Abstract
Most known starfish species possess a compound eye at the tip of each arm, which, except for the lack of true optics, resembles an arthropod compound eye. Although these compound eyes have been known for about two centuries, no visually guided behaviour has ever been directly associated with their presence. There are indications that they are involved in negative phototaxis but this may also be governed by extraocular photoreceptors. Here, we show that the eyes of the coral-reef-associated starfish Linckia laevigata are slow and colour blind. The eyes are capable of true image formation although with low spatial resolution. Further, our behavioural experiments reveal that only specimens with intact eyes can navigate back to their reef habitat when displaced, demonstrating that this is a visually guided behaviour. This is, to our knowledge, the first report of a function of starfish compound eyes. We also show that the spectral sensitivity optimizes the contrast between the reef and the open ocean. Our results provide an example of an eye supporting only low-resolution vision, which is believed to be an essential stage in eye evolution, preceding the high-resolution vision required for detecting prey, predators and conspecifics.
Keywords: echinoderm, Linckia, compound eye, coral reef, navigation
1. Introduction
Though still debated, there are many indications of image-forming eyes having evolved several times within Metazoa [1–3]. One example of assumed independently evolved eyes is found in the cnidarian lineage peaking in complexity in the image-forming lens eyes in cubozoans [4,5]. Another example is found within echinoderms, where only starfish and a single species of holothurian possess true eyes with putative image-forming capacity [6,7]. Interestingly, there is evidence of image formation without eyes in sea urchins possibly using directional shading of epidermal opsin by the spines [8,9]. The behavioural significance of the image formation in sea urchins has not yet been established.
Although certainly convergently evolved, the compound eyes of starfish resemble the compound eyes of arc clams, which also lack lenses [10] in contrast to the more advanced compound eyes of arthropods. Depending on species, the fully grown starfish eyes are composed of 50–200 ommatidia, each holding several photoreceptors. The eye is presumably supporting true image vision, but this has not been verified. From immunocytochemistry and physiology, it has been indicated that vision in these eyes is opsin based as in all other known animal eyes [11–13]. Relatively few behavioural experiments have been conducted with starfish and almost none dealing with their visual performance. In the 1960s, Yoshida & Ohtsuki [14] made experiments studying their phototaxis and found some indications that the eyes are possibly involved in this behaviour. Still, no behaviour has ever been directly associated with the eyes and image formation, and today it is still unknown why they possess such prominent eyes.
Here, we studied the eyes and behaviour of Linckia laevigata, a circum tropical species of starfish closely associated with coral reefs [15]. We tested the hypothesis that they use vision to find their way back to the reef if displaced. We also obtained functional data on the physiology and spatial resolution of their eyes, and correlated this with the behavioural data.
2. Material and methods
(a). Experimental animals
For the morphological and optical studies, animals were purchased from an aquarium store in Copenhagen, Denmark. For the electrophysiological and behavioural experiments, animals were collected in their natural habitat on the coral reef at Akajima, Okinawa, Japan, and kept in a 300 l holding tank with running seawater at 29°C and salinity of 34 psu at Akajima Marine Science Laboratory. These animals were used for the experiments within 2 days of collection.
(b). Eye morphology
Pictures of the eyes were taken in vivo using a Nikon D300 camera equipped with a 105 mm Micro Nikkor lens and a Leica dissection microscope equipped with a digital camera (Evolution MP v. 5.0, MediaCybernetics, MD, USA). These pictures were used to determine possible influence on the visual field by the ossicles, etc. sitting next to the eye. Further, the pictures revealed in which direction the eye pointed relative to the long axis of the arm. Four eyes from four specimens were fixed in 2.5% glutaraldehyde, 1.5% paraformaldehyde and 3% sucrose in 0.1 M phosphate buffered saline (PBS) overnight. The eyes were postfixed in 1% osmium tetraoxide in 0.1 M PBS overnight at 4°C, dehydrated in a series of ethanol and acetone, and embedded in Epon 812 resin following standard procedures. Two eyes were cut in 2 µm sections, stained with toluidine blue and used for light microscopy (LM). The two last eyes were used for ultrathin sections (70 nm) and contrasted with lead citrate and uranyl acetate and observed in a Jeol 1010 transmission electron microscope (TEM) equipped with a SC1000 Gatan digital camera. Longitudinal sections of three randomly chosen, fully developed ommatidia were used to estimate ommatidial receptive fields.
(c). Visual field measurements
Two eyes from two different specimens were placed in a custom-made underwater goniometer, and observed through a dissection microscope. The visual field of the bilaterally symmetric eye was determined by finding the midline of the eye along with the optical centres of six peripheral ommatidia situated at regular intervals along one-half of the eye (figure 3). As the eyes are bilaterally symmetric, we mirrored the data from the one-half to obtain the complete visual field, which was too large for the range of the goniometer. We also measured the angles between the optical axes of neighbouring ommatidia in different parts of the eye (n = 16), and used this to calculate the average sampling density.
