Abstract
Infectious agents are part of food webs and ecosystems via the relationship with their host species that, in turn, interact with both hosts and non-hosts. Through these interactions, infectious agents influence food webs in terms of structure, functioning and stability. The present literature shows a broad range of impacts of infectious agents on food webs, and by cataloguing that range, we worked towards defining the various mechanisms and their specific effects. To explore the impact, a direct approach is to study changes in food-web properties with infectious agents as separate species in the web, acting as additional nodes, with links to their host species. An indirect approach concentrates not on adding new nodes and links, but on the ways that infectious agents affect the existing links across host and non-host nodes, by influencing the ‘quality’ of consumer–resource interaction as it depends on the epidemiological state host involved. Both approaches are natural from an ecological point of view, but the indirect approach may connect more straightforwardly to commonly used tools in infectious disease dynamics.
Keywords: infectious agents, infection dynamics, interaction strengths, food webs, energy flow, ecosystem
1. Introduction
The concept of a food web in community ecology provides a conceptual framework to study and understand relationships between species [1–9]. Species that infect other species have received relatively little attention in these studies, but in recent years this has been changing (promoted notably by Lafferty et al. [10–12]). Here, we discuss approaches for studying infectious agents as part of the food-web framework.
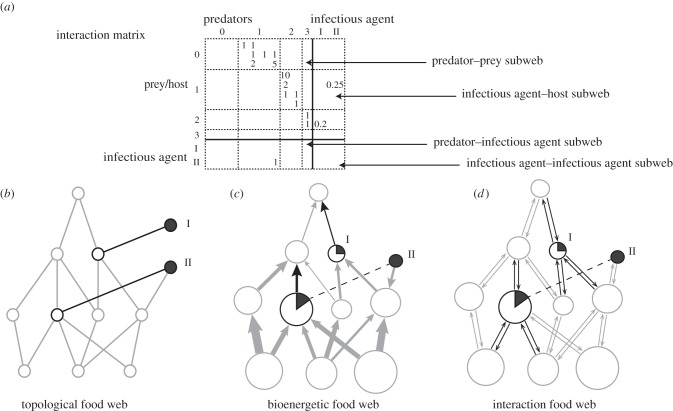
Food webs can typically be thought of in three different ways (figure 1), with increasing detail in data required [12–15]. First, in the form of diagrams or networks, where organisms (species or functional groups of species) are represented in the form of nodes, and where feeding relationships between consumers and resources are represented as links (topological webs). Second, as flows of energy and matter (bioenergetics webs). Third, in terms of interaction strengths across the species links (interaction webs)—that is, combining biomass estimates, usually at some (assumed) steady state, and empirical data on key physiological traits of the species (such as lifespan, energy-conversion efficiencies and diet preferences). Many food-web studies have shown that interaction strengths are strongly patterned [8,15–17], and that both distribution of interaction strengths and the topological structure of the web are important for stability in ecosystems [15,18].
Figure 1.
Illustrations of topological, bioenergetic and interaction food webs (for an imaginary system). (a) Interaction matrix for the imaginary web (with species ordered from left to right in each trophic level) and its four subwebs (e.g. [10]). (b–d) These subwebs are included in different way into different types of food webs: in black, a pathogen (I) and a parasite (II). (b) Infectious agents can be directly included in the food web (here illustrated for the topological food web only) through new nodes (black filled, infectious agent; black open, host species; grey open, non-host species) and links (grey, predator–prey; black, infectious agent–host); in this case, we would use a binary form for the interaction matrix. (c,d) The bioenergetic and interaction food webs here illustrate the indirect inclusion only. The black part of a circle represents the infected proportion of the population of that host species, and black arrows are examples of energy flow/interaction strength affected; the dashed line highlights that the free-living stages and the within-host stages of a parasite represent the same biological species, with separate bookkeeping for the different stages.
The initial papers highlighting the need to incorporate infectious agents into food-web analysis are largely concentrated on empirical work on parasites in aquatic systems. Papers have mostly either provided empirical data [12,19–22] or highlighted the need to consider parasites in our efforts to understand food webs and ecosystems [10–12,23–27]. As parasites in aquatic systems have, as a rule, a life cycle with one or more obligatory free-living stages (either different sequential manifestations of the same parasite individual or offspring of an individual, produced inside a host), the parasite as a species is (at least partly) free living and quantifiable (e.g. in terms of biomass). Consequently, it is natural to take a direct approach and incorporate an infectious species as a (special type of consumer) node in the web, with links to the host species it uses as resource, with possible free-living stages ingested as ‘prey’ (figure 1b). Infectious agents potentially change the topological properties of the host and non-host species' network, thus generating insight into their effects on the food web.
One could also incorporate infectious agents indirectly by thinking of them as living inside their hosts and influencing the energy flow and interaction strengths of the existing consumer–resource links in the host/non-host network (figure 1c,d).
The main part of this review (§2) is devoted to a systematic classification of mutual influences of infectious agents on energy flow and interaction strength in food webs, covering a range of parasites and pathogens, in a broad range of host species. Such classifications may well reveal unexpected differences between apparently closely related infectious agents, and notably also show similarities between (apparently) unrelated infectious agents that become clear only at the ecosystem level. Such a focus improves on the pairwise ‘one agent–one host’ interactions in many epidemiological studies, also in those related to wildlife, which may so far have ‘hidden’ these aspects. In §3, we briefly return to the various ways in which to extend the theoretical food-web framework to address effects of infectious agents, for future understanding of the many examples that have been documented.
2. Infectious agents and interaction strengths: a catalogue of examples
We systematically group (mostly empirical) papers studying effects of infectious agents in ecosystems and on food-web interaction. Other reviews of such studies have been published, focusing mainly on parasites [28]. As a classification principle, interaction between infectious agents and the ecosystem is represented at various levels of biological integration. The studies we review have specifically highlighted these interactions, but in fact, because of their ubiquitous nature, infectious agents possibly impact on life-history traits, behaviour, feeding or other individual-level aspects, and thus influence, to some extent, all ecological quantification. It would be rare, if not impossible, to obtain field data from an infection-free system.
(a). Infectious agents and energy (flow and biomass)
Infectious agents can lead to increased or decreased energy flow through food webs by affecting feeding rates, growth, mortality, fecundity, behaviour and other properties of individuals.
Most infectious agents force a host to redirect parts of its energy, assimilated for biomass production and maintenance, towards investment in immune response [10,29]. Parasitoids and parasitic castrators use their host's energy directly for their own maintenance and production. Infectious agents also affect growth rates at the host level by changing food consumption, or by affecting the assimilation and production efficiency of the consumed food [30]. One possible effect is altering feeding behaviour (e.g. infected hosts eat less/more or change the size or species of prey). An example of the former is the herbivorous snail Littorina littorea, which is frequently parasitized by the trematode Cryptocotyle lingua; uninfected snails consumed 40% more ephemeral macroalgal biomass than infected snails in the laboratory, possibly because the digestive system of infected snails is compromised by C. lingua infection [31]. Another example is the tapeworm, which causes infected sticklebacks, Gasterosteus aculeatus, to eat smaller prey [32]. Similar examples exist in hosts from terrestrial ecosystems. Avian malaria causes Hawaiian honeycreepers (Drepanidinae) to consume less food and consequently to lose body weight [33]. Gastrointestinal parasites and many other infectious disease agents can reduce the ability of a host to absorb nutrients, altering digestive efficiency and compromising the host's nutritional status [34].
