Abstract
The trajectory of an animal's growth in early development has been shown to have long-term effects on a range of life-history traits. Although it is known that individual differences in behaviour may also be related to certain life-history traits, the linkage between early growth or development and individual variation in behaviour has received little attention. We used brief temperature manipulations, independent of food availability, to stimulate compensatory growth in juvenile three-spined sticklebacks Gasterosteus aculeatus. Here, we examine how these manipulated growth trajectories affected the sexual responsiveness of the male fish at the time of sexual maturation, explore associations between reproductive behaviour and investment and lifespan and test whether the perceived time stress (until the onset of the breeding season) influenced such trade-offs. We found a negative impact of growth rate on sexual responsiveness: fish induced (by temperature manipulation) to grow slowest prior to the breeding season were consistently quickest to respond to the presence of a gravid female. This speed of sexual responsiveness was also positively correlated with the rate of development of sexual ornaments and time taken to build a nest. However, after controlling for effects of growth rate, those males that had the greatest sexual responsiveness to females had the shortest lifespan. Moreover, the time available to compensate in size before the onset of the breeding season (time stress) affected the magnitude of these effects. Our results demonstrate that developmental perturbations in early life can influence mating behaviour, with long-term effects on longevity.
Keywords: compensatory growth, longevity, trade-off, phenotypic plasticity, sexual behaviour, three-spined stickleback
1. Introduction
Environmental conditions contribute to the shaping of the adult phenotype [1], and the individual phenotypic differences that arise as a response to early developmental conditions, often most obviously reflected in terms of adult size or morphology, can directly affect life-history fitness. For instance, individuals that have experienced early environmental perturbations to their growth (e.g. through fluctuations in food availability, population density or temperature in the case of ectotherms) can rapidly compensate in size when conditions improve (so-called compensatory growth; [2]). Compensatory growth is assumed to be an adaptation to environmental perturbation early in life [3]. However, while growth compensation can have not only beneficial effects on some life-history traits (e.g. future reproduction, escape from predation), but it can also have adverse effects on other traits, for example longevity [4–6]. Compensatory growth can also have consistent, long-term effects on behaviour through altering energy allocation to growth at the expense of activity [7], swimming endurance [8] or aggression in adulthood, which may in turn affect an individual's later reproductive performance [9,10].
It has recently been suggested that the trade-off between life-history strategies at the origin of within-population variation along a fast–slow continuum may have come along with a whole suite of co-adapted behaviour and physiological traits [11–13]. At one end of the spectrum, fast individuals/genotypes grow quickly, and mature, breed and die early, whereas, at the other end, slow individuals experience slower growth, delay their reproduction over a longer period and enjoy a longer life. Although such an integration of behaviour traits with life-history strategies is possible at the genetic level, an organism's behavioural and life-history decisions may also respond in a plastic way to early environmental constraints [12]. The extent to which adult behaviour and life-history traits are influenced by developmental trajectories has implications for our understanding of the trade-off between short- and long-term consequences of responses to adverse environmental conditions. Yet, we know little about the fitness consequences of growth responses to early environmental conditions.
It is known that rapid growth (e.g. during a phase of growth compensation) may be dependent on elevated rates of feeding activity and consumption [7]. However, rapid growth may lead to greater levels of oxidative damage to tissues, either because of higher rates of metabolism leading to a greater generation of reactive oxygen species or because resources have been diverted away from tissue repair in order to promote new growth [6]. As a consequence, growth rate can negatively affect important performance traits such as locomotion (e.g. swimming endurance in fishes; [8]). What is less documented is whether aspects of behaviour that affect reproductive success are influenced by the growth trajectory. Any effects of growth perturbations on individual differences in behaviour may also be influenced by the perceived time available for growth compensation to occur (so-called time stress; [14]). The time stress is greater when less time is available for growth compensation prior to a key event in life (e.g. breeding season, migration) when body size is a key determinant of fitness. For example, long winters involving prolonged low temperatures may constrain the growth of ectothermic individuals over a longer period when compared with short winters and thus force compensatory growth to occur over a shorter time prior to the breeding season. Therefore, the degree of time stress may have a negative impact on the relationship between compensatory growth and behaviour.
We have shown elsewhere (results summarized in the electronic supplementary material, table S1) that compensatory growth trajectories manipulated by a brief temperature treatment (cold or warm spell; see Materials and methods for more details) influence swimming endurance, reproductive investment (sexual ornamentation, speed of building nests, first clutch-size and egg size) and lifespan of three-spined sticklebacks Gasterosteus aculeatus. These previous results showed that catch-up growth (induced by a brief cold spell, resulting in subsequent growth acceleration) had negative effects in comparison with steadily growing control fish, whereas slowdown growth (induced by a brief warm spell that prompted subsequent growth deceleration) led to improved performance relative to the control [6,8,15]. In addition, the negative effects of catch-up growth were found to be reduced if fish had more time in which to compensate prior to the breeding season by a different season of experiment and different shifts of the annual photoperiod cycle [6,8,15].
In this paper, we further analyse data from these previous experimental fish to focus on the effect of their early growth trajectory on male sexual responsiveness, a trait not previously addressed. This trait is particularly relevant because it can provide further insights in how early-life development can have long-term ecological and potentially evolutionary consequences. We examine for the first time, to our knowledge: (i) how the growth trajectory early in life affected mating behaviour and the onset of sexual maturity, (ii) the consequences of both compensatory growth trajectory and reproductive effort associated with mating behaviour on lifespan, and (iii) whether such effects are influenced by time stress. Furthermore, using an appropriate statistical approach [16], we tested whether the relationships between compensatory growth and fitness traits are similar at the between- and within-treatment levels. Our hypothesis was that differences in early environmental conditions may have long-term effects on reproductive behaviour and that these effects would be influenced by the degree of time stress, being the more pronounced the shorter the time available for size recovery prior to a key life-history event (in this case, the breeding season). By using a short-lived ectotherm, we were able to alter growth trajectories through temperature manipulations without altering nutritional condition and could determine the long-term effects, including the effect on longevity.