Figure 3.
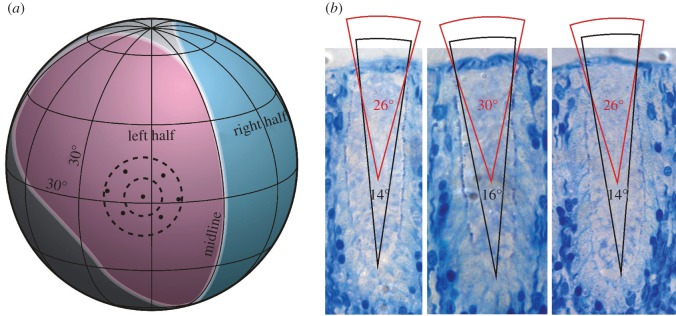
The visual field of a starfish compound eye. (a) Visual field measured with a goniometer. The left (pink) and right (blue) halves of the eye are symmetric around the midline. Altogether, the eye covers about 170° vertically and 120°–210° horizontally. To illustrate the sampling array, optical axes from a central and six neighbouring ommatidia are plotted. The average separation between the central ommatidia and the neighbours is 16°. The dashed circles illustrate the estimated acceptance cone of individual receptors in the middle and bottom of an ommatidium, respectively. (b) Estimates of acceptance angles from the middle and bottom part of the ommatidia.
(d). Electrophysiology
Extracellular electroretinogram (ERG) recordings were obtained from seven ommatidia from five individuals. The eye including the modified tube foot was dissected from an animal and transferred to a Petri dish in the electrophysiological set-up containing seawater at 29°C and a salinity of 3.4%. A custom-made glass suction electrode was placed on the edge of one of the fully developed ommatidia and suction was applied until a slight migration of pigment into the electrode was observed. The pore diameter of the electrode was 1–3 µm, resulting in an impedance of 2–5 Mohm. Recordings were amplified 1000 times and filtered (0.01 Hz high pass and 1000 Hz low pass) via a differential alternating current amplifier (1700, A-Msystems Inc., WA, USA) and recorded using a custom-made program for Labview (Labview v. 8.5, National Instruments, TX, USA). The light stimulus was provide by an ultra bright white LED (Luxeon III star, Philips, San Jose, CA, USA) placed in a Linos microbench system (Linos, Goettingen, Germany). The microbench was equipped with a series of neutral density filters and interference colour filters (half-width = 12 nm, CVI Laser, Bensheim, Germany). The stimulus was presented to the ommatidium of interest using a 1 mm light guide to assure a close to even illumination of its entire field of view.
The experimental protocol started with 15 min of dark adaptation. Then an intensity series was presented covering 5 log units in steps of 0.3 or 0.7 log units starting at the low intensity end (1.1 W sr−1 m−2). This was followed by an equal quanta (6 × 1018 photons s−1 sr−2 m−2) spectral series covering 400–660 nm in 22 steps, and the protocol ended with a second intensity series to ensure that the sensitivity had not changed during the experiment. Each stimulus lasted 100 ms and the stimuli were presented with 2 min in between. Only data from eyes lasting a full protocol, where the second intensity series differed less than 10% from the first, were used for the analysis. The data were analysed manually in the program Igor Pro v. 6.12A (Wavematrics, Lake Oswego, OR, USA). The spectral data were transformed by the V/logI-curve to obtain the relative sensitivity (see [16] for details on this procedure).
(e). Behavioural experiments
The behavioural experiments were performed on two different coral reefs at Akajima, Okinawa, Japan. A first set of experiments was made with three groups of intact specimens of L. laevigata placed at 4, 2 and 1 m in front of the reef front, respectively (locality 1, reef front facing east). The reef was approximately 6 m wide at the base and 3 m tall at the middle part. The vertical slope of the front was about 45°, which resulted in the tallest central part of the reef taking up about 23°, 31° and 40° of the visual field vertically depending on the distance. Only at 1 m (visual angle = 40°) did the starfish walk straight back to the reef (see the electronic supplementary material, figure S2), and here all starfish reached it in less than 25 min. Accordingly, 1 m was chosen to be the distance to the reef in the following experiments. Five adult-sized specimens (25–30 cm) had their eyes removed with a pair of scissors (two ossicles also had to be removed to get access to the eyes), while five others were sham operated (each arm had two tube feet and two ossicles removed from the middle part). The animals were left to recover and were tested the day after. The five animals having the same treatment were tested simultaneously, placing them 1 m from the reef front with 1 m in between (locality 1). Their movements were then monitored for 25 min using an underwater video camera (GoPro HERO3, Woodman Laboratories, Inc., USA). The water depth was 5 m and the observer was lying at the surface. The experiments were repeated with 10 new animals at another location (locality 2, reef front facing south). The water depth here was 6 m. The average speed of movements was determined in each experimental trial by path integration.