Infectious agents may also change consumption in the opposite way, causing infected individuals to eat more. One example is the parasitic castrator trematode Cercaria batillariae in mud snails, Batillaria cumingi, which increases the food consumption of its hosts for its own energy needs and induces gigantism [35,36]. Similarly, air-breathing freshwater snails Physa acuta infected with the parasitic castrator trematode Posthodiplostomum minimum grazed more rapidly than uninfected snails [37]. This is even more interesting when we consider that biomass of a parasitically castrated host population can exceed the biomass of the uninfected host population [21].
Infectious agents can affect biomass of the host population through lower fecundity and castration. Some infectious agents can increase survival and growth rate, but reduce fecundity. An example is the fungus Atkinsonella hypoxylon, which infects ramets of the grass Danthonia spicata [38]. An example of castration is the previously described trematode C. batillariae, which castrates its host so that after infection snails can only produce larval trematodes [36].
In any interaction between two trophic levels, a change in energy flow due to the presence of an infectious agent at one level may influence the other level. Examples include infections in prey resulting in additional predation driven by unusual behaviour in infected individuals [39–42]. Changes in grouse behaviour can result from intestinal helminth parasites, which may contribute to higher mortality through increased predation by mammalian predators [43]. The behaviour-changing effects of the pathogen Toxoplasma gondii have been well documented: infected rodents behave in ways that make them more vulnerable to predation, thus increasing the odds for the pathogen to reach cats, to complete its life cycle [44]. Infection in prey that induces mortality may also increase energy available to consumers. This was observed in 1994 in Serengeti National Park, when severe drought in 1993 and a Babesia sp. infection in Cape buffalo led to increased numbers of buffalo carcasses available to be consumed by lions. The lions, partly immune-compromised by an outbreak of canine distemper virus (CDV), became additionally infected with Babesia sp., through ixodid ticks with a broad host range, possibly causing substantial mortality by this combination of factors [45]. A similar combination, involving plague in prairie dogs and CDV in black-footed ferrets, possibly led to the decline in the wild of the black-footed ferret [46], where plague in prairie dogs currently appears to frustrate re-introduction programmes [47].
Parasites may also be consumed directly as an energy resource without infecting their predator. Such predation on parasites is a natural process that happens in many communities and ecosystems, and can occur in at least three ways: as concomitant predation of parasites with prey; predation on living stages; and grooming [48]. An example of this is that 16–408 ticks per bird were found in the stomach contents of captured oxpeckers [49–51]. We discuss another grooming example related to ticks and opossums below. A different mechanism is the mistletoe, a parasite that has fruits available year round, flowers with abundant nectar and foliage rich in nutrients [52]. This parasite is an important food source for many species of birds and mammals [53]. Furthermore, cercariae (larval forms of trematodes) that do not find suitable hosts within a certain time, and thus die, contribute to the detrital pools in aquatic ecosystems [37] or become food for non-host species of fish [54].
Between ecosystems, infectious agents may also contribute indirectly to changes in energy flow. For example, the Dutch elm disease fungus increases energy flow between the terrestrial and aquatic ecosystems through increased mortality of hosts, which contributes to coarse woody debris in streams [55]. Similarly, nematomorph parasitoids change the behaviour of crickets, which then enter streams and become prey for trout [56].
The importance of the functional role of the host in the ecosystem can be seen, for example, with detrivores. Helminth parasites may alter behaviour post-infection of this functional group and decrease consumption of detritus, which further enhances energy flow through communities and ecosystems [57].
Pathogens are abundant in aquatic environments. The oceans contain an estimated 1030 virus particles, with 1023 infections occurring each second [58]. Influences of viruses in such ecosystems are still underexplored, but several examples exist that show these influences to be varied and complex. For example, viruses infecting primary producers (phytoplankton) in marine ecosystems can have a substantial negative effect on productivity [59]. In this respect, a fascinating system is the use of resource chloroplasts to gain energy from photosynthesis within the consumer's cells by the solar-powered sea slug, Elysia chlorotica. The synchronized sudden death of slugs appears to be connected to a release of viral particles from the chloroplasts, suggesting that these parasitoid viruses play a role in regulating the life history of these consumers through infection of the resource [60,61].
(b). Infectious agents and species (biodiversity)
At the species level, the term biodiversity is frequently used to account for species richness, described as the number of species of a particular taxon or life form that characterizes a particular biological community, habitat or ecosystem [7]. Biodiversity of food web/ecosystem is influenced by infectious agents directly through their own diversity, as well as through their influence on host and non-host diversity. Diversity of infectious agents is difficult to assess, and complicated further by the discovery of cryptic species [62].
Infectious agents can have great influence if they are introduced to a new ecosystem. Examples include spill-over from terrestrial to marine ecosystems, such as toxoplasmosis of sea otters, aspergillosis of sea fans and many others [63,64]. Usually, introductions of agents are connected with introductions of infected hosts. Apart from transmission among the newly imported hosts, sometimes the infectious agent is also transmitted to a native species that may be even more susceptible. One of the most well-studied examples is the replacement of red squirrel with grey squirrel, possibly mediated by parapoxvirus, which causes higher mortality in red squirrel [65].
Although infectious agents are considered to be one of the main factors directing species extinctions in natural ecosystems, research has shown that of 833 known species extinctions in the last 500 years [66] only 31 were known to be partly owing to infectious disease agents. It is very rare for an infectious disease to be listed as the single factor that contributed to extinction of a particular species [66]. For example, the chytrid fungus Batrachochytrium dendrobatidis in combination with environmental factors has led to the extinction of dozens of frog species in tropical regions in recent decades [67,68].
(c). Infectious agents and community (interactions)
Infectious agents in communities interact with their hosts, but also mediate negative and positive interactions between host and non-host species. Within hosts, parasites and pathogens form interaction networks, and may modify each other's dynamics [45,69,70]. More fieldwork is needed to elucidate such interactions between infectious agents, but effects may be difficult to disentangle. For example, Maas et al. [71] looked at more than 600 lions in Kruger Park, searching for possible synergistic effects between bovine tuberculosis and feline immunodeficiency virus, potentially mimicking the influence of tuberculosis and HIV established in humans, but found no evidence for a similar relation in lions.
Community-level changes are frequently forced by decline in a community's keystone population and affect predator–prey, competitive, mutualistic and other community interactions. As examples of other parasite-mediated interactions are well reviewed elsewhere [72–74], we restricted our interest to infectious agents that mediate predator–prey interactions.