2. Material and methods
(a). Fish and rearing condition
We captured wild juvenile three-spined sticklebacks with a dip net and minnow traps in the River Endrick, UK (56°04′ N, 4°23′ W) on 1 November 2007 and 29 January 2008 for use in two independent experiments: we ran the same experiment with either a long (=winter experiment) or short (=spring experiment) period between the time of the growth manipulation and the onset of the breeding season in order to examine the effect on reproduction of time available for compensatory growth prior to the breeding season. All fish were transferred to 80 l acclimatization aquaria (at a density of two fish × l–1) for three weeks prior to the start of each experiment and fed ad libitum with frozen chironomid larvae. The temperature was initially maintained at 9.7 ± 0.1°C prior to the start of experiments, whereas the photoperiod was initially ambient. On 21 November 2007 and 21 February 2008 for the winter and spring experiment, respectively, we anaesthetized and measured 120 fish for standard length (±0.01 mm) and wet mass (±0.001 g) for each experiment. We then sorted the fish into groups of five individuals of differing size (to aid identification; regular measurements throughout the experiment confirmed that size ranks never changed within a tank), with each group of five in a separate small tank with aeration, a filter and artificial plants (335 × 170 × 185 mm). Each week we changed 1.75 l (25% of the total volume of water) and also added 62.5 ml of seawater per tank in order to prevent the risk of whitespot infection (Ichthyophthirius multifiliis).
(b). Temperature and time-stress manipulation
In both the winter and spring experiments, we used three temperature manipulations (i.e. high (14°C), low (6°C) and intermediate temperatures (10°C)) for a period of four weeks (period 1), following which all fish experienced the same temperature regime. This was initially 10°C (period 2) but was increased to 14°C at the onset of each breeding season to allow the fish to breed, before being returned to 10°C during non-breeding seasons; full details of the experimental regimes are given in references [8,15] and in the electronic supplementary material, figure S1.
In order to test how the amount of time available for growth compensation prior to breeding influenced sexual responsiveness, we replicated the above three groups under two different photoperiod regimes: a natural photoperiod regime (=ambient photoperiod treatment, AP) or a delayed photoperiod (=a day length corresponding to a point 35 days earlier in the year, DP). We used fluorescent lights controlled by electronic timers to produce simulated daylight for all fish, with blackout plastic sheeting around the tanks being used to achieve independent lighting regimes. The same seasonal rate of progression of the photoperiod for both the AP and DP groups after the initial adjustment of photoperiods was followed, so that the DP group was continually at a state 35 days earlier in the season (giving the DP group in both the winter and spring experiments longer to recover prior to the breeding season from the growth perturbation caused by the temperature manipulation). Overall, there were six manipulation groups in each experiment (three temperature × two photoperiod treatments, each with four replicate tanks). While the experiments involved a total initial sample size of 240 juvenile fish, only 85 developed as sexually mature male fish for which we recorded details of breeding behaviour (winter—8, 15 and 19 individuals; spring—13, 15 and 15 individuals for low-, high- and steady- (as control group) temperature treatment, respectively). Note that originally we had 90 males but five individuals that died during the period of behavioural observations are not included in the statistical analyses. Only males are considered because it is much more problematic to measure the degree and rate of development of sexual responsiveness in female sticklebacks [17]. Details of the effects of the treatments on the swimming and reproductive performance and lifespan of all of the fish in these experiments are given in [6,8,15].
(c). Compensatory growth rate and survival
Length and mass of all fish were measured every two weeks during the temperature manipulations and every three weeks thereafter; all fish were starved for 24 h prior to measuring to prevent variation in the weight of stomach contents. In order to record lifespan, we monitored each tank daily and noted when each fish died.
We calculated compensatory growth rate after the temperature manipulation as: compensatory growth rate = 100 × [ln(Lc × Li−1)] × t−1, where Li is the initial length at the end of period 1 and Lc is the standard length when fish in the different manipulation groups had converged on the same mean size prior to breeding (based on inspection of growth trajectories—i.e. when there was no statistical difference in standard length between manipulation groups—see [15] and the electronic supplementary material, text S2). t is the interval in days between Lc and Li, being 105 and 84 days in the winter and spring experiment, respectively.
The initial four-week temperature manipulations caused compensatory growth responses, with the 6°C group subsequently showing growth acceleration to make up their size deficit (hereafter termed the ‘catch-up’ treatment group), whereas the 14°C group showed growth deceleration (hereafter termed the ‘slowdown’ group) relative to the 10°C (control) fish; as a result all groups reached the same average size prior to the breeding season (full details of the growth responses are given in [8,15] and in the electronic supplementary material, text S2 and figure S1).
(d). Assessment of sexual responsiveness
In order to assay male sexual responsiveness, on 16 May 2008 and 3 July 2008 (for the winter and spring experiment, respectively), we assigned males that had started to develop the typical sexual ornamentation (blue eye coloration and reddish throats; [18]) to individual tanks of the same size as those in which they had previously been held. Each tank was given an artificial plant and a Petri dish containing fine sand (i.e. a nesting dish) and nest material (50 × 5 cm lengths of thread; see the electronic supplementary material, figure S2 for details of tank design). The walls of these tanks were covered by white opaque paper to prevent fish from seeing their neighbours or the experimenter.
