose fruit colours non-randomly, we used Monte Carlo simulations to sample combinations of fruit species within meals according to the number of fruits (1–11) present in each meal. We ran separate simulations for each month and year, because Monte Carlo simulations are weighted by the availability of each fruit species, i.e. the number of fruits of each species present in Hato Ratón at a given time based on the fruit counts. In other words, the likelihood that a fruit species is represented in the simulated meals is a function of its abundance in a given month. For each month, we ran 5000 simulations of each number of fruits (1–11) in the meals, where the {x,y,z} coordinates of each meal are determined by summing the values of each species represented in the meal according to its proportion. We used MANOVA analyses with Wilk's test to test for differences in {x,y,z} coordinates as dependent variables between the observed and simulated meals for each species, month and year separately. We determined whether the observed colour profile of meals was within the 95% CI of simulated meals expected under the assumption that birds forage randomly.
3. Results
Avian seed dispersers can visually evaluate the lipid rewards of western Mediterranean fruits but not their sugar or protein rewards. Fruits with higher lipid contents were significantly darker than congeneric fruits with lower lipid contents (sister species comparison that eliminates biases caused by phylogenetic relatedness, paired t-test, n = 23, t = 2.71, d.f. = 22, p < 0.05), a relationship significantly consistent across the congeneric comparisons (19 of 23 contrasts showing higher lipid content in the congener with darker fruits; binomial test, p = 0.003). Fruits with higher lipid contents also differed in their chromatic properties, scoring lower (mean = 0.063) on the x-axis of avian colour space—which captures variation from blue (negative values) to red (positive values) in our fruits—than congeners with lower lipid content (mean = 0.155; paired t-test, t = 2.27, d.f. = 22, p < 0.05). Again, this relationship was consistent across the congeneric comparisons (binomial test, p = 0.003). There were no differences in the relationship between other parameters of avian colour space (y-, z-coordinates) and lipid (p > 0.2). The contents of carbohydrates and proteins did not correlate with any parameter of avian colour space (x,y,z axes and perceived brightness, all p > 0.3). Thus, the previously reported association between carbohydrates and fruit brightness [13] does not hold within plant genera. Overall, lipid-rich fruits were darker, and both significantly and consistently less chromatic on the Iberian Peninsula compared with fruits with low lipid content that were redder.
Because fruit colour is related to lipid contents in the western Mediterranean area, we then asked whether the colours of fruits simultaneously present in a local community consistently indicate lipid contents. Variation in fruit brightness indicated very reliably the lipid contents of 16 fleshy-fruited species (see electronic supplementary material, table S2) that blackcaps and garden warblers consumed in Hato Ratón (regressions with permutation test, F1,14 = 53.5, r2 = 0.778; p < 0.001). Colour variation was likewise associated with the lipid contents of these fruits. This association was strongest for the y-axis of avian colour space, which ranges from purple (negative values) to green (positive values) (F1,14 = 29.7, r2 = 0.606; p = 0.0002). UV reflectance was also associated with lipid contents (z-axis: F1,14 = 20.7, r2 = 0.567, p = 0.0004), as was–albeit to a lesser extent–variation in the reflectance in the blue part of the spectrum relative to the red part of the spectrum (x-axis: F1,14 = 13, r2 = 0.445, p = 0.003). None of the colour parameters of the 16 fleshy-fruited species indicated the contents of sugars and proteins (regressions with permutation test, F1,14 = r2 < 0.11, p > 0.1), revealing that these nutritional rewards are not indicated at Hato Ratón. Now that we have shown a reliable relationship between lipids and fruit colours in the fruits ingested by birds, we tested our second prediction that birds use fruit colour as a reliable advertisement to optimize foraging.