Results of predator–prey interaction mediated by infection depend on the trophic position of the infected species. First, infection may occur at a low trophic level and influence consumers at upper trophic levels. Second, infection may influence species at mid-trophic level, possibly leading to changes in species populations of the same functional group, or propagate up and down trophic levels and produce trophic cascades. Effects may include prey switches by predators, when one prey species is reduced in abundance because of an infectious disease, leading to decreased interaction with that particular prey. Examples of infection-mediated predator–prey interactions are widespread in various ecosystems. In an aquatic ecosystem, an outbreak of unidentified infectious (possibly bacterial) agent in a keystone herbivore, the sea urchin (Diadema antilarum), induces high mortality. Because of its controlling effects of algal abundance [75], the loss of Diadema from coral reef systems where they had previously been abundant encouraged growth of their main resource, benthic algae; this was characterized as a main ecological phase shift from a coral-dominated system to an algal-dominated system [76]. Similarly, in a terrestrial system, anthrax, during the wet season, fatally infects zebras, springboks, wildebeest and oryx, as well as, during the dry season, elephants. As a consequence, infected carcasses are available for vultures, hyenas, lions and black-backed jackals year round. Decline in populations of major herbivore species such as zebras and elephants force cascading effects in the ecosystem [27,77]. These types of change are moreover affected by outbreaks in the top predator population. An example is canine parvovirus (CPV) in wolves, which induced a shift from top-down to bottom-up control of moose population dynamics: as CPV decimated the wolf population, moose growth rate is regulated by bottom-up effects and climate [78].
Infectious agents may also indirectly influence predator–prey interaction and drive community composition, altering behaviour of the host. As already stated, nematomorph parasitoids affect the behaviour of crickets, causing them to enter streams to become available as prey for trout. This indirectly influences trout, causing them to eat fewer benthic invertebrates, thus inducing an increase of benthic algae and a decrease in the rate of leaf breakdown [56].
(d). Infectious agents and ecosystem (physical characteristics, ecosystem engineering)
Infectious agents sometimes influence physical characteristics of the ecosystem to which they belong, and the term ‘ecosystem engineering’ has been used as a metaphor to describe these effects. This phenomenon was first defined by Jones et al. [79,80] to represent direct or indirect control of resource availability mediated by an organism's ability to cause physical state changes in abiotic or biotic materials. This definition includes space among the resources an organism can use for growth, maintenance and (re)production. Diverse examples of infectious agents as ecosystem engineers, with either direct or indirect influence, have been described [81,82].
Among the infectious agents that are themselves ecosystem engineers would be parasitic plants such as mistletoes. They change physical characteristics of an ecosystem (e.g. by providing nests for many animals). In southwest Oregon, mistletoe (Arceuthobium douglasii) brooms provide nests for the northern spotted owl [83]. Likewise, the Dutch elm disease fungus affects forest structure by changing the amount of standing material and creating canopy gaps that alter microclimate; tree defoliation increases the amount of light reaching the bottom and promotes herb and scrub growth; dead trees contribute to coarse woody debris in streams and decreased availability of nest sites [55,84]. In the same way, the fungus Phytophthora cinnamomi converted large areas of eucalyptus forest to monocot-dominated open savannah, eliminating nest sites and food for many animals [85].
Infections may also have indirect impacts on the ecosystem. For instance, rinderpest outbreaks in the 1960s caused a decline of ungulates in Maasai Mara National Park. The lack of herbivores facilitated an increase in dry-grass fires, which led to a significant decrease in acacia trees, an important part of the ecosystem [86]. These trees provide shade for ungulates, as well as nest sites for raptors, owls, vultures and a variety of other bird species [87]. Holdo et al. [88] found the opposite effect in Serengeti National Park, after rinderpest was eradicated there as a strong regulator of wildebeest. Similarly, rabbits, after being introduced to Great Britain in the eleventh century as a domestic animal, were by the 1950s sufficiently abundant in the wild that they were preventing regeneration of woody plants in many habitats. This led to a habitat transition from a forest-dominated to a grassland-dominated ecosystem. Introduction of the myxoma virus led to decline of the rabbit population and the re-establishment of forest after 20 years [89].
One can also find these examples in aquatic ecosystems. Larval trematodes that encyst the foot of the cockle Austrovenus stutchburyi reduce the burrowing ability of cockle, making them easier prey for host birds, and the shells of stranded cockles create habitat for a rich and distinctive epibiont community [90,91]. The impact of cockles on the benthic community is governed by reduced sediment disturbance, increased surface structural complexity and availability of larval trematodes as an additional food resource. The shells of dead cockles are so abundant that they offer important new habitat in the mudflat ecology.
(e). Effects of different levels of biological integration on infectious agents
Infectious agents are also influenced by energy flow, biodiversity, community structure and abiotic characteristics of an ecosystem.
With regard to biomass and energy, for example, hantavirus prevalence in small rodent host species increases as a result of boosts in primary food production in mast years [92,93], and a similar effect is observed for plague prevalence in small rodent host species when climate (notably rainfall) induces boosts of primary producers, leading to host population growth [94], and subsequent plague outbreaks [95]. Susceptibility to Metschnikowia bicuspidata infection, its evolution and the sizes of outbreaks among host individuals of Daphnia dentifera (an interaction to be discussed in more detail below) is influenced by the level of ecosystem productivity in lakes and the quality of the algal resource for the Daphnia [96,97].
Perhaps the most hotly debated effect concerns the influence of community biodiversity on the infectious agent. Biodiversity and the ratio of suitable (competent) and unsuitable (incompetent) hosts direct the survival of infectious disease agents in food webs [98]. This diversity involves vectors and (other) hosts of infectious agents, as well as non-hosts.
Vector diversity, as the research on transmission of vector-borne diseases shows, is relevant because the potential for infectious agents to persist increases in multi-species host populations. For example, for fleas transmitting the plague bacterium among rodents, the presence of multiple vector species able to infest multiple susceptible host species creates a more connected host network [99]. In West Nile virus transmission, there might be season-dependent shifts in feeding behaviour of the mosquito vector species, shifting from (virus-competent) birds to (less competent) mammals [100].
Whether individual species are suitable/unsuitable as hosts will determine the ability of the infectious agent to become established in a community [101]. For example, certain plant pathogens showed reduced prevalence but also reduced diversity in host species that are threatened, compared with non-threatened host species [102].
Indirect influences on infectious agents can result from increased diversity of predator species, which may change the behaviour of prey. For instance, the deer mouse spends more time in shelters as the number of predators increases, thus decreasing infection spread [103].
Transmission of an agent may significantly increase after loss of non-host species: prevalence of Sin Nombre virus rose 2–14% with decline of diversity [103]. Lyme disease is transmitted by blacklegged ticks, but Virginia opossums can predate on 83–96% of the ticks that attach to them and engorge, and loss of this weakly competent host species has led to increased Lyme prevalence in ticks that switch to feeding on mice, which are both strongly competent and weakly grooming [104].
Although the influence of biodiversity on prevalence in specific host species is no longer questioned, there is considerable debate concerning the generality of the effect, what factors determine whether the relationship is positive or negative, and what the mechanisms and causes are [105,106]. With respect to the latter, mechanisms often referred to are either transmission- and/or contact-related. But increased bird biodiversity did not reduce transmission or reduce encounters between mosquitoes infected with West Nile virus and competent hosts, for instance, even though a negative relationship was found between bird diversity and human incidence [107].