In order to quantify the courtship behaviour of the males, twice a day, we presented each male with a female enclosed in an airtight Plexiglas container (for preventing an odour cue, see [19]), placed inside the male's tank. Each female was only put once in five different male tanks on a given day in order to prevent them from becoming stressed by confinement or becoming habituated to the presence of males. The standard length of females used in total (n = 30, mean ± s.e. = 51.6 ± 0.3 mm; electronic supplementary material, table S2) was similar between temperature treatments (repeated measures ANOVA, effect of treatment, F2,79 = 0.258, p = 0.773), as male sticklebacks prefer larger or more distended females [20]. Prior to placing the female's container in the front half of the male's tank, we confined the male at the rear of the tank (next to the artificial plant) using a piece of black opaque plastic that temporarily formed a lateral divider across the tank. This was removed (allowing the male to approach the female) 1 min after the female's container had been added to the male's tank. The distinct phases of the courtship behaviour of the male three-spined stickleback (i.e. zigzags, biting, creeping, gluing and fanning) are well documented [21–23].
Once we took out the black opaque plastic divider, we measured a male's interest in mating (and hence sexual responsiveness) as the time taken by a male to respond to a female, defined as: (i) until the male first touched (kissed) the wall of the female's container, or (ii) until he passed the middle line (see the electronic supplementary material, figure S1) of the tank and first began nest-building, fanning or courtship behaviours without kissing the wall. Note that we defined the male's sexual responsiveness as whichever of these two times was the shortest. However, on rare occasions (n = 7, 0.3% across all trials), the male then immediately (within 5 s) retreated beyond the midline of the tank towards the artificial plant and remained there for over 30 s. We interpreted this initial erratic movement as a mild fright response to the movement of the plastic divider, and so excluded it, instead defining his responsiveness as the time from the start until he next came and either touched the female's container or began nest-building, fanning or courtship. If the male never came towards the female, then he was given the maximum score of 5 min. After 5 min, the female and her container were removed from the male's tank. The response of each male to a gravid female was tested in this way twice daily, repeated for 30 days, at the start of both the first and second breeding seasons. Males were returned to their original group tank at the end of the breeding season (once they had lost their sexual ornamentation; [15]).
To quantify the sexual responsiveness of each male, we first calculated his daily residual response time, which was defined as the difference between his response time to the female for a given day and the mean response time for all n recorded males that day (the population mean). The population mean (i.e. all individuals from both winter and spring experiments combined) for the jth day was computed as 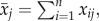 where xij is the response time of individual i that day. We used the sum of an individual male's daily residuals from the population mean over the 30-day study period as a metric of his sexual responsiveness, i.e.
where xij is the response time of individual i that day. We used the sum of an individual male's daily residuals from the population mean over the 30-day study period as a metric of his sexual responsiveness, i.e. 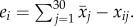 We describe a male's sexual responsiveness to the female as being either high or low when the sum of the daily residuals was positive (greater than 0) or negative (less than 0), respectively.
We describe a male's sexual responsiveness to the female as being either high or low when the sum of the daily residuals was positive (greater than 0) or negative (less than 0), respectively.
(e). Nest-building and secondary sexual traits
In order to measure a male's nest-building behaviour, the status of his nest was checked daily for the first week after placing males in individual tanks, and a record made of the day on which the nest was completed (i.e. threads gathered and glued together). While we have previously shown a negative effect of catch-up growth on the speed of nest-building elsewhere [15], this measurement of building behaviour is included in this study, because nest-building could be considered as part of a male's behavioural investment in reproduction, and so here we test how this behaviour is associated with a male's sexual responsiveness.
Every week for 16 weeks during the breeding season, we recorded the red throat of the males by placing them in a small tank (170 × 70 × 105 mm with 50 ml water) and photographed them using a standardized photography protocol described by Frischknecht [24]. Note that in our earlier studies where we were interested in the effect of growth trajectory on reproductive investment and senescence (summarized in [15] and the electronic supplementary material, text S1) we quantified total reproductive investment of the males over the full breeding season as the number of weeks that they exceeded the mean redness score for the population. Here, we are concerned only with how earlier growth trajectories affect the strength of a male's sexual responsiveness towards a potential mate. As this behaviour was assessed by placing a gravid female in each male's tank—which also had the effect of stimulating him to produce the red throat ornamentation—it makes sense in this case to relate this sexual responsiveness to the rate of development of the red throat rather than the duration of the season that he remained red (which is more related to senescence than interest in mating). The rate of development of the red throat was measured as follows: for each male, we performed regression analysis of the intensity score for his red throat against date over the 30 days of observation and used the slope of that regression as an index of the rate of development of sexual ornamentation.
(f). Statistical analysis
Any associations between early growth trajectories (catch-up or slowdown), male mating behaviour and life-history fitness could be caused by an effect of treatment (i.e. a between-treatment group effect) or an effect owing to variation in individuals within a treatment group (i.e. a within-treatment group effect). To distinguish within- from between-group effects, we used the technique of ‘within-group centring’ [16]. A first variable that expresses only the between-group variance component was given by the mean value of the trait in question, calculated over all males in the same growth treatment group. A second variable that expresses only the within-group variance component was computed by subtracting this treatment mean value from the individual values for all males in a given growth treatment group.