Birds responded to fruit colours non-randomly (table 1), and that response predicted the total lipid intake in their meals (blackcap F5,398 = 763.2, adjusted r2 = 0.90, p < 0.0001; garden warbler F5,144 = 406.5, adjusted r2 = 0.93, p < 0.0001), which was above the value for random foragers (blackcap, t = 301, p < 0.0001; garden warbler, t = 154, p < 0.0001). Lipid-rich fruits did not differ from less rewarding fruits in Hato Ratón in their size or other parameters. Although the variation in the reflectance in the blue part of the spectrum relative to the red part (x-axis) was a less reliable indicator of the lipid contents of fruits in Hato Ratón than other colour parameters, this variation was the best predictor of the lipid rewards of the meals of both warblers (blackcap: x-axis: t = 16.34, p < 0.0001; y-axis: t = 0.4 p > 0.6; z-axis: t = 7.65, p < 0.0001; brightness profile: t = 0.3, p > 0.7, number of fruits: t = 16.52, p < 0.0001; garden warbler: x-axis: t = −21.22, p < 0.0001, y-axis: t = 12.2, p < 0.0001; z-axis: t = 7.52, p < 0.0001; brightness profile: t = 172, p < 0.0001, number of fruits: t = −4.64, p < 0.0001). This result shows that the colour profile of meals predicted their lipid reward as much as (in blackcaps) or more than (in garden warblers) the number of fruits eaten per meal (mean = 2). Birds preferentially consumed fruits that were less chromatic than randomly expected (figure 2) and those colour combinations predicted lipid rewards but not the rewards of carbohydrates or proteins.
Table 1.
MANOVA analyses showed that the colour patterns in the meals of the two warbler species differed from simulated random compositions in all months except for one in blackcaps (see also figure 2). Columns indicate the frequencies of observed meals (in %) that are outside the 2.5% and 97.5% percentiles of the simulated values for the achromatic, x, y and z components of the colour space.
| species and period(Wilk's lambda) | p | %A | %x | %y | %z |
|---|---|---|---|---|---|
| Sylvia atricapilla | |||||
| October 1981, n = 44 (0.9990) |
** | 4.5 | 6.8 | 4.5 | 9.0 |
| November 1981, n = 36 (0.9999) |
n.s. | 5.5 | 5.6 | 2.8 | 16.7 |
| October 1982, n = 91 (0.9837) |
** | 26.4 | 34.1 | 4.4 | 14.3 |
| November 1982, n = 206 (0.9963) |
** | 15.5 | 12.6 | 3.4 | 20.4 |
| Sylvia borin | |||||
| September 1981, n = 27 (0.9991) |
** | 14.8 | 11.1 | 7.4 | 25.9 |
| October 1981, n = 17 (0.9998) |
* | 11.8 | 35.3 | 5.9 | 17.6 |
| September 1982, n = 73 (0.9272) |
** | 26 | 75.3 | 26.2 | 8.2 |
| October 1982, n = 17 (0.9886) |
** | 35.3 | 41.2 | 5.9 | 11.8 |
**p < 0.0001; *p < 0.05; n.s., non-significant.
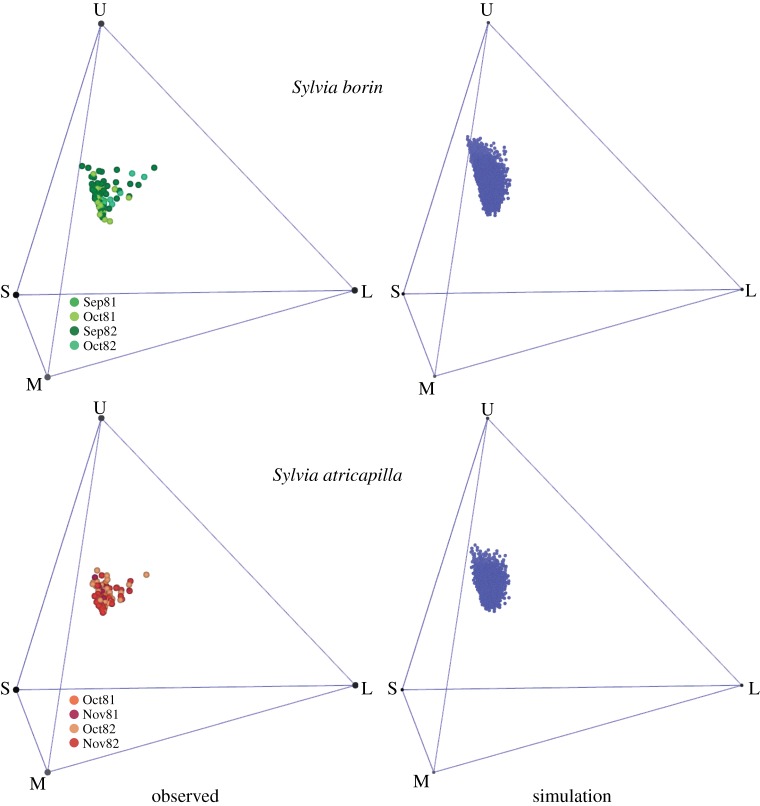
Figure 2.