One known large influence of community diversity on the infectious agents is through extinction or decline of key species for highly host-specific agents. If their population sizes are under threshold, the agents cannot persist [108]. For example, extinction of five North American carnivore species is predicted to lead to extinction of 56 parasite species [109].
Community structure affects infectious agents also through the behavioural and social characteristics of hosts. This is seen in the behaviour that some species of host develop to avoid infectious agents (e.g. mammals smelling infected faeces and avoiding contaminated areas [110]). Various social behaviours like mating strategies, social avoidance, group size and group isolation have different consequences for transmission [111]. For instance, mating behaviour may increase host susceptibility: male field crickets Gryllus lineaticeps produce chirped songs to attract mates, and the parasitoid fly Ormia ochracea uses this song to locate them [112]. Furthermore, species that live in high-density populations facilitate transmission because of the frequency of contacts between individuals [113]. Increased frequency of contacts can be induced not only in social groups of the same species but also between different species that share a place of foraging, water or shelter [114]. Thus, for example, different rodent species may become infected with the plague bacterium by indirect contact because they frequent the same burrows [115].
A particularly interesting interaction between Daphnia dentifera, one of its invertebrate predators (Chaoborus midge larvae) and a yeast parasitoid (Metschnikowia bicuspidata) [116] is an example of association of community and ecosystem influence. Here, the predator produces a chemical compound that has two effects. The direct effect is that it induces growth of the Daphnia prey individuals. Owing to this growth in size, these individuals become more susceptible to the fungus, in the sense that bigger individuals filter a larger volume of water, and therefore ingest larger numbers of spores. A second indirect effect of this is that, once infected, larger individuals increase transmission rates, as they produce more spores than would infect Daphnia of a normal size.
3. Consequences for thinking about infectious agents in food webs
The examples above illustrate both the breadth and depth of various types of interaction between infectious agents and food webs/ecosystems. This motivates the need for a theoretical framework, as an additional tool to generate robust insight into the mechanisms behind, and consequences of, this interaction, and to explore possible generic principles.
Many of the examples (table 1) involve effects on energy flow and interaction strength, and it is possible that studying infectious agents indirectly (i.e. via the way they influence interaction strength) is an effective approach to such insight. This is especially relevant for pathogens.
Table 1.
Selected examples from the review.
| infectious agent/disease | host | type of infectious agent | type of ecosystem | reference |
|---|---|---|---|---|
| infectious agents influencing different levels of biological integration | ||||
| trematode | snail | parasitic castrator | aquatic | [31] |
| tapeworm | fish | parasite | aquatic | [32] |
| malaria | birds | pathogen | terrestrial | [33] |
| CDV+Babesia | lion | pathogen | terrestrial | [45] |
| mistletoe | tree | parasite | terrestrial | [52] |
| nematomorph parasitoid | crickets | parasitoid | terrestrial | [56] |
| toxoplasmosis | sea otter | pathogen | aquatic | [63,64] |
| parapoxvirus | squirrels | pathogen | terrestrial | [65] |
| fungus | frog | pathogen | terrestrial | [67,68] |
| unknown (suspected bacterial) | sea urchin | pathogen | aquatic | [75,76] |
| CPV | wolves | pathogen | terrestrial | [78] |
| fungus | eucalyptus tree | pathogen | terrestrial | [85] |
| rinderpest | wildebeest | pathogen | terrestrial | [88] |
| trematode | cockle | trophically transmitted parasite | aquatic | [90,91] |
| biological integration influencing infectious agents | ||||
| hantavirus | rodent | pathogen | terrestrial | [91,92] |
| plague | rodent | pathogen | terrestrial | [98] |
| West Nile virus | birds | pathogen | terrestrial | [99] |
| Borrelia burgdorferi | rodent | pathogen | terrestrial | [103] |
| parasitoid fly | cricket | parasitoid | terrestrial | [111] |
| yeast | Daphnia | parasitoid | aquatic | [115] |
Conceptual research on effects of infectious disease agents in food webs has so far been mainly directed at a direct topological approach by explicitly incorporating the agents, notably parasites, as species in the web of host and non-host interaction. Lafferty et al. [10] distinguish various sorts of links, such as parasite–parasite, parasite–host and predator–prey, also making a useful distinction between ‘possible’ and ‘realized’ links. They show that doing a careful accounting of such different links clarifies the large influence that parasites can have when included as species in the topological web.
Many pathogens hardly have individual biomass (although the total biomass of pathogens as species is underexplored and may be substantial) and generally have no free-living stage, and their transmission is not explicitly considered, but is assumed to occur inside populations of a host species that is presented by one node. Here, an approach where the agent is incorporated indirectly, through its effect on hosts, may be fruitful. Consider, as an example, a system with a consumer species and a resource species, with a pathogen that can infect the consumer in an immunizing infection. Instead of treating all consumer individuals the same, as one would do when studying this interaction in the absence of the pathogen, we now differentiate the consumer individuals by epidemiological state, differentiating susceptible, infectious and recovered/immune consumers of this species in the most basic case. The interaction strength quantifying the link between a particular class of consumers and the resource will now depend on the epidemiological state of those consumers. Similar reasoning also applies to the inclusion of parasites, using parasite load as epidemiological state [117], or parasitoids.
Which approach is most feasible will depend on the food web and infectious agent studied, the type of question one wants to address, and the level of detail available in data. The direct approach allows one to explore infectious agents as biological species—for example, exploring the ecological influence of a new species of infectious agent, as did Dunne et al. [118], the influence of parasite mortality on biomass redistribution in the web, the distribution of infectious species over trophic levels and the role of infectious species in maintaining biodiversity. The resulting changes in topological structure and ecosystem stability deserve substantial future attention. If one is interested in questions that require quantification of within- and between-host species spread of infection, the indirect approach may be a more natural point of departure. Examples are questions on the evolution of virulence, jumps to/emergence in new host species, the ability to invade a given ecosystem, persistence, effects of control strategies and changes in prevalence over various host species in the web. Viewing interactions between hosts and non-hosts as being mediated by the (dynamic) epidemiological status of the individuals involved is close to the established methodology in epidemic theory of infectious diseases, thus suggesting a feasible theoretical framework combining community ecology and epidemiology [119].
Extending and combining epidemiological and ecological theoretical frameworks of analysis would allow us to understand the observed types of behaviour at the ecosystem level and to explain them in terms of lower (e.g. one consumer–one resource, species or even individual) level interactions, mechanisms and processes. Both fields have considerably increased their methodology in recent decades and are able to address very complex phenomena with low-dimensional models—for example, in the case of population dynamics of structured populations, emerging behaviour from complexity in ecosystems or infectious agents in highly heterogeneous structured populations of hosts, including heterogeneity in contacts, modelled in networks. These approaches have shown that infectious agents are able to both stabilize and destabilize predator–prey interaction, mediate coexistence of resources and consumers, affect spatial patterning of populations, as well as have regulatory and other conservation consequences [119–124]. Such studies in the mathematical biology literature, often aiming at insight for generic systems, and mostly focusing on just two interacting species (apart from the infectious agent), have indeed mostly taken the approach of differentiating between epidemiological states in a consumer or a resource, or in both. There are a number of studies where models are analysed for specific systems to interpret empirical data (for example, the work of Hall et al. [96] and Duffy et al. [97], and other papers by these authors mentioned therein). The studies so far have hardly integrated epidemiology and food-web ecology by thinking in terms of interaction strength or energy flow (with the work of Getz on anthrax being one of the exceptions [77]).