A linear mixed-effect model (LME) based on within-group centring was used to analyse the factors influencing sexual responsiveness. The full model included experiment (winter or spring) and photoperiod treatment (AP or DP) as fixed effects and compensatory growth rate and manipulated fish length (at the end of the temperature manipulation) as covariates; this compensatory growth rate was divided into two variables: the mean for each growth treatment (as a between-group effect) and the within-group variation about those treatment means (as the within-group effect). We also included the identity of the tank in which a male was held before the start of the breeding season as a random effect, plus their two-way interactions.
Separate LME models subsequently explored the factors influencing (i) the rate of change in male red throat coloration, (ii) the rate of nest-building, and (iii) lifespan. Because fish were captured in the wild, we assigned to all individuals the same nominal birth date (1 June 2007) for the purpose of statistical analysis. In these analyses, the full models contained experiment and photoperiod treatment as fixed effects, and compensatory growth rate (partitioned into within- and between-growth treatment effects as above) and sexual responsiveness (similarly partitioned into within- and between-growth treatment effects) as covariates.
For all models, we started with a full model and sequentially dropped non-significant variables, so that the final models included only significant terms (or terms that were components of significant interactions). All means are presented with standard deviation, and all analyses were performed with the software R version 2.15.0 [25] and the package lme4 [26]. All experiments were performed under licence from the UK Home Office (PIL 60/11377).
3. Results
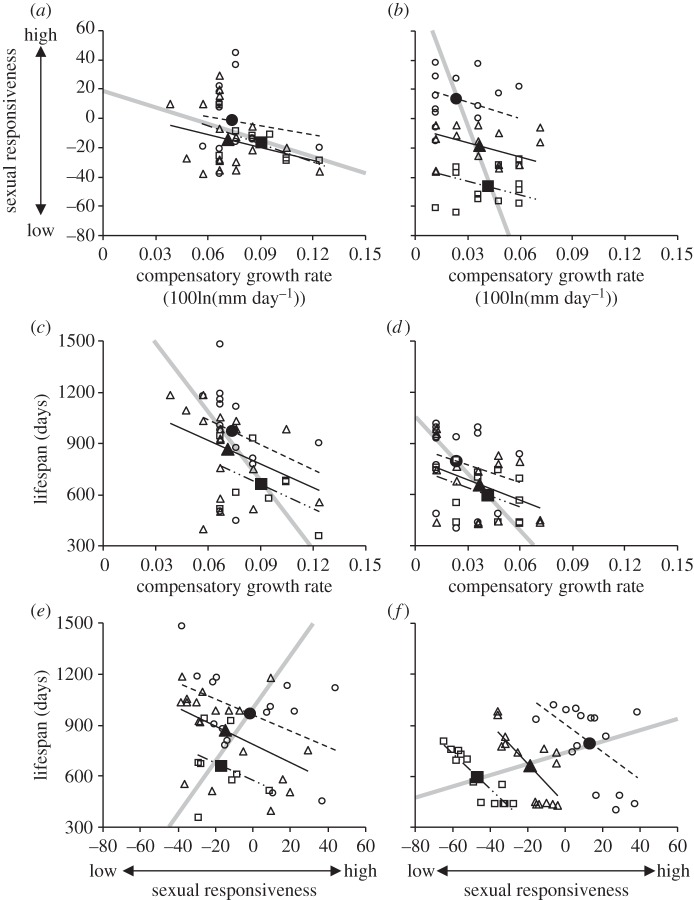
There were significant differences in the level of male sexual responsiveness between the winter and spring experiment (table 1): males that had undergone the growth manipulation in the winter were more attracted to gravid females than were those males that had undergone growth manipulation in the spring. While the photoperiod manipulation (LME, F1,80 = 0.11, p = 0.737), the within-treatment variation in compensatory growth rate (F1,80 = 0.51, p = 0.476), and manipulated fish length (F1,80 = 0.10, p = 0.750) had no effect on male sexual responsiveness, it was significantly affected by the variation between growth treatments (table 1): the slowdown males were quicker to respond to females than the catch-up males (figure 1a,b). There was a significant interaction between experiment and the between-treatment effect of compensatory growth (table 1): the effect of compensatory growth rate on sexual responsiveness was much greater in the spring than the winter experiment (figure 1a,b).
Table 1.
Male sexual responsiveness of three-spined sticklebacks in relation to growth treatment (catch-up, control or slowdown). (The results are shown of a linear mixed-effect (LME) analysis based on within-group centring to assess the relative importance of within-treatment and between-treatment effects of growth rate. Variation in compensatory growth rate was thus partitioned into that owing to treatment (‘between-group effect of growth’) and the residual within-treatment variation owing to the individual. The full model included experiment (winter or spring) and photoperiod (AP or DP) as fixed effects, compensatory growth rate (partitioned into within- and between-growth treatment effects) as covariates, plus their two-way interactions, and original rearing tank as a random effect. Non-significant variables were dropped from the final model, which is shown here. The parentheses represent the reference coding of the categorical variable.)
| final model | estimate ± s.e. | F | d.f. | p |
|---|---|---|---|---|
| intercept | 4.077 ± 0.512 | |||
| experiment (winter) | −3.198 ± 1.317 | 5.90 | 1, 81.0 | 0.017 |
| between-group effect of growth | −123.907 ± 14.778 | 38.12 | 1, 81.0 | <0.001 |
| experiment (winter) × between-group effect of growth | 113.764 ± 21.711 | 27.46 | 1, 81.0 | <0.001 |
Figure 1.