For analysing the behavioural data, still pictures were grabbed from the videos with 5 min intervals (at 0, 5, 10, 15, 20 and 25 min) to calculate the speed of movement. The direction perpendicular to the reef front from their starting position was determined for each individual and set to 0°. Their overall direction of movement was then measured in degrees relative to this 0. For the individuals reaching the reef, the direction of movement was determined to the point of first contact with the reef. For the others, the direction of movement was determined to the end position after the 25 min. The results were then analysed with circular statistics. First, a Rayleigh test was performed to test whether the animals moved randomly or not. If this test returned the direction to be non-random, a V-test was used to check whether the direction was different from 0 (towards the reef front).
3. Results and discussion
(a). Eye morphology
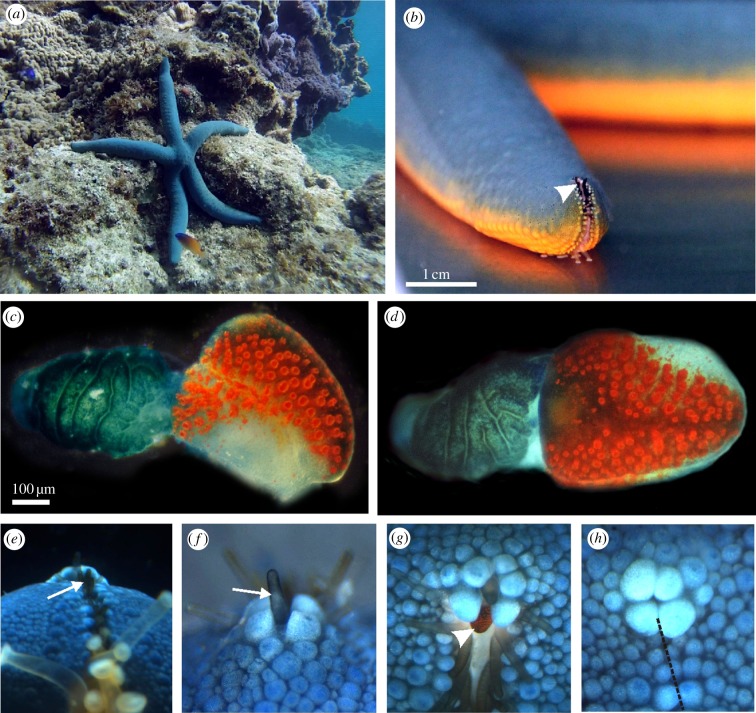
To better understand the functional significance of the compound eyes present in most starfish, we started out by examining the eye morphology, and used this for estimations of receptive fields and spatial resolution. As in all other known starfish, the eyes of L. laevigata are situated at the very tip of each arm and in moving animals the tip bends 80°–120° upwards which raise the eyes from the substrate (figure 1a,b). The eye sits on a modified tube foot, and fully grown it holds 150–200 ommatidia, distributed in a bilaterally symmetric pattern on the so-called optical cushion (figure 1c,d). As the eye is sitting on the base of a modified tube foot, it is drawn into the ambulaceral groove so that the visual field is narrowed laterally (figure 1b). The visual field is further shaped by two rows of modified black tube feet surrounding the eye (figure 1e–h), potentially blocking light coming from below (figure 1e) and from above (figure 1f). These tube feet are spread out when the animal is active (figure 1g), but interestingly when the animal is disturbed they can be withdrawn into the ambulaceral groove which closes and covers the eye leaving the animal blind (figure 1h).
Figure 1.
Visual system of the starfish L. laevigata. (a) Linckia laevigata in its natural coral reef habitat at Akajima, Japan, where it feeds on detritus and algae. (b) As in other starfish species, the compound eye of L. laevigata is situated on the tip of each arm (arrowhead). It sits in the ambulaceral groove which continues to the top of the arm tip. (c) Lateral view of the compound eye, also called the optical cushion, which is sitting on the base of a modified tube foot. The eye has approximately 150 separate ommatidia with bright red screening pigment. (d) Frontal view of the compound eye showing its bilateral symmetry. (e) The tip of the arm seen from below. The view of the compound eye is obscured by a double row of modified black tube feet (arrow). (f) The arm tip seen straight from above. Note that the eye is again obscured from view by a modified black tube foot (arrow). (g) The compound eye (arrowhead) seen from 45° above horizontal in a freely behaving animal. When the animal is active, the modified black tube feet spread out to allow vision. (h) If the animal is disturbed, it closes the ambulaceral groove (broken line) at the arm tip and withdraws the modified tube feet. The compound eye is then completely covered, leaving the animal blind.