If we are to understand observed patterns as reviewed in this paper, and ultimately predict what repercussions changes to ecosystems may have with regard to infectious disease prevalence and distribution, including emergence in human populations that may result, then developing such theories for realistic systems is essential.
Acknowledgements
We want to thank the reviewers, whose critical and detailed comments greatly improved this paper.
Funding statement
We acknowledge financial support from the Complexity programme of The Netherlands Organisation for Scientific Research (NWO).
References
- 1.Odum EP. 1971. Fundamentals of ecology. London, UK: Toppan Co [Google Scholar]
- 2.Pimm S. 1982. Food webs. London, UK: Chapman and Hall [Google Scholar]
- 3.Polis GA, Winemiller KO. 1996. Food webs: integration of patterns and dynamics. London, UK: Chapman and Hall [Google Scholar]
- 4.Pimm SL. 2002. Food webs. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.de Ruiter PC, Wolters V, Moore JC, Winemiller KO. 2005. Food web ecology: playing Jenga and beyond . Science 309, 68–71 (doi:10.1126/science.1096112) [DOI] [PubMed] [Google Scholar]
- 6.Begon M, Townsend C, Harper J. 2009. Ecology: from individuals to ecosystems, 4th edn London, UK: Blackwell Publishing [Google Scholar]
- 7.Levin SA, Carpenter SR, Godfray HCJ, Kinzig AP, Loreau M, Losos JB, Walker B, Wilcove DS. 2009. The Princeton guide to ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- 8.McCann KS. 2011. Food webs (MPB-50). Princeton, NJ: Princeton University Press [Google Scholar]
- 9.Moore JC, de Ruiter PC. 2012. Energetic food webs: an analysis of real and model ecosystem. Oxford, UK: Oxford University Press [Google Scholar]
- 10.Lafferty KD, Dobson AP, Kuris AM. 2006. Parasites dominate food web links . Proc. Natl Acad. Sci. USA 103, 11 211–11 216 (doi:10.1073/pnas.0604755103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafferty KD, et al. 2008. Parasites in food webs: the ultimate missing links . Ecol. Lett. 11, 533–546 (doi:10.1111/j.1461-0248.2008.01174.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafferty KD, Hechinger RF, Shaw JC, Whitney K, Kuris AM. 2006. Food webs and parasites in a salt marsh ecosystem. In Disease ecology: community structure and pathogen dynamics (eds Collinge SK, Ray C.), pp. 119–134 Oxford, UK: Oxford University Press [Google Scholar]
- 13.O'Neill RV. 1969. Indirect estimation of energy fluxes in animal food webs . J. Theor. Biol. 22, 284–290 (doi:10.1016/0022-5193(69)90006-X) [DOI] [PubMed] [Google Scholar]
- 14.Hunt H, et al. 1987. The detrital food web in a shortgrass prairie . Biol. Fert. Soils 3, 57–68 [Google Scholar]
- 15.De Ruiter PC, Neutel A, Moore JC. 1995. Energetics, patterns of interaction strengths, and stability in real ecosystems ability in real ecosystems. Science 269, 1257–1257 (doi:10.1126/science.269.5228.1257) [DOI] [PubMed] [Google Scholar]
- 16.McCann K, Yodzis P. 1994. Biological conditions for chaos in a three-species food chain . Ecology 75, 561–564 (doi:10.2307/1939558) [Google Scholar]
- 17.Emmerson MC, Raffaelli D. 2004. Predator–prey body size, interaction strength and the stability of a real food web . J. Anim. Ecol. 73, 399–409 (doi:10.1111/j.0021-8790.2004.00818.x) [Google Scholar]
- 18.Neutel AM, Heesterbeek JA, De Ruiter PC. 2002. Stability in real food webs: weak links in long loops . Science 296, 1120–1123 (doi:10.1126/science.1068326) [DOI] [PubMed] [Google Scholar]
- 19.Huxham M, Raffaelli D, Pike A. 1995. Parasites and food web patterns . J. Anim. Ecol. 64, 168–176 (doi:10.2307/5752) [Google Scholar]
- 20.Thompson RM, Mouritsen KN, Poulin R. 2004. Importance of parasites and their life cycle characteristics in determining the structure of a large marine food web . J. Anim. Ecol. 74, 77–85 (doi:10.1111/j.1365-2656.2004.00899.x) [Google Scholar]
- 21.Kuris AM, et al. 2008. Ecosystem energetic implications of parasite and free-living biomass in three estuaries . Nature 454, 515–518 (doi:10.1038/nature06970) [DOI] [PubMed] [Google Scholar]
- 22.Amundsen PA, Lafferty KD, Knudsen R, Primicerio R, Klemetsen A, Kuris AM. 2009. Food web topology and parasites in the pelagic zone of a subarctic lake . J. Anim. Ecol. 78, 563–572 (doi:10.1111/j.1365-2656.2008.01518.x) [DOI] [PubMed] [Google Scholar]
- 23.Arias-González JE, Morand S. 2006. Trophic functioning with parasites: a new insight for ecosystem analysis. Mar. Ecol. Prog. Ser. 320, 43–53 (doi:10.3354/meps320043) [Google Scholar]
- 24.Edeline E, Ari TB, Vøllestad LA, Winfield IJ, Fletcher JM, James JB, Stenseth NC. 2008. Antagonistic selection from predators and pathogens alters food-web structure . Proc. Natl Acad. Sci. USA 105, 19 792–19 796 (doi:10.1073/pnas.0808011105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byers JE. 2009. Including parasites in food webs. Trends Parasitol. 25, 55–57 (doi:10.1016/j.pt.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 26.Beckerman AP, Petchey OL. 2009. Infectious food webs . J. Anim. Ecol. 78, 493–496 (doi:10.1111/j.1365-2656.2009.01538.x) [DOI] [PubMed] [Google Scholar]
- 27.Getz WM. 2009. Disease and the dynamics of food webs . PLoS Biol. 7, e1000209 (doi:10.1371/journal.pbio.1000209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatcher MJ, Dick JTA, Dunn AM. 2012. Diverse effects of parasites in ecosystems: linking interdependent processes . Front. Ecol. Environ. 10, 186–194 (doi:10.1890/110016) [Google Scholar]
- 29.Anderson RM, May RM. 1979. Population biology of infectious diseases. I . Nature 280, 361–367 (doi:10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 30.Otto SB, Rall BC, Brose U. 2007. Allometric degree distributions facilitate food-web stability . Nature 450, 1226–1229 (doi:10.1038/nature06359) [DOI] [PubMed] [Google Scholar]
- 31.Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AM. 2007. Parasites alter community structure . Proc. Natl Acad. Sci. USA 104, 9335–9339 (doi:10.1073/pnas.0700062104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergersen R. 1996. Sticklebacks from Greenland . J. Fish Biol. 48, 799–801 (doi:10.1111/j.1095-8649.1996.tb01474.x) [Google Scholar]