(a,b) Sexual responsiveness by male three-spined sticklebacks in relation to their earlier rate of compensatory growth. Positive values for sexual responsiveness indicate males that were consistently faster to approach a gravid female, while negative values indicate slow males. Lifespan of males in relation to (c,d) their earlier rate of compensatory growth and (e,f) their sexual responsiveness. Individual data points and within-treatment regression lines are plotted for males from the (a,c,e) winter and (b,d,f) spring experiments, categorized by growth treatment (slowdown: circle and dashed line, catch-up: square and double dashed line, control: triangle and solid line). Larger symbols and grey thicker lines denote treatment mean values and between-treatment regression lines from the estimates of the coefficients of the final models (see [16] for statistical explanation, and tables 1 and 2 for full statistical analysis).
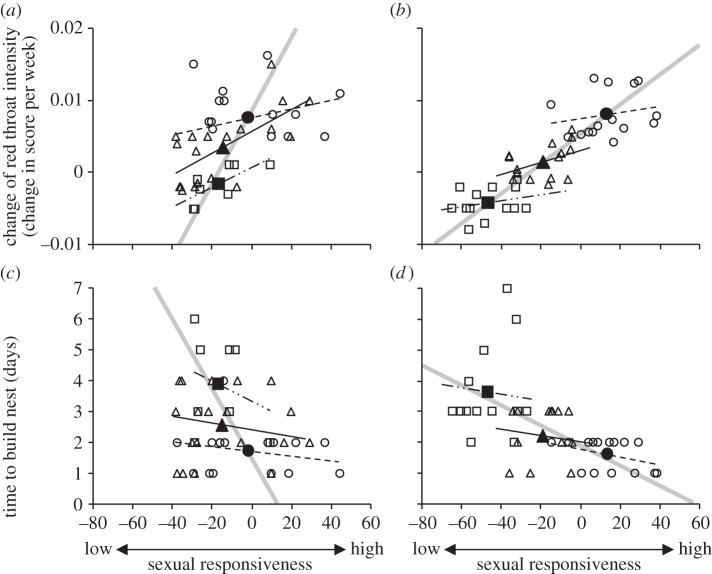
All males began developing a red throat coloration at the onset of the breeding season, but the temporal rate of change in red throat intensity (R × week−1) over the first 30 days of the season was affected by the experimental regime (table 2): the development of the red throat was faster in the winter fish (change in score of 0.005 ± 0.001 R × week−1) than in the spring fish (0.001 ± 0.001 R × week−1). The rate of change in throat intensity was negatively affected by compensatory growth rate, at both between-group and within-group levels (table 2): the greater the rate of compensatory growth in the period leading up to the breeding season, the slower the development of the red throat. The rate of change in throat coloration was also related to sexual responsiveness, at both between-group and within-group levels (table 2): males that showed the greatest sexual responsiveness also showed the fastest rate of development of their red throats (figure 2a,b). In addition, there was a significant interaction between experiment and the between-growth treatment effect (table 2): the effect of growth treatment on the development of the red throat was greater in the spring than in the winter experiment (figure 2a,b). However, the change in red throat intensity was independent of photoperiod (F1,65 = 0.01, p = 0.93).
Table 2.
Red throat intensity, time taken by males to build a nest and lifespan of three-spined sticklebacks in related to growth treatment (catch-up, control or slowdown) and male sexual responsiveness. (The full linear mixed-effect (LME) models included experiment (winter or spring) and photoperiod (AP or DP) as fixed effects, covariates of compensatory growth rate and sexual responsiveness (both of which were partitioned into within- and between-growth treatment effects; see table 1), plus their two-way interactions, and original rearing tank as a random effect. Non-significant variables were dropped from the final model. The parentheses represent the reference coding of the categorical variable.)
| analysis | final model | estimate ± s.e. | F | d.f. | p |
|---|---|---|---|---|---|
| Red throat intensity | intercept | 0.012 ± 0.002 | |||
| experiment (winter) | −0.013 ± 0.004 | 11.51 | 1, 70.8 | 0.001 | |
| between-group effect of growth | −0.220 ± 0.081 | 7.43 | 1, 73.4 | 0.008 | |
| within-group effect of growth | −0.046 ± 0.023 | 3.98 | 1, 77.9 | 0.049 | |
| between-group effect of sexual responsiveness | 0.003 ± 0.001 | 23.81 | 1, 75.4 | <0.001 | |
| within-group effect of sexual responsiveness | 0.001 ± 0.001 | 15.44 | 1, 76.9 | <0.001 | |
| experiment (winter) × between-group effect of sexual responsiveness | 0.001 ± 0.001 | 6.31 | 1, 6.3 | 0.015 | |
| nest-building effort | intercept | 6.407 ± 1.973 | |||
| experiment (winter) | −9.178 ± 2.457 | 13.96 | 1, 79.0 | <0.001 | |
| between-group effect of growth | 156.069 ± 67.344 | 1.66 | 1, 79.0 | 0.201 | |
| within-group effect of growth | 16.920 ± 6.295 | 7.23 | 1, 79.0 | 0.009 | |
| between-group effect of sexual responsiveness | −0.081 ± 0.020 | 15.55 | 1, 79.0 | <0.001 | |
| experiment × between-group effect of growth | −214.675 ± 66.081 | 10.55 | 1, 79.0 | 0.002 | |
| lifespan | intercept | 0.975 ± 0.448 | |||
| experiment (winter) | −2.709 ± 0.696 | 15.13 | 1, 78.0 | <0.001 | |
| between-group effect of growth | −38.421 ± 14.937 | 6.62 | 1, 78.0 | 0.012 | |
| within-group effect of growth | −18.978 ± 4.214 | 20.28 | 1, 78.0 | <0.001 | |
| between-group effect of sexual responsiveness | 0.001 ± 0.006 | 4.78 | 1, 78.0 | 0.032 | |
| within-group effect of sexual responsiveness | −0.026 ± 0.004 | 38.54 | 1, 78.0 | <0.001 | |
| experiment (winter) × between-group effect of sexual responsiveness | −0.038 ± 0.016 | 5.42 | 1, 78.0 | 0.023 |
Figure 2.