Colour profiles of warblers’ meals within the three-dimensional tetrahedral representation of the visual space. For each species, meals of different years and months are identified by colour codes (inset). The locations of the observed colour profiles of fruit meals are compared with the locations of simulated meals (n = 10 000), representing the expected patterns for avian foragers consuming fruits in proportion to their actual availability in the habitat. The three-dimensional scatter of observed data (left) for the two warbler species consistently deviates from the simulated, null model expectation (right), indicating that birds were selecting highly distinctive and non-random combinations of fruit colours in their meals. In turn, these fruit combinations consistently resulted in higher lipid intake when compared with simulated meals, despite marked changes (between years and months) in the fruit supply. U, S, M and L refer to the ultraviolet-, short-, medium- and long-wavelengths cones of birds, respectively.
4. Discussion
Here, we have shown for the first time that communication in a seed–dispersal mutualism is regionally and locally reliable and that mutualistic seed dispersers apparently forage using fruit colours as indicators of lipid rewards and thereby maximize their lipid intake. These results highlight that (i) frugivorous animals could use colour to optimize foraging returns and hence partner choice in seed dispersal mutualism; (ii) reliability does not need to be very strong to structure the consumer–resource interactions that characterize mutualisms.
(a). Reliability and selection by mutualists
Because visual discrimination is the first of several steps in hierarchical decision-making by frugivorous birds [22], the striking covariance of the visual and lipid profiles of the meals of the two Sylvia warblers facilitates the maximization of birds' intake of lipid, a critical nutrient for their survival during autumn and winter [16]. Interestingly, neither carbohydrate nor protein rewards were associated with variation in fruit colour, even though our previous study found an association between carbohydrates and fruit brightness in 111 species from southern Spain [13]. The lack of an association between carbohydrates and fruit brightness within the community indicates that broad-scale comparisons including species from different areas and seasons can overestimate the reliability of fruit colours that are locally available to animals while foraging.
Our conclusion that birds used dark and less chromatic fruit colours to optimize lipid rewards is supported by two facts. First, the relationship between less chromatic colours and lipid rewards was stronger in the meals of birds than among the fruits available at Hato Ratón. This difference illustrates that birds preferentially consumed fruits with a tight association between fruit colour and lipid rewards. Second, varying fruit availability among different months and years represents a natural experiment to examine birds’ preferences. That birds’ non-random selection remained significant even if analysed separately over monthly periods (table 1) indicates very consistent choices independent of temporal variations in the fruit supply.
Our analyses revealed congruent associations between fruit colour and lipid rewards on the regional and local scale. Yet, we expect the reward–colour association to vary according to spatial and temporal variation in fruit abundance and ripening phenology. Importantly, such variation does not prevent birds from responding to (and potentially selecting) reliable plant communication. Experiments manipulating colour–reward associations have shown that garden warblers associate colours with their specific rewards within 1–2 days [23], allowing birds quickly to develop preference rankings according to the local fruit supply. Thus, in repeated interactions, mutualists can cope with variation in signal–reward associations because they necessarily evaluate the quality of a resource by taste and post-ingestive feedbacks, enabling them to learn the quality of rewards.
Reliability in plant–animal communication can arise as a by-product of a shared biochemistry of fruit pigments and nutrients [12,13]. For example, covariance between carotenoids and lipid molecules is explicable, because carotenoids depend on a phospholipid environment [24]. However, the covariance in our study is not explicable by fruit biochemistry, because the dark and less chromatic fruits associated with lipid contents are mainly pigmented by anthocyanins, a hydrophilic class of pigments unrelated to lipids.