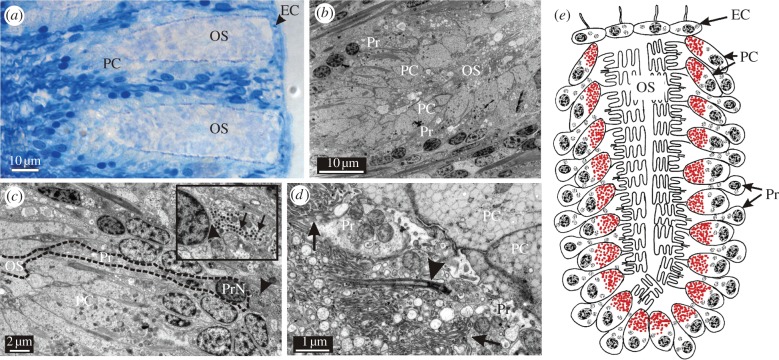
The ommatidia vary in size, with the largest being 25 µm wide and 60 µm deep (figure 2a). The large ommatidia have about 120 photoreceptors and a similar number of pigment cells arranged in seven to eight layers along the long axis (figure 2b,e). This is in good accordance with what is known from the similar-sized starfish Patiria miniata and Nepanthia belcheri [17,18]. From the LM and TEM-micrographs, it is evident that the ommatidia lack any focusing optics, which contrasts with earlier suggestions from a range of species [7,19]. They are covered by a single layer of epithelial cells (figure 2a), and the receptive fields are thus determined by the screening pigment alone, just as in a pinhole eye. Because the outer segments of the photoreceptors are arranged in layers, the width of their receptive fields depends on their position in the ommatidia (figure 3b). Interestingly, it looks as though visual information is already being processed in the retina, as indicated by afferent synapses contacting the photoreceptors at the soma (figure 2c). It is not known, though, whether the synaptic contacts are made by interneurons or by neighbouring photoreceptors. Morphologically, the receptors are of an intermediate type with microvilli originating both from the cell surface and from a modified cilium (figure 2d).
Figure 2.
Morphology of the starfish eye. (a) LM of two ommatidia sectioned longitudinally. Each of the fully developed ommatidia is composed of 100–150 photoreceptors and about the same number of pigment cells (PC). Note the thin layer of epithelial cells (EC, arrowhead) covering the outer segments (OS). The receptors are arranged in seven to eight layers perpendicular to the surface (b). (c) Longitudinal section of a photoreceptor (Pr, dashed black line). Interestingly, it receives feedback from the nervous system as indicated by afferent synapses (insert, arrowhead indicates synapse, arrows indicate synaptic vesicles). PrN, photoreceptor nucleus. (d) The outer segments of the starfish photoreceptors are morphologically a mixture of the rhabdomeric- and ciliary-type receptors. They are formed by microvilli coming both directly from the cell membrane (arrows) and from a modified cilium (arrowhead). (e) Schematic drawing of an ommatidium showing the layered arrangement of the photoreceptors and pigment cells.
(b). Low spatial resolution
From the goniometric measurements of the visual field, each compound eye is shown to cover about 210° horizontally and about 170° vertically (figure 3). When the position of the eye on the bent arm tip is taken into account, it is clear that the starfish eyes monitor the surroundings from the water surface to the substrate just in front of it. Horizontally, there is a large overlap between eyes on neighbouring arms. In total, this gives the animals the ability to simultaneously view their entire surroundings. However, the visual field is probably dynamic, because the eyes are surrounded by the highly flexible black tube feet in all directions, potentially narrowing the visual field.
The optical axes of the ommatidia are more or less evenly distributed in the visual field, resulting in a sampling density of 10°–20° across the eye, with an average of 16° ± 4.5° (n = 16; figure 3a). Together with the large acceptance angles of 15°–30°, this means that the eyes can only resolve low spatial frequencies, and thus only detect large structures in the environment. In their natural habitat, this would be large coral structures. It eliminates the possibility that L. laevigata uses vision to detect fine details in the habitat e.g. for finding food, predators or conspecifics. Such low spatial resolution is similar to that found in other animals with limited brain power [5].