Rate of development of red throat ornamentation (a,b) and time taken by males to build a nest (c,d) of male three-spined sticklebacks in relation to their earlier rate of compensatory growth and their sexual responsiveness. Data are plotted separately for the (a,c) winter and (b,d) spring experiments. Within-treatment regression lines are shown by thin dashed lines and are categorized by growth treatment (slowdown: circle and dashed line, catch-up: square and double dashed line, control: triangle and solid line). Larger symbols and grey thicker lines denote treatment mean values and between-treatment regression lines from the estimates of the coefficients of the final models (see [16] for statistical explanation, and table 2 for full statistical analysis).
All males completed their nests within 7 days (mean ± s.d. = 2.47 ± 1.31 days). Males were faster at completing their nests in the spring than the in winter experiment (table 2), but there was no difference between photoperiod treatment groups (F1,78 = 1.34, p = 0.251). While this result is the same as that presented previously [15], the more sophisticated analysis presented here was able to separate within- from between-treatment group effects. There was no between-treatment group effect of compensatory growth rate on the rate of nest-building, but a negative effect within-treatment groups (table 2): individual males with the fastest rate of compensatory growth within a treatment group subsequently took longer to build their nests. The time taken to build a nest was also related to the between-group variation in sexual responsiveness (table 2): the greater the responsiveness to the female, the shorter the time taken to build the nest (figure 2c,d). A male's building behaviour was also affected by the interaction between experiment and this between-group effect (table 2), with a stronger between-group effect under the spring experiment (figure 2c,d). However, there was no within-group effect of variation in sexual responsiveness on nest-building time (F1,78 = 0.11, p = 0.739). Overall, there was a significant correlation between the rate of nest-building and the rate of development of the red throat (Pearson correlation, r = −0.356, 83 d.f., p < 0.001), with males that developed their red throat fastest being quickest to build a nest.
Sexual responsiveness was positively related to lifespan, because the slowdown fish (which showed greater interest in mating) survived longer than the control fish, which survived longer than the catch-up fish (which were the slowest to respond to the female; table 2 and figure 1). However, relationships within growth treatment groups went in the opposite direction, because the males that showed the greatest interest in females in each treatment group tended to have the shortest lifespans (table 2). Lifespan was also related to an interaction between experiment and between-treatment effect of sexual responsiveness (table 2 and figure 1e,f): as with the development of throat coloration and rate of nest-building, the treatment effect of sexual responsiveness was greater under the spring than under the winter experiment.
4. Discussion
Although long-term effects of different juvenile growth trajectories using three-spined sticklebacks have previously been documented after exposed to brief warm or cold spells [6,8,15], this study is, to our knowledge, the first to report relationships between growth trajectory early in life and an important aspect of behaviour (adult male responsiveness to females) later in life. We showed a negative impact of growth rate on sexual responsiveness: fish induced (by temperature manipulation) to grow the slowest prior to the breeding season were consistently the quickest to respond to the presence of a gravid female. This speed of sexual responsiveness was also positively correlated with rate of development of sexual ornaments and time taken to build a nest. Moreover, rapid growth resulted in a shorter lifespan—but there was a negative relationship between sexual responsiveness and lifespan once the effect of growth rate had been taken into account. While the manipulation of photoperiod had no effect in any analyses, almost all of the adverse effects of growth rate were stronger in the spring experiment than in the one conducted in winter. This strongly supports the time-stress hypothesis that the amount of time available until a life-history event (here the breeding season) influences the strength of both the compensatory response to an environmental perturbation and its consequences [14].
Growth rate can have immediate effect on behaviour through its direct effect on activity; the most usual means by which growth is accelerated is through a hyperphagic response which will represents an increase in foraging activity [27]. In this study, the effects on behaviour (i.e. sexual responsiveness, red throat coloration and nest-building) were apparent long after the period of growth manipulation had ended. It is clear that the early-life environment can have strong impacts on the development of behaviours that are only expressed at a later life stage [28], especially if there are sensitive developmental periods during which adult behavioural patterns are shaped [29,30]. While further studies are needed to establish whether traits such as sexual responsiveness are formed during sensitive periods in development, evidence against a sensitive period occurs from the fact that broadly similar results were obtained in this from manipulating growth at two different times in development (i.e. the winter and spring experiments, corresponding to when the fish were approximately six and nine months old, respectively).
An alternative explanation for the effect of growth rate on subsequent behaviour is that it had permanent effects on physiological traits, which themselves influenced behaviour. It is known that rapid growth can have long-term adverse effects on immune responses, metabolic rate and starvation resistance [31–34]. These could result in an altered behavioural phenotype that is less able to engage in the physical activities associated with attracting a mate. It is well documented that rapid growth leads to an impairment of locomotor performance [8,35,36], which may lead to an inability to perform at the level required of sexual displays [37]. Moreover, locomotor performance can have further implications to natural conditions—as mating displays may increase conspicuousness to predators, males that have undergone rapid growth may be more cautious in their displays as their speed of escape is lower.