Animals can also select for reliability in mutualisms as part of a self-serving strategy. For example, hawkmoths reduce probing time on less rewarding Petunia flowers to increase their foraging efficiency and thereby reduce the pollination and seed set of relatively unrewarding flowers [25]. It is currently unknown whether seed dispersers remove more fruits from more rewarding plants on an intraspecific level, but birds possess fine-tuned discrimination abilities for variation in the concentration of the main nutrients [26]. Because fruit colours and nutrients are both evolutionary labile traits [13], the covariance between both can evolve according to selection by mutualists if these remove fewer fruits of less rewarding plant individuals.
(b). Communication in mutualisms
We suggest that communication could structure mutualisms, even if its reliability is limited. We suggest limited reliability to be a very general mechanism structuring the consumer–resource interactions that characterize mutualisms where partners repeatedly exchange resources to their mutual benefit. This is because fair trade among mutualists can always ensue if the summed effects of repeated interactions have pronounced influence on the fitness of each partner, but a single interaction does not strongly determine their fitness. This occurs not only among members of a social group, as in cooperation and dominance, but also in mutualisms such as pollination and seed dispersal.
Conversely, selection for reliability is more pronounced in one-time communication systems such as mate choice and prey–predator interactions, where a single error of choice can have remarkable fitness consequences. The current debate on the general reliability of communication [5,7] does not reflect the fact that selection upon reliability will vary depending on the interaction frequency among communicators. Finally, we note that our results explain why the coloration of fruit products influences how humans perceive their nutritional quality [27,28].
Acknowledgements
This project was supported by bilateral agreement between Ministerio de Ciencia e Innovación and Deutscher Akademischer Austausch Dienst (HA2006-0038 and DE2009-0091). A.V. is supported by the research postdoctoral programme ‘Ramón y Cajal’ (Ministerio de Ciencia e Innovación; RYC-2007-00620). The Consejería de Medio Ambiente, Junta de Andalucía and Severo Ochoa Program for Centres of Excellence in R&D&I (SEV-2012-0262) provided facilities and access and sampling permissions for this study. H.M.S., A.V. and P.J. designed the study, P.J. did the fieldwork and most statistical analyses, colour measurements were primarily done by A.V., colour analyses by H.M.S., and H.M.S. wrote the draft of the manuscript. All authors contributed intensively to revisions.
References
- 1.Schaefer HM, Ruxton GD. 2011. Plant–animal communication, p. 320 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Searcy WA, Nowicki S. 2005. The evolution of animal communication. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Arnegard ME, McIntrye PB, Harmon LJ, Zelditch ML, Crampton WGR, Davis JK, Sullivan JP, Lavoué S, Hopkins CD. 2010. Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am. Nat. 176, 335–356 (doi:10.1086/655221) [DOI] [PubMed] [Google Scholar]
- 4.Stoddard MC, Prum RO. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of New World buntings. Am. Nat. 171, 755–776 (doi:10.1086/587526) [DOI] [PubMed] [Google Scholar]
- 5.Carazo P, Font E. 2010. Putting information back into biological communication. J. Evol. Biol. 23, 661–669 (doi:10.1111/j.1420-9101.2010.01944.x) [DOI] [PubMed] [Google Scholar]
- 6.Dawkins R, Krebs JR. 1978. Animal signals: information or manipulation. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 282–309 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 7.Rendall D, Owren MJ, Ryan MJ. 2009. What do animal signals mean? Anim. Behav. 78, 233–240 (doi:10.1016/j.anbehav.2009.06.007) [Google Scholar]