(c). Temporal and spectral response
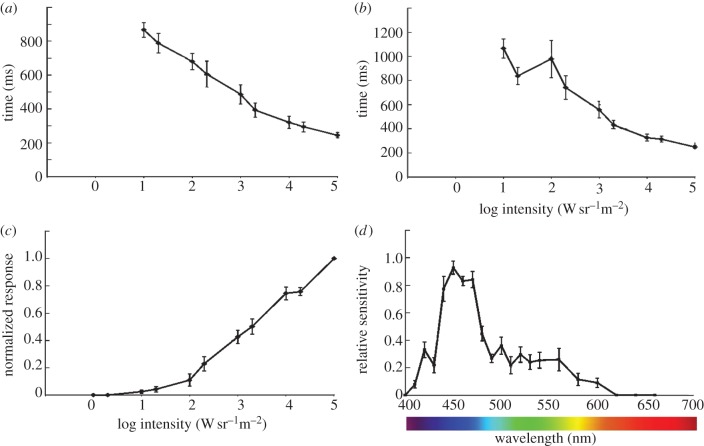
Using ERGs, we went on to test the temporal response from the photoreceptors and their spectral sensitivity (figure 4; electronic supplementary material, S1). The response time turned out to be very long and with flashes shorter than 50 ms; there was almost no response even at the highest intensity used (1.1 × 105 W sr−1 m−2). We therefore used 100 ms flashes, but at the highest intensity, the time to peak was still 250 ms and the half-width of the response was about the same (figure 4). These very slow responses did not come as a surprise, because they make a good match with the slow behaviour of the animals. The slow responses will remove any fast-moving objects from sight. Further, the ERGs suggest that the eyes of L. laevigata are colourblind and use a single opsin with peak sensitivity in the blue part of spectrum around 450 nm (figure 4). The spectral sensitivity curve is somewhat narrower than for a typical opsin with the best fit to the absorption curve for a theoretical 452 nm opsin [20], resulting in a regression coefficient (r2) of 0.69. We speculate that this is owing to some kind of spectral filter present in the eye. It is not unusual that the lens or cornea absorb damaging ultraviolet-light [21]. Our morphological data show that no lens is present, only a thin layer of epithelial cells (figure 2a,e), making us unable to point to a specific structure responsible for the filtering.
Figure 4.
Temporal resolution and spectral sensitivity. (a) The temporal resolution of the photoreceptors was examined with ERGs. The half-width of the impulse response had a minimum of approximately 250 ms, which is comparatively very slow. (b) The slow response is supported by the time to peak measurements of the impulse response. Again the minimal time to peak was about 250 ms. (c) It is seen from the V/logI-curve that the dynamic range of the eyes was at least 4 log units, but it is also seen from the curve that the receptors were not saturated at the maximum test intensity (1.1 × 105 W sr−1 m2). (d) The spectral sensitivity of the receptors showed a single and relatively narrow peak in the deep blue (450 nm) part of the spectrum. The half-width is about 50 nm, which is somewhat narrower than the typical opsin, indicating the presence of spectral filters.
Interestingly, the maximum sensitivity at 450 nm matches the dominant wavelength of clear ocean water in the horizontal and upward directions [22]. This means that the open ocean will appear bright to the starfish. But the coral reef will appear dark, because light reflected from it mostly contains the longer wavelength part of the spectrum [23,24]. In general, little light below 470 nm is reflected from reef components.
(d). Navigation
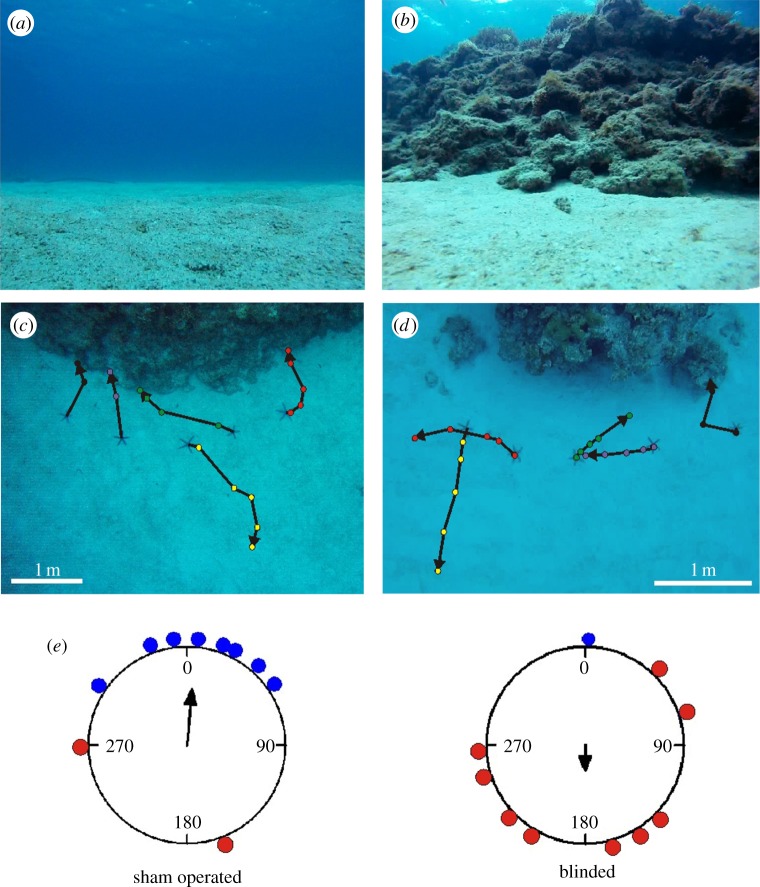
From the optical and physiological results, we hypothesize that L. laevigata starfish use vision to detect large stationary objects. Given their ecology and close association with coral reefs, it seems likely that they use visual cues to discriminate reef structures from the open sea in order to navigate towards their preferred habitat. We initially tested this hypothesis by placing intact specimens at different distances (1, 2 and 4 m) in front of a reef front taking up between 23° and 40° vertically of the visual space (see the electronic supplementary material, figure S2). At 4 m, they walked randomly (length of mean vector = 0.31, Rayleigh test: p = 0.4) but a distance of 2 m seemed to be very close to the limit of successful navigation (length of mean vector = 0.46, Rayleigh test: p = 0.1). At 2 m, the reef takes up 31o of the visual field vertically which is approaching the resolving power of the eyes as indicated by our morphological and goniometry data. When the animals were placed 1 m in front of the same reef front (maximum height approx. 40°), they all walked more or less straight back to the reef (length of mean vector = 0.99, Rayleigh test p = 0.008, V-test p < 0.001; electronic supplementary material, figure S2). It was obvious, therefore, that at short distances, they were able to detect the reef and find their way back. Next, we made a new series of experiments at two different localities, one with the reef front facing south and one with the front facing east. At both localities, the navigational abilities of five blinded (modified tube foot with the eye and two ossicles surgically removed from each arm) and five sham-operated (two tube feet and two ossicles removed from the middle of each arm) animals were tested at a distance of 1 m from the reef front (maximum height approx. 