Long-term costs of compensatory growth on reproduction and longevity could arise both through direct effects on growth plasticity or indirect effects on mating behaviour. We have shown elsewhere that the juvenile growth rate induced by differential temperature regimes affects subsequent breeding investment and lifespan [6,15]: negative effects of catch-up growth have been presumed to be owing to the decrease in resources available for reproduction. It is interesting to note that we observed in several instances opposite effects of sexual responsiveness on lifespan between- and within-temperature treatments (figures 1 and 2; more specifically, a positive relationship between temperature treatments and a negative relationship within treatments). These differences in sexual responsiveness between-growth treatments may be related to trade-offs between reproductive investment and survival, because early growth condition (e.g. temperature) cannot directly be compared with variation in resource acquisition (i.e. foraging), but it may increase the ability to acquire resource and energy efficiently [38]. Conversely, the trade-off between reproduction and growth can be detected when comparing individuals that underwent the same growth conditions in early life as environmental condition causing growth plasticity may affect the ability to acquire resources and energy efficiently [38,39]: fish in slowdown growth groups show both higher sexual responsiveness and longer lifespan than those in catch-up growth groups.
Few studies have investigated how animal behaviour interacts with lifespan. A theoretical study [13] suggested that bold or aggressive individuals should have a shorter lifespan than shyer ones, which was supported by a meta-analysis of available data [40], although the opposite relationship has also been found (but, see [41]). These studies suggested that the evolution and maintenance of variation in animal behaviour might be related to the trade-off between reproduction and survival. In this study, males with the greatest sexual responsiveness tended to also be those that built their nests fastest and developed their sexual ornamentation fastest. However, when examining variation within-treatment groups, these males showing the fastest sexual responses had the shortest lifespans, suggesting a trade-off between speed in attracting a mate and future longevity, although the direction of causality cannot be determined.
In conclusion, an environmental perturbation early in life changed the tempo and pattern of growth and influenced aspects of animal behaviour related to reproduction, with more rapid growth being associated with more reticent reproductive behaviour. Moreover, the time available before the onset of the breeding season (time stress) affected the magnitude of these effects. While this study did not explore the causal mechanisms underlying these effects, they may relate to changes in resource allocation that could alter levels of oxidative damage to tissues and hence rates of senescence [6,7]. The finding of a negative correlation between sexual responsiveness and lifespan suggests a further resource allocation trade-off, but additional empirical studies are required to investigate these relationships in more detail.
Acknowledgements
We thank Graham Law, John Laurie and Alastair Kirk for help with fish husbandry, and two referees for comments that improved an earlier version of the manuscript.
All experiments were performed under licence from the UK Home Office (PIL 60/11377).
Funding statement
W.S.L. was supported by a University of Glasgow Postgraduate Scholarship and an Overseas Research Student award, and his analysis works at UQAM was partly supported by the FQRNT Merit Postdoc Scholarship in Québec, Canada.
References
- 1.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645 (doi:10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 3.Mangel M, Munch SB. 2005. A life-history perspective on short- and long-term consequences of compensatory growth. Am. Nat. 166, E155–E176 (doi:10.1086/444439) [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940 (doi:10.1016/s0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 5.Dmitriew CM. 2011. The evolution of growth trajectories: what limits growth rate? Biol. Rev. 86, 97–116 (doi:10.1111/j.1469-185X.2010.00136.x) [DOI] [PubMed] [Google Scholar]
- 6.Lee W-S, Monaghan P, Metcalfe NB. 2013. Experimental demonstration of the growth rate–lifespan trade-off. Proc. R. Soc. B 280, 20122370 (doi:10.1098/rspb.2012.2370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W-S, Metcalfe NB, Monaghan P, Mangel M. 2011. A comparison of dynamic-state-dependent models of the trade-off between growth, damage, and reproduction. Am. Nat. 178, 774–786 (doi:10.1086/662671) [DOI] [PubMed] [Google Scholar]
- 8.Lee W-S, Monaghan P, Metcalfe NB. 2010. The trade-off between growth rate and locomotor performance varies with perceived time until breeding. J. Exp. Biol. 213, 3289–3298 (doi:10.1242/jeb.043083) [DOI] [PubMed] [Google Scholar]
- 9.Woodgate JL, Bennett ATD, Leitner S, Catchpole CK, Buchanan KL. 2010. Developmental stress and female mate choice behaviour in the zebra finch. Anim. Behav. 79, 1381–1390 (doi:10.1016/j.anbehav.2010.03.018) [Google Scholar]
- 10.McGhee KE, Travis J. 2011. Early food and social environment affect certain behaviours but not female choice or male dominance in bluefin killifish. Anim. Behav. 82, 139–147 (doi:10.1016/j.anbehav.2011.04.009) [Google Scholar]