- 33.Atkinson CT, Dusek RJ, Woods KL, Iko WM. 2000. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi . J. Wildl. Dis. 36, 197–204 (doi:10.7589/0090-3558-36.2.197) [DOI] [PubMed] [Google Scholar]
- 34.Koski KG, Scott ME. 2001. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral . Annu. Rev. Nutr. 21, 297–321 (doi:10.1146/annurev.nutr.21.1.297) [DOI] [PubMed] [Google Scholar]
- 35.Byers JE. 2000. Competition between two estuarine snails: implications for invasions of exotic species. Ecology 81, 1225–1239 (doi:10.1890/0012-9658(2000)081[1225:CBTESI]2.0.CO;2) [Google Scholar]
- 36.Miura O, Kuris AM, Torchin ME, Hechinger RF, Chiba S. 2006. Parasites alter host phenotype and may create a new ecological niche for snail hosts . Proc. R. Soc. B 273, 1323–1328 (doi:10.1098/rspb.2005.3451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernot RJ, Lamberti GA. 2008. Indirect effects of a parasite on a benthic community: an experiment with trematodes, snails and periphyton . Freshw. Biol. 53, 322–329 [Google Scholar]
- 38.Clay K. 2006. The effect of the fungus Atkinsonella hypoxylon (Clavicipitaceae) on the reproductive system and demography of the grass Danthonia spicata . New Phytol. 98, 165–175 (doi:10.1111/j.1469-8137.1984.tb06106.x) [DOI] [PubMed] [Google Scholar]
- 39.Lafferty KD, Morris AK. 1996. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts . Ecology 77, 1390–1397 (doi:10.2307/2265536) [Google Scholar]
- 40.Seppälä O, Karvonen A, Tellervo Valtonen E. 2004. Parasite-induced change in host behaviour and susceptibility to predation in an eye fluke–fish interaction . Anim. Behav. 68, 257–263 (doi:10.1016/j.anbehav.2003.10.021) [Google Scholar]
- 41.Kuris AM. 2005. Trophic transmission of parasites and host behavior modification . Behav. Proc. 68, 215–218 (doi:10.1016/j.beproc.2004.08.012) [DOI] [PubMed] [Google Scholar]
- 42.Thomas F, Adamo S, Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Proc. 68, 185–199 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 43.Isomursu M, Rätti O, Helle P, Hollmén T. 2008. Parasitized grouse are more vulnerable to predation as revealed by a dog-assisted hunting study. Ann. Zool. Fenn. 45, 496–502 (doi:10.5735/086.045.0604) [Google Scholar]
- 44.Vyas A, Kim SK, Sapolsky RM. 2007. The effects of Toxoplasma infection on rodent behavior are dependent on dose of the stimulus . Neuroscience 148, 342–348 (doi:10.1016/j.neuroscience.2007.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munson L, et al. 2008. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions . PLoS ONE 3, e2545 (doi:10.1371/journal.pone.0002545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams ES, Thorne ET, Appel M, Belitsky DW. 1988. Canine distemper in black-footed ferrets (Mustela nigripes) from Wyoming . J. Wildl. Dis. 24, 385–398 (doi:10.7589/0090-3558-24.3.385) [DOI] [PubMed] [Google Scholar]
- 47.Matchett MR, Biggins DE, Carlson V, Powell B, Rocke T. 2010. Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana . Vector Borne Zoonot. 10, 27–35 (doi:10.1089/vbz.2009.0053) [DOI] [PubMed] [Google Scholar]
- 48.Johnson PT, et al. 2010. When parasites become prey: ecological and epidemiological significance of eating parasites . Trends Ecol. Evol. 25, 362–371 (doi:10.1016/j.tree.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 49.Van Someren V. 1951. The red-billed oxpecker and its relation to stock in Kenya . East Afr. Agric. J. 17, 1–11 [Google Scholar]
- 50.Bezuidenhout J, Stutterheim C. 1980. A critical evaluation of the role played by the red-billed oxpecker Buphagus erythrorhynchus in the biological control of ticks . Onderstepoort J. Vet. Res. 47, 51–75 [PubMed] [Google Scholar]
- 51.Samish M, Ginsberg H, Glazer I. 2004. Biological control of ticks . Parasitology 129, S389–S403 (doi:10.1017/S0031182004005219) [DOI] [PubMed] [Google Scholar]
- 52.Watson DM. 2001. Mistletoe—a keystone resource in forests and woodlands worldwide . Annu. Rev. Ecol. Syst. 32, 219–249 (doi:10.1146/annurev.ecolsys.32.081501.114024) [Google Scholar]
- 53.Press MC, Phoenix GK. 2005. Impacts of parasitic plants on natural communities . New Phytol. 166, 737–751 (doi:10.1111/j.1469-8137.2005.01358.x) [DOI] [PubMed] [Google Scholar]
- 54.Kaplan AT, Rebhal S, Lafferty K, Kuris A. 2009. Small estuarine fishes feed on large trematode cercariae: lab and field investigations . J. Parasitol. 95, 477–480 (doi:10.1645/GE-1737.1) [DOI] [PubMed] [Google Scholar]
- 55.Peterken G, Mountford E. 1998. Long-term change in an unmanaged population of wych elm subjected to Dutch elm disease . J. Ecol. 86, 205–218 (doi:10.1046/j.1365-2745.1998.00255.x) [Google Scholar]
- 56.Sato T, et al. 2012. Nematomorph parasites indirectly alter the food web and ecosystem function of streams through behavioural manipulation of their cricket hosts . Ecol. Lett. 15, 786–793 (doi:10.1111/j.1461-0248.2012.01798.x) [DOI] [PubMed] [Google Scholar]
- 57.Hernandez AD, Sukhdeo MVK. 2008. Parasite effects on isopod feeding rates can alter the host's functional role in a natural stream ecosystem . Int. J. Parasitol. 38, 683–690 (doi:10.1016/j.ijpara.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 58.Suttle CA. 2007. Marine viruses—major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812 (doi:10.1038/nrmicro1750) [DOI] [PubMed] [Google Scholar]
- 59.Suttle CA, Chan AM, Cottrell MT. 1990. Infection of phytoplankton by viruses and reduction of primary productivity . Nature 347, 467–469 (doi:10.1038/347467a0) [Google Scholar]
- 60.Pierce SK, Maugel TK, Rumpho ME, Hanten JJ, Mondy WL. 1999. Annual viral expression in a sea slug population: life cycle control and symbiotic chloroplast maintenance . Biol. Bull. 197, 1–6 (doi:10.2307/1542990) [DOI] [PubMed] [Google Scholar]
- 61.Rohwer F, Thurber RV. 2009. Viruses manipulate the marine environment . Nature 459, 207–212 (doi:10.1038/nature08060) [DOI] [PubMed] [Google Scholar]
- 62.Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W. 2008. Homage to Linnaeus: How many parasites? How many hosts? Proc. Natl Acad. Sci. USA 105, 11 482–11 489 (doi:10.1073/pnas.0803232105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harvell CD, et al. 1999. Emerging marine diseases—climate links and anthropogenic factors . Science 285, 1505–1510 (doi:10.1126/science.285.5433.1505) [DOI] [PubMed] [Google Scholar]