40°). While the blinded starfish walked randomly (length of mean vector = 0.25, Rayleigh test p = 0.56), the sham operated were still able to navigate back to the reef (length of mean vector = 0.57, Rayleigh test p = 0.034, V-test p = 0.005; figure 5). To verify that the blinded starfish were not just impaired by the surgery, we compared the walking speed during the experiments from intact, sham-operated and blinded animals. The average walking speeds were 5.2, 4.0 and 4.3 cm min−1, respectively, and none of the differences were significant (one-way ANOVA, F2,21 = 3.34, p = 0.25). This allowed us to conclude that the blinded animals were just as active but walked in random directions. To get additional evidence for visual navigation, we tested the ability of 10 intact animals during a starry but moonless night where our V/logI-curve (figure 4a) suggests they can no longer see. Indeed, they also walked randomly in the dark (length of mean vector = 0.08, Rayleigh test p = 0.94, electronic supplementary material, figure S3). Previous studies have found that another starfish Oreaster reticulates is also able to walk more or less straight back to their feeding grounds, but here is was suggested to be based on olfaction [25,26].
Figure 5.
Navigation experiments. In the behavioural experiments, the test animals were removed from their natural reef habitat and placed 1 m from the reef edge. (a) The visual scene away from the coral reef habitat was the open ocean. (b) The visual scene towards the natural habitat (coral reef) at a distance of 1 m. (c) Trajectories from the sham-operated animals tested at locality 1 (reef front to the east). Most of the animals quickly found their way back to the habitat. (d) Trajectories from the eye-ablated animals tested at locality 2 (reef front to the south). They moved at the same pace as the intact and sham-operated animals but in random directions. (e) Circular statistics of the behavioural experiments show that the direction of movement of the sham-operated animals differed significantly from random and correlated with the direction to the reef front. The direction of movement of the eye-ablated animals did not differ significantly from random. Blue dots represent animals reaching the reef within the 25 min, red dots represent animals that did not. See experimental procedure for details of the statistical analysis.
(e). Eye evolution
In the light of eye evolution, echinoderms are interesting because they constitute an early branch within the deuterostomes and might therefore shed light on the split between the two major photoreceptor types, ciliary and rhabdomeric, with their different photo-transduction pathways [3,27]. Morphologically, the starfish eyes are unusual, because their photoreceptors are neither ciliary nor rhabdomeric but a combination [17]. This agrees with molecular data showing that they have both ciliary and rhabdomeric types of opsins [11,28,29]. It should be kept in mind, though, that little is known about the expression pattern and functionality of these opsins. The ultrastructural data we present here from L. laevigata support previous observations that the outer segments of the photoreceptors are composed of both ciliary and microvillar projections (figure 2d). Our data also shed light on another aspect of eye evolution. It has been proposed that major steps in eye evolution, such as implementation of spatial resolution and true optics, have been tightly connected to the acquisition of new visually guided behaviours [30]. It is suggested that early in eye evolution, animals possessed few and simple visually guided behaviours, requiring only simple eyes and simple nervous systems. According to this hypothesis, one of the first visual tasks to evolve was habitat recognition, which requires relatively little in terms of spatial and temporal resolution as well as visual processing. Our results here offer an example of a visual system at this early stage of evolution of true vision used for habitat recognition (class III according to Nilsson [31]).
4. Conclusion
To our knowledge, the results we present here are the first to directly demonstrate a visually guided behaviour in starfish involving their compound eyes. We show that if displaced from their natural coral habitat, L. laevigata uses vision to navigate back, but only when the reef takes up more than 30° vertically, which matches our estimates of a 15°–30° resolution. Interestingly, this means that the behaviour only works at relatively short distances from the reef, and we suggest that the importance of the behaviour is not primarily to seek new reefs, or to handle major displacements from the reef, but rather to ensure that they do not move away from the reef.