- 11.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 12.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063 (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 (doi:10.1038/Nature05835) [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe NB, Bull CD, Mangel M. 2002. Seasonal variation in catch-up growth reveals state-dependent somatic allocations in salmon. Evol. Ecol. Res. 4, 871–881 [Google Scholar]
- 15.Lee W-S, Monaghan P, Metcalfe NB. 2012. The pattern of early growth trajectories affects adult breeding performance. Ecology 93, 902–912 (doi:10.1890/11-0890.1) [DOI] [PubMed] [Google Scholar]
- 16.van de Pol MV, Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758 (doi:10.1016/j.anbehav.2008.11.006) [Google Scholar]
- 17.Kraak SBM, Bakker TCM, Mundwiler B. 1999. Sexual selection in sticklebacks in the field: correlates of reproductive, mating, and paternal success. Behav. Ecol. 10, 696–706 (doi:10.1093/beheco/10.6.696) [Google Scholar]
- 18.Wootton RJ. 1976. The biology of the sticklebacks. London, UK: Academic Press [Google Scholar]
- 19.Mclennan DA. 2003. The importance of olfactory signals in the gasterosteid mating system: sticklebacks go multimodal. Biol. J. Linn. Soc. 80, 555–572 (doi:10.1111/j.1095-8312.2003.00254.x) [Google Scholar]
- 20.Kraak SBM, Bakker TCM. 1998. Mutual mate choice in sticklebacks: attractive males choose big females, which lay big eggs. Anim. Behav. 56, 859–866 (doi:10.1006/anbe.1998.0822) [DOI] [PubMed] [Google Scholar]
- 21.McPhail JD, Hay DE. 1983. Differences in male courtship in freshwater and marine sticklebacks (Gasterosteus aculeatus). Can. J. Zool. 61, 292–297 (doi:10.1139/z83-039) [Google Scholar]
- 22.Hay DE, Mcphail JD. 2000. Courtship behaviour of male threespine sticklebacks (Gasterosteus aculeatus) from old and new hybrid zones. Behaviour 137, 1047–1063 (doi:10.1163/156853900502420) [Google Scholar]
- 23.Tibergen N. 1951. The study of instinct. Oxford, UK: Clarendon Press [Google Scholar]
- 24.Frischknecht M. 1993. The breeding coloration of male 3-spined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigor. Evol. Ecol. 7, 439–450 (doi:10.1007/Bf01237640) [Google Scholar]
- 25.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Stastistical Computing; See http://www.r-project.org [Google Scholar]
- 26.Bates D, Maechler M, Bolker B. 2012. lme 4: linear mixed-effects models using S4 classes. R package version 0999999-0 See http://cranr-projectorg/package=lme4
- 27.Ali M, Nicieza A, Wootton RJ. 2003. Compensatory growth in fishes: a response to growth depression. Fish Fish. 4, 147–190 (doi:10.1046/j.1467-2979.2003.00120.x) [Google Scholar]
- 28.Crews D. 2008. Epigenetics and its implications for behavioral neuroendocrinologry. Front. Neuroendocrinol. 29, 344–357 (doi:10.1016/j.yfrne.2008.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bateson P. 1979. How do sensitive periods arise and what are they for? Anim. Behav. 27, 470–486 (doi:10.1016/0003-3472(79)90184-2) [Google Scholar]
- 30.Immelmann K, Prove R, Lassek R, Bischof HJ. 1991. Influence of adult courtship experience on the development of sexual preferences in zebra finch males. Anim. Behav. 42, 83–89 (doi:10.1016/S0003-3472(05)80608-6) [Google Scholar]
- 31.Criscuolo F, Monaghan P, Nasir L, Metcalfe NB. 2008. Early nutrition and phenotypic development: ‘catch-up’ growth leads to elevated metabolic rate in adulthood. Proc. R. Soc. B 275, 1565–1570 (doi:10.1098/rspb.2008.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupont-Prinet A, Chatain B, Grima L, Vandeputte M, Claireaux G, McKenzie DJ. 2010. Physiological mechanisms underlying a trade-off between growth rate and tolerance of feed deprivation in the European sea bass (Dicentrarchus labrax). J. Exp. Biol. 213, 1143–1152 (doi:10.1242/Jeb.037812) [DOI] [PubMed] [Google Scholar]
- 33.De Block M, Stoks R. 2008. Short-term larval food stress and associated compensatory growth reduce adult immune function in a damselfly. Ecol. Entomol. 33, 796–801 (doi:10.1111/j.1365-2311.2008.01024.x) [Google Scholar]
- 34.Fischer K, Zeilstra I, Hetz SK, Fiedler K. 2004. Physiological costs of growing fast: does accelerated growth reduce pay-off in adult fitness? Evol. Ecol. 18, 343–353 (doi:10.1007/s10682-004-2004-3) [Google Scholar]
- 35.Billerbeck JM, Lankford TE, Conover DO. 2001. Evolution of intrinsic growth and energy acquisition rates. I. Trade-offs with swimming performance in Menidia menidia. Evolution 55, 1863–1872 (doi:10.1554/0014-3820(2001)055[1863:Eoigae]2.0.Co;2) [DOI] [PubMed] [Google Scholar]
- 36.Arendt JD. 2003. Reduced burst speed is a cost of rapid growth in anuran tadpoles: problems of autocorrelation and inferences about growth rates. Funct. Ecol. 17, 328–334 (doi:10.1046/j.1365-2435.2003.00737.x) [Google Scholar]
- 37.Clark CJ. 2012. The role of power versus energy in courtship: what is the ‘energetic cost’ of a courtship display? Anim. Behav. 84, 269–277 (doi:10.1016/j.anbehav.2012.04.012) [Google Scholar]
- 38.De Jong G, van Noordwijk AJ. 1992. Acquisition and allocation of resources: genetic (co)variances, selection, and life histories. Am. Nat. 139, 749–770 (doi:10.1086/285356) [Google Scholar]
- 39.Roff DA, Fairbairn DJ. 2007. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447 (doi:10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 40.Smith BR, Blumstein DT. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 (doi:10.1093/beheco/arm144) [Google Scholar]
- 41.Réale D, Martin J, Coltman DW, Poissant J, Festa-Bianchet M. 2009. Male personality, life-history strategies and reproductive success in a promiscuous mammal. J. Evol. Biol. 22, 1599–1607 (doi:10.1111/j.1420-9101.2009.01781.x) [DOI] [PubMed] [Google Scholar]