- 64.Burge CA, Kim CJ, Lyles JM, Harvell CD. 2013. Special issue oceans and humans health: the ecology of marine opportunists . Microb. Ecol. 65, 869–879 (doi:10.1007/s00248-013-0190-7) [DOI] [PubMed] [Google Scholar]
- 65.Rushton S, Lurz P, Gurnell J, Fuller R. 2001. Modelling the spatial dynamics of parapoxvirus disease in red and grey squirrels: a possible cause of the decline in the red squirrel in the UK? J. Appl. Ecol. 37, 997–1012 (doi:10.1046/j.1365-2664.2000.00553.x) [Google Scholar]
- 66.Smith KF, Sax DF, Lafferty KD. 2006. Evidence for the role of infectious disease in species extinction and endangerment . Conserv. Biol. 20, 1349–1357 (doi:10.1111/j.1523-1739.2006.00524.x) [DOI] [PubMed] [Google Scholar]
- 67.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America . Proc. Natl Acad. Sci. USA 95, 9031–9036 (doi:10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health . Nature 484, 186–194 (doi:10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jolles AE, Ezenwa VO, Etienne RS, Turner WC, Olff H. 2008. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo . Ecology 89, 2239–2250 (doi:10.1890/07-0995.1) [DOI] [PubMed] [Google Scholar]
- 70.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. . Science 330, 243–246 (doi:10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maas M, Keet D, Rutten V, Heesterbeek J, Nielen M. 2012. Assessing the impact of feline immunodeficiency virus and bovine tuberculosis co-infection in African lions. Proc. R. Soc. B 279, 4206–4214 (doi:10.1098/rspb.2012.1503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatcher MJ, Dick JTA, Dunn AM. 2006. How parasites affect interactions between competitors and predators . Ecol. Lett. 9, 1253–1271 (doi:10.1111/j.1461-0248.2006.00964.x) [DOI] [PubMed] [Google Scholar]
- 73.Lafferty KD. 2008. Effects of disease on community interactions and food web structure. In Infectious disease ecology: the effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld RS, Keesing F, Eviner VT.), pp. 205–222 Princeton, NJ: Princeton University Press [Google Scholar]
- 74.Daskin JH, Alford RA. 2012. Context-dependent symbioses and their potential roles in wildlife diseases . Proc. R. Soc. B 279, 1457–1465 (doi:10.1098/rspb.2011.2276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carpenter R. 1990. Mass mortality of Diadema antillarum . Mar. Biol. 104, 67–77 (doi:10.1007/BF01313159) [Google Scholar]
- 76.Hughes TP. 1994. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551 (doi:10.1126/science.265.5178.1547) [DOI] [PubMed] [Google Scholar]
- 77.Getz WM. 2011. Biomass transformation webs provide a unified approach to consumer–resource modelling . Ecol. Lett. 14, 113–124 (doi:10.1111/j.1461-0248.2010.01566.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilmers CC, Post E, Peterson RO, Vucetich JA. 2006. Predator disease out-break modulates top-down, bottom-up and climatic effects on herbivore population dynamics . Ecol. Lett. 9, 383–389 (doi:10.1111/j.1461-0248.2006.00890.x) [DOI] [PubMed] [Google Scholar]
- 79.Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers . Oikos 69, 373–386 (doi:10.2307/3545850) [Google Scholar]
- 80.Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers . Ecology 78, 1946–1957 (doi:10.1890/0012-9658(1997)078[1946:PANEOO]2.0.CO;2) [Google Scholar]
- 81.Thomas F, Poulin R, de Meeüs T, Guégan J, Renaud F. 1999. Parasites and ecosystem engineering: what roles could they play? Oikos 84, 167–171 (doi:10.2307/3546879) [Google Scholar]
- 82.Hatcher MJ, Dunn AM. 2011. Parasites in ecological communities: from interactions to ecosystems. Cambridge, UK: Cambridge University Press [Google Scholar]
- 83.Marshall K, Mamone M, Barclay R. 2003. A survey of Douglas-fir dwarf mistletoe brooms used for nests by northern spotted owls on the Applegate Ranger District and Ashland Resource Area in southwest Oregon . West. J. Appl. For. 18, 115–117 [Google Scholar]
- 84.Hanula JL. 1996. Relationship of wood-feeding insects and coarse woody debris. In Biodiversity and coarse woody debris in Southern forests (eds McMinn JW, Crossley DA.), pp. 55–81 Asheville, NC: Southern Research Station, United States Department of Agriculture [Google Scholar]
- 85.Weste G, Marks G. 1987. The biology of Phytophthora cinnamomi in Australasian forests . Annu. Rev. Phytopathol. 25, 207–229 (doi:10.1146/annurev.py.25.090187.001231) [Google Scholar]
- 86.Dublin HT. 1991. Dynamics of the Serengeti-Mara woodlands: an historical perspective. For. Conser. Hist. 35, 169–178 [Google Scholar]
- 87.Tews J, Brose U, Grimm V, Tielbörger K, Wichmann M, Schwager M, Jeltsch F. 2004. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures . J. Biogeogr. 31, 79–92 (doi:10.1046/j.0305-0270.2003.00994.x) [Google Scholar]
- 88.Holdo RM, Sinclair AR, Dobson AP, Metzger KL, Bolker BM, Ritchie ME, Holt RD. 2009. A disease-mediated trophic cascade in the Serengeti and its implications for ecosystem C . PLoS Biol. 7, e1000210 (doi:10.1371/journal.pbio.1000210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dobson A, Crawley M. 1994. Pathogens and the structure of plant communities . Trends Ecol. Evol. 9, 393–398 (doi:10.1016/0169-5347(94)90062-0) [DOI] [PubMed] [Google Scholar]
- 90.Thomas F, Poulin R, Renaud F. 1998. Nonmanipulative parasites in manipulated hosts: 'hitch-hikers’ or simply 'lucky passengers’? J. Parasitol. 84, 1059–1061 (doi:10.2307/3284648) [PubMed] [Google Scholar]
- 91.Mouritsen KN, Poulin R. 2010. Parasitism as a determinant of community structure on intertidal flats . Mar. Biol. 157, 201–213 (doi:10.1007/s00227-009-1310-2) [Google Scholar]
- 92.Clement J, Vercauteren J, Verstraeten WW, Ducoffre G, Barrios JM, Vandamme AM, Maes P, Van Ranst M. 2009. Relating increasing hantavirus incidences to the changing climate: the mast connection. Int. J. Health Geogr. 8, 1 (doi:10.1186/1476-072X-8-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jonsson CB, Figueiredo LTM, Vapalahti O. 2010. A global perspective on hantavirus ecology, epidemiology, and disease . Clin. Microbiol. Rev. 23, 412–441 (doi:10.1128/CMR.00062-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samia NI, Kausrud KL, Heesterbeek H, Ageyev V, Begon M, Chan KS, Stenseth NC. 2011. Dynamics of the plague–wildlife–human system in Central Asia are controlled by two epidemiological thresholds . Proc. Natl Acad. Sci. USA 108, 14 527–14 532 (doi:10.1073/pnas.1015946108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davis S, Trapman P, Leirs H, Begon M, Heesterbeek J. 2008. The abundance threshold for plague as a critical percolation phenomenon . Nature 454, 634–637 (doi:10.1038/nature07053) [DOI] [PubMed] [Google Scholar]