Our data also show that the physiology and morphology of the eyes are well suited for such a behavioural task. The slow walking speed of starfish (cm min−1) and the large size of the reef structures mean that fast vision with high spatial resolution is not needed. It is likely that this type of low-resolution vision is essential for the starfish, and it is also at par with the limited central nervous system [28,32–34], which is likely to have a limited processing capacity. Low spatial and temporal resolution is often seen as an adaptation to vision at low light intensities [35]. Even though it may expand the active period of L. laevigata, our night-time behavioural experiments suggest its vision is not sensitive enough for navigation in the 8 log units lower intensities present on a starry but moonless night [36]. The spectral sensitivity, with a single peak at about 450 nm, also seems well suited for navigation towards coral reefs. It causes the undesired open ocean to appear as bright as possible while the desired habitat, the coral reef, appears dark, thereby maximizing the contrast needed to locate the reef.
Acknowledgements
The authors are grateful for all the help supplied by Dr Kenji Iwao at AMSL and the input offered by MSc Jan Bielecki and Dr Ronald Petie.
Funding statement
A.G. acknowledges financial support from the VILLUM Foundation (grant no. VKR022166). D.-E.N. is supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation.
References
- 1.Arendt D, Hausen H, Purschke G. 2009. The ‘division of labour’ model of eye evolution. Phil. Trans. R. Soc. B 364, 2809–2817 (doi:10.1098/rstb.2009.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozmic Z, Swamynathan SK, Ruzickova J, Jonasova K, Paces V, Vlcek C, Piatigorsky J. 2008. Cubozoan crystallins: evidence for convergent evolution of pax regulatory sequences. Evol. Dev. 10, 52–61 (doi:10.1111/j.1525-142X.2007.00213.x) [DOI] [PubMed] [Google Scholar]
- 3.Nilsson DE. 2004. Eye evolution: a question of genetic promiscurity. Curr. Opin. Neurobiol. 14, 407–414 (doi:10.1016/j.conb.2004.07.004) [DOI] [PubMed] [Google Scholar]
- 4.O'Connor M, Garm A, Nilsson DE. 2009. Structure and optics of the eyes of the box jellyfish Chiropsella bronzie. J. Comp. Physiol. A 195, 557–569 (doi:10.1007/s00359-009-0431-x) [DOI] [PubMed] [Google Scholar]
- 5.Nilsson DE, Gislén L, Coates MM, Skogh C, Garm A. 2005. Advanced optics in a jellyfish eye. Nature 435, 201–205 (doi:10.1038/nature03484) [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Yoshida M. 1978. Fine structure of the ocelli of a synaptid holothurian, Opheodesoma spectabilis, and the effects of light and darkness. Zoomorphologie 90, 1–17 (doi:10.1007/BF00993740) [Google Scholar]
- 7.Smith JE. 1937. On the nervous system of the starfish Marthasterias glacialis. Phil. Trans. R. Soc. Lond. B 227, 111–173 (doi:10.1098/rstb.1937.0002) [Google Scholar]
- 8.Yerramilli D, Johnsen S. 2010. Spatial vision in the purple sea urchin Strongylocentrotus purpuratus (Echinoidea). J. Exp. Biol. 213, 249–255 (doi:10.1242/jeb.033159) [DOI] [PubMed] [Google Scholar]
- 9.Blevins E, Johnsen S. 2004. Spatial vision in the echinoid genus Echinometra. J. Exp. Biol. 207, 4249–4253 (doi:10.1242/jeb.01286) [DOI] [PubMed] [Google Scholar]
- 10.Nilsson DE. 1994. Eyes as optical alarm systems in fan worms and ark clams. Phil. Trans. R. Soc. Lond. B 346, 195–212 (doi:10.1098/rstb.1994.0141) [Google Scholar]
- 11.Ullrich-Lüter EM, Dupont S, Arboleda E, Hausen H, Arnone MI. 2011. Unique system of photoreceptors in sea urchin tube feet. Proc. Natl Acad. Sci. USA 108, 8367–8372 (doi:10.1073/pnas.1018495108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnsen S. 1997. Identification and localization of a possible rhodopsin in the echinoderms Asterias forbesi (Asteroidea) and Ophioderma brevispinum (Ophiuroidea). Biol. Bull. 193, 97–105 (doi:10.2307/1542739) [DOI] [PubMed] [Google Scholar]
- 13.Yoshida M. 1966. Compound ocellus of a starfish: its function. Science 1953, 197–198 (doi:10.1126/science.153.3732.197) [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Ohtsuki H. 1968. The phototactic behavior of the starfish Asterias amurensis Lütken. Biol. Bull. 134, 516–532 (doi:10.2307/1539869) [Google Scholar]