- 8.Sergio F, Blas J, Tanferna A, López L, Lemus JA, Hiraldo F. 2011. Raptor nest decorations are a reliable threat against conspecifics. Science 331, 327–330 (doi:10.1126/science.1199422) [DOI] [PubMed] [Google Scholar]
- 9.Számado S. 2011. The cost of honesty and the fallacay of the handicap principle. Anim. Behav. 81, 3–10 (doi:10.1016/j.anbehav.2010.08.022) [Google Scholar]
- 10.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature 432, 218–222 (doi:10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 11.Edwards DP, Yu DW. 2007. The roles of sensory traps in the origin, maintenance, and breakdown of mutualism. Behav. Ecol. Sociobiol. 61, 1321–1327 (doi:10.1007/s00265-007-0369-3) [Google Scholar]
- 12.Cazetta E, Galetti M, Rezende EL, Schaefer HM. 2012. On the reliability of visual communication in vertebrate-dispersed fruits. J. Ecol. 100, 277–286 (doi:10.1111/j.1365-2745.2011.01901.x) [Google Scholar]
- 13.Valido A, Schaefer HM, Jordano P. 2011. Colour, design and reward: phenotypic integration of fleshy fruit displays. J. Evol. Biol. 24, 751–760 (doi:10.1111/j.1420-9101.2010.02206.x) [DOI] [PubMed] [Google Scholar]
- 14.Benitez-Vieyra S, Ordano M, Fornoni J, Boege K, Domínguez CA. 2010. Selection on signal–reward correlation: limits and opportunities to the evolution of deceit in Turnera ulmifolia L. J. Evol. Biol. 23, 2760–2767 (doi:10.1111/j.1420-9101.2010.02132.x) [DOI] [PubMed] [Google Scholar]
- 15.Jordano P. 1987. Frugivory, external morphology and digestive-system in Mediterranean Sylviid warblers Sylvia spp. Ibis 129, 175–189 (doi:10.1111/j.1474-919X.1987.tb03199.x) [Google Scholar]
- 16.Jordano P. 1988. Diet, fruit choice and variation in body condition of frugivorous warblers in Mediterranean scrubland. Ardea 76, 193–209 [Google Scholar]
- 17.Simons D, Bairlein F. 1990. Neue Aspekte zur zugzeitlichen Frugivorie der Gartengrasmücke (Sylvia borin). J. Ornithol. 131, 381–401 (doi:10.1007/BF01639815) [Google Scholar]
- 18.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer HM, Schaefer V, Vorobyev M. 2007. Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? Am. Nat. 169, S159–S169 (doi:10.1086/510097) [DOI] [PubMed] [Google Scholar]
- 20.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 (doi:10.1111/j.1095-8312.2005.00540.x) [Google Scholar]
- 21.Hart NS. 2001. The visual ecology of avian photoreceptors. Prog. Ret. Eye Res. 20, 675–703 (doi:10.1016/S1350-9462(01)00009-X) [DOI] [PubMed] [Google Scholar]
- 22.Sallabanks R. 1993. Hierarchical mechanisms of fruit selection by an avian frugivore. Ecology 74, 1326–1336 (doi:10.2307/1940063) [Google Scholar]
- 23.Schaefer HM, Spitzer K, Bairlein F. 2008. Long-term effects of previous experience determine nutrient discrimination abilities in birds. Front. Zool. 5, 4 (doi:10.1186/1742-9994-5-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cazzonelli C. 2011. Carotenoids in nature: insights from plants and beyond. Funct. Plant Biol. 38, 833–847 (doi:10.1071/FP11192) [DOI] [PubMed] [Google Scholar]
- 25.Brandenburg A, Kuhlemeier C, Bshary R. 2012. Hawkmoth pollinators decrease seed set of a low-nectar Petunia axillaris line through reduced probing time. Curr. Biol. 22, 1635–1639 (doi:10.1016/j.cub.2012.06.058) [DOI] [PubMed] [Google Scholar]
- 26.Schaefer HM, Schmidt V, Bairlein F. 2003. Discrimination abilities for nutrients: which difference matters for choosy birds and why? Anim. Behav. 65, 531–541 (doi:10.1006/anbe.2003.2089) [Google Scholar]
- 27.Bayarri S, Calvo C, Costell E, Durán L. 2001. Influence of color on perception of sweetness and fruit flavor of fruit drinks. Food Sci. Tech. Int. 7, 399–404 (doi:10.1106/JJWN-FFRQ-JBMC-LQ5R) [Google Scholar]
- 28.Clydesdale FM. 1993. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 33, 81–101 (doi:10.1080/10408399309527614) [DOI] [PubMed] [Google Scholar]