- 96.Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Cáceres CE. 2008. Quality matters: resource quality for hosts and the timing of epidemics . Ecol. Lett. 12, 118–128 (doi:10.1111/j.1461-0248.2008.01264.x) [DOI] [PubMed] [Google Scholar]
- 97.Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR. 2012. Ecological context influences epidemic size and parasite-driven evolution . Science 335, 1636–1638 (doi:10.1126/science.1215429) [DOI] [PubMed] [Google Scholar]
- 98.Johnson PT, Thieltges DW. 2010. Diversity, decoys and the dilution effect: how ecological communities affect disease risk . J. Exp. Biol. 213, 961–970 (doi:10.1242/jeb.037721) [DOI] [PubMed] [Google Scholar]
- 99.Eisen RJ, et al. 2012. Flea diversity as an element for persistence of plague bacteria in an East African plague focus . PLoS ONE 7, e35598 (doi:10.1371/journal.pone.0035598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior . PLoS Biol. 4, e82 (doi:10.1371/journal.pbio.0040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leung TL, Poulin R. 2008. Size-dependent pattern of metacercariae accumulation in Macomona liliana: the threshold for infection in a dead-end host . Parasitol. Res. 104, 177–180 (doi:10.1007/s00436-008-1166-2) [DOI] [PubMed] [Google Scholar]
- 102.Gibson AK, Mena-Ali JI, Hood ME. 2010. Loss of pathogens in threatened plant species . Oikos 119, 1919–1928 (doi:10.1111/j.1600-0706.2010.18616.x) [Google Scholar]
- 103.Dizney LJ, Ruedas LA. 2009. Increased host species diversity and decreased prevalence of Sin Nombre virus . Emerg. Infect. Dis. 15, 1012–1018 (doi:10.3201/eid1507.081083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keesing F, Brunner J, Duerr S, Killilea M, Logiudice K, Schmidt K, Vuong H, Ostfeld RS. 2009. Hosts as ecological traps for the vector of Lyme disease . Proc. R. Soc. B 276, 3911–3919 (doi:10.1098/rspb.2009.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Randolph SE, Dobson AD. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm . Parasitology 139, 847–863 (doi:10.1017/S0031182012000200) [DOI] [PubMed] [Google Scholar]
- 106.Ostfeld RS, Keesing F, Eviner VT. 2008. Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton, NJ: Princeton University Press [Google Scholar]
- 107.Swaddle JP, Calos SE. 2008. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect . PLoS ONE 3, e2488 (doi:10.1371/journal.pone.0002488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT. 2009. Epidemic dynamics at the human–animal interface. Science 326, 1362–1367 (doi:10.1126/science.1177345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS. 2009. The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. R. Soc. B 276, 3037–3045 (doi:10.1098/rspb.2009.0413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hutchings MR, Judge J, Gordon IJ, Athanasiadou S, Kyriazakis I. 2006. Use of trade-off theory to advance understanding of herbivore–parasite interactions . Mamm. Rev. 36, 1–16 (doi:10.1111/j.1365-2907.2006.00080.x) [Google Scholar]
- 111.Loehle C. 1995. Social barriers to pathogen transmission in wild animal populations . Ecology 76, 326–335 (doi:10.2307/1941192) [Google Scholar]
- 112.Beckers OM, Wagner WE. 2012. Divergent preferences for song structure between a field cricket and its phonotactic parasitoid. J. Insect Behav. 25, 1–11 (doi:10.1007/s10905-011-9312-6) [Google Scholar]
- 113.Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M. 2008. Poor condition and infection: a vicious circle in natural populations . Proc. R. Soc. B 275, 1753–1759 (doi:10.1098/rspb.2008.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Craft M, Hawthorne P, Packer C, Dobson A. 2008. Dynamics of a multihost pathogen in a carnivore community . J. Anim. Ecol. 77, 1257–1264 (doi:10.1111/j.1365-2656.2008.01410.x) [DOI] [PubMed] [Google Scholar]
- 115.Collinge SK, et al. 2005. Testing the generality of a trophic-cascade model for plague. EcoHealth 2, 102–112 (doi:10.1007/s10393-005-3877-5) [Google Scholar]
- 116.Duffy MA, Housley JM, Penczykowski RM, Cáceres CE, Hall SR. 2011. Unhealthy herds: indirect effects of predators enhance two drivers of disease spread . Funct. Ecol. 25, 945–953 (doi:10.1111/j.1365-2435.2011.01872.x) [Google Scholar]
- 117.Diekmann O, Heesterbeek H, Britton T. 2012. Mathematical tools for understanding infectious disease dynamics. Princeton, NJ: Princeton University Press [Google Scholar]
- 118.Dunne JA, et al. 2013. Parasites affect food web structure primarily through increased diversity and complexity . PLoS Biol. 11, e1001579 (doi:10.1371/journal.pbio.1001579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roberts M, Heesterbeek JAP. 2012. Characterizing the next-generation matrix and basic reproduction number in ecological epidemiology . J. Math. Biol. 66, 1–20 [DOI] [PubMed] [Google Scholar]
- 120.Venturino E. 1994. The influence of diseases on Lotka–Volterra systems . Rocky Mt. J. Math. 24, 381–402 (doi:10.1216/rmjm/1181072471) [Google Scholar]
- 121.Hadeler K, Freedman H. 1989. Predator–prey populations with parasitic infection . J. Math. Biol. 27, 609–631 (doi:10.1007/BF00276947) [DOI] [PubMed] [Google Scholar]
- 122.Beltrami E, Carroll T. 1994. Modeling the role of viral disease in recurrent phytoplankton blooms . J. Math. Biol. 32, 857–863 (doi:10.1007/BF00168802) [Google Scholar]
- 123.Oliveira NM, Hilker FM. 2010. Modelling disease introduction as biological control of invasive predators to preserve endangered prey . Bull. Math. Biol. 72, 444–468 (doi:10.1007/s11538-009-9454-2) [DOI] [PubMed] [Google Scholar]
- 124.Malchow H, Petrovskii SV, Venturino E. 2008. spatiotemporal patterns in ecology and epidemiology: theory, models, and simulation. London, UK: Chapman and Hall/CRC Press [Google Scholar]