- 15.Mueller B, Bos AR, Graf G, Gumanao GS. 2011. Size-specific locomotion rate and movement pattern of four common Indo-Pacific sea stars (Echinodermata; Asteroidea). Aquat. Biol. 12, 157–164 (doi:10.3354/ab00326) [Google Scholar]
- 16.Coates MM, Garm A, Theobald JC, Thompson SH, Nilsson DE. 2006. The spectral sensitivity in the lens eyes of a box jellyfish, Tripedalia cystophora. J. Exp. Biol. 209, 3758–3765 (doi:10.1242/jeb.02431) [DOI] [PubMed] [Google Scholar]
- 17.Pen PE, Alexander CG. 1980. Fine structure of the optic cusion in the asteroid Nepanthia belcheri. Mar. Biol. 58, 251–256 (doi:10.1007/BF00390773) [Google Scholar]
- 18.Eakin RM, Brandenburger JL. 1979. Effects of light on ocelli of seastars. Zoomorphologie 92, 191–200 (doi:10.1007/BF00994084) [Google Scholar]
- 19.Pfeffer W. 1901. Die Sehorgane der Seesterne. Zoologische Jahrbuecher Abteilung fuer Anatomie und Ontogenie der Tiere 14, 523–550 [Google Scholar]
- 20.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528 (doi:10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 21.O'Connor M, Garm A, Marshall JN, Hart NS, Ekström P, Skogh C, Nilsson D-E. 2010. Visual pigment in the lens eyes of the box jellyfish Chiropsella bronzie. Phil. Trans. R. Soc. B 277, 1843–1848 (doi:10.1098/rspb.2009.2248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarland WN, Munz FW. 1975. Part II: the photic environment of clear tropical seas during the day. Vis. Res. 15, 1063–1070 (doi:10.1016/0042-6989(75)90002-4) [DOI] [PubMed] [Google Scholar]
- 23.Hochberg EJ, Atkinsonn MJ, Apprill AA. 2004. Spectral reflectance of coral. Coral Reefs 23, 84–95 (doi:10.1007/s00338-003-0350-1) [Google Scholar]
- 24.Schalles JF, Maeder JA, Rundquist D, Narumalani S, Keck J. 2000. Close range, hyperspectral reflectance measurement of coral and other reef substrates In Proc. 9th Int. Coral Reef Symp. Bali, vol. 2 (ed. MK Moosa), pp. 1017–1024. Bali, Indonesia: Indonesian Institute of Sciences [Google Scholar]
- 25.Scheibling RE. 1981. Optimal foraging movements of Oreaster reticulatus. J. Exp. Mar. Biol. Ecol. 51, 173–185 (doi:10.1016/0022-0981(81)90127-1) [Google Scholar]
- 26.Scheibling RE. 1980. Homing movements of Oreaster reticulatus when experimentally translocated from a sand patch habitat. Mar. Behav. Physiol. 7, 213–223 (doi:10.1080/10236248009386982) [Google Scholar]
- 27.Arendt D, Wittbrodt J. 2001. Reconstructing the eyes of Urbilateria. Phil. Trans. R. Soc. Lond. B 356, 1545–1563 (doi:10.1098/rstb.2001.0971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke RD, et al. 2006. A genomic view of the sea urchin nervous system. Dev. Biol. 300, 434–460 (doi:10.1016/j.ydbio.2006.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raible F, Fessmar-Raible K, Arboleda E, Kaller T, Bork P, Arendt D, Arnone MI. 2006. Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461–475 (doi:10.1016/j.ydbio.2006.08.070) [DOI] [PubMed] [Google Scholar]
- 30.Nilsson D.-E. 2009. The evolution of eyes and visually guided behaviour. Proc. R. Soc. B 364, 2833–2847 (doi:10.1098/rstb.2009.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson DE. 2013. Eye evolution and its functional basis. Vis. Neurosci. 30, 5–20 (doi:10.1017/S0952523813000035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mashanov VS, Zueva OR, Heinzeller T, Dolmatov IY. 2006. Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology 125, 27–38 (doi:10.1007/s00435-005-0010-9) [Google Scholar]
- 33.Garcia-Arrarás JE, Rojas-Soto M, Jiménez LB, Diaz-Miranda L. 2001. The enteric nervous system of echinoderms: unexpected complexity revealed by neurochemical analysis. J. Exp. Biol. 204, 865–873 [DOI] [PubMed] [Google Scholar]
- 34.Elphick MR, Emson RH, Thorndyke MC. 1989. FMRFamide-like immunoreactivity in the nervous system of the starfish Asterias rubens. Biol. Bull. 177, 141–145 (doi:10.2307/1541841) [Google Scholar]
- 35.Warrant EJ. 2004. Vision in the dimmest habitats on Earth. J. Comp. Physiol. A 190, 765–789 (doi:10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- 36.Land MF, Nilsson DE. 2012. Animal eyes, 2nd edn Oxford, UK: Oxford University Press [Google Scholar]