Abstract
Cholesterol is implicated in the development of late onset Alzheimer’s disease (AD). We sought to determine the associations between beta amyloid (Aβ) plaque deposition in vivo using the Pittsburgh Compound B (PiB) and several indices of cholesterol homeostasis (i.e., total cholesterol, HDLc, LDLc, triglycerides, ApoE, clusterin, oxysterol metabolites of cholesterol and previously reported genes associated with late-onset AD) in 175 non-demented elderly subjects. High Aβ deposition was significantly associated with having a lower mini-mental state exams score (<27, p=0.04), high systolic blood pressure (p=0.04), APOE*4 (p<0.01), lower plasma ApoE levels (p=0.02) and variation in the ABCA7 gene (p=0.02) and EPHA1 genes (p=0.02). Cholesterol measures were not related to Aβ deposition in this cohort of non-demented elderly adults. However, plasma and genetic factors relating to cholesterol transport were associated with Aβ deposition in the brain. The better understanding of cholesterol transport mechanisms may lead to the design of potential targets for the prevention of Aβ deposition in the brain.
1. Background
Considerable resources and time are being invested in understanding the pathophysiology of late-onset form of Alzheimer’s disease (AD)1. Cholesterol levels and factors related to its homeostasis have been implicated in the development of AD in observational and genetic studies; however, the mechanisms underlying the relationship between cholesterol and AD pathology are not well understood1. Intervention trials to lower blood low-density lipoprotein cholesterol (LDLc) by statins have shown little effect on treatment and prevention of dementia2,3. This may be because cholesterol homeostasis and metabolism are separated in the periphery and brain due to the blood brain barrier (BBB). Oxysterol metabolites of cholesterol, 24S-hydroxycholesterol (24OHC) and 27-hydroxycholesterol (27OHC) are important exceptions to this rule, as they cross the BBB directly by diffusion and are involved in regulating cholesterol synthesis via Liver X receptors4.
Factors related to cholesterol homeostasis have shown promise in genetic and observational studies. The E4 allele in apolipoprotein E (APOE*4) remains the strongest risk factor for AD, accounting for 20-30% of the genetic risk. Additionally, recent genome-wide association studies (GWAS) studies have identified several new single nucleotide polymorphisms (SNPs) associated with AD5-8; of particular interest, were associations between AD and several SNPs within genes related to cholesterol homeostasis, including: CLU (a.k.a.clusterin or ApoJ), ABCA1 and ABCA7.
Several blood biomarkers related to cholesterol homeostasis have also been associated with AD9. Circulating levels of apolipoproteins (ApoE and clusterin) have been associated with Aβ deposition and severity of AD10, 11 as well as progression of AD and MCI12,13. Additionally, oxysterol metabolites of cholesterol, including 24S-hydroxycholesterol (24OHC) may indicate the presence of neurodegeneration14; are associated with AD15, 16, vascular dementia (VaD)17 and mild cognitive impairment (MCI)16, 17; and are correlated with brain volume in cognitively normal adults18. Recently, our group observed that plasma oxysterols concentrations were higher among cognitively normal individuals who went on to develop cognitive impairment (AD & MCI) over 8 years of follow-up19.
The use of amyloid-specific radiolabeled ligands (e.g. Pittsburgh compound B (PiB-PET)) have opened new avenues of inquiry in both AD and aging through the direct visualization and quantification of fibrillar Aβ plaques in the brain in vivo. This technique has allowed the study of risk factors and determinants of Aβ deposition in the brain of older adults. The aim of this study was to evaluate several indices of cholesterol homeostasis including genetic and blood biomarkers and their relationship with neuroimaging of Aβ deposition in non-demented elderly adults.
2.0 Methods
2.1 Participants
The Ginkgo Evaluation of Memory (GEM, 2000-2008) Study was a multi-site, placebo-controlled, double-blind, randomized clinical trial of daily use of Ginkgo biloba in 3069 community-dwelling participants 72-96 years old20. In 2009, approximately 10 (±3) months following the GEM study closeout, n=194 participants from the Pittsburgh site underwent MR imaging and Aβ PET scans as part of the GEM Neuroimaging Sub-Study. The design of the Neuroimaging Sub-Study and comparisons to the total Pittsburgh site (n=671) were detailed by Mathis et al.21.
2.2 Final GEM Study Visit
All GEM Study participants who returned to the clinics for their final evaluation between October 2007 and March 31, 2008 had a final clinic visit that included: resting blood pressure, blood draw and inventory of the participant’s prescription and over-the counter medications. Statin use was assessed at each GEM Study visit20. For the purpose of this study, participants were categorized as (ever versus never) statin users during the GEM Study (2000-2008) and a separate variable was created to assess current statin use at the final GEM study visit.
2.3 PET Imaging of Brain Aβ Deposition
Details of PiB-PET data acquisition have been described previously21, 22. We used the iterative mild outlier cutoff method (SUVR was >1.57)23 to determine Aβ positivity and compared these results to those obtained using the sparse k-means approach24 and found them to be nearly identical. Results from the iterative outlier method are presented herein.
2.4 GEM Neuroimaging Sub-Study Visit Assessments
At the time of neuroimaging in 2009, Sub-Study participants underwent a shortened visit which included an abbreviated neuropsychological (NP) battery a global measure of cognition (Mini-Mental State Examination (MMSE)25, and tests of memory, visuospatial and visuoconstructional, language and executive functions. In addition, we obtained a 10-question CES-D for depression, timed walk, and inventory of the participant’s prescription and over-the counter medications.
2.5 Cognitive Status
Cognitive Adjudication Committee was blinded to neuroimaging results by the Cognitive Diagnostic Center and took into account historical serial cognitive assessments from the parent GEM Study26 in addition to the Neuroimaging Sub-Study. Criteria for mild cognitive impairment (MCI) included 1 - 3 tests impaired at below 1.5 standard deviations below age and education adjusted means, according to the Winblad criteria27.
2.6 Assay of Genetic Markers
SNP genotyping assays were done using the TaqMan procedure using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). PCR amplification was done using a PTC-200 MJThermal Cycler (Biorad) or a GeneAmp 9700 (Applied Biosystems), and the endpoint fluorescence reading was done on an ABI Prism 7900HT instrument. Genetic markers included: ABCA1 (rs2230806), ABCA7 (rs3752246), ARID5B (rs2588969), BIN1 (rs7561528), CD33 (rs3865444), CD2AP (rs9349407), CLU (rs1532278), CR1 (rs6701713), EPHA1 (rs11767557), haptoglobin genotype, MS4A4A (rs4938933), and PICALM (rs561655).
2.7 Assay of Apolipoproteins
Frozen plasma samples were thawed for the assay of apolipoproteins E and J (clusterin). Apolipoprotein E was analyzed using an immunoturbidimetric procedure developed by Kamiya Biomedical Company (Seattle, WA). Diluted serum was incubated with anti-human-apolipoprotein E antibody and the resulting turbidity measured at 340 nm and 700 nm. Blanks, calibrators and control pools were run with each set of samples. The inter-assay and intra-assay coefficients of variation were 2.6% and 2.2%, respectively. Clusterin was measured using a competitive ELISA procedure developed by ALPCO Diagnostics (Salem, NH). Briefly, diluted serum samples were incubated for 1 hour at 37°C in microtiter plates coated with recombinant human clusterin in the presence of polyclonal antibodies to clusterin. The plates were washed x3 and HRP conjugated anti-rabbit IgG added and incubated for 1 hour at 37°C and then washed x5. Substrate solution was added and the plates incubated for 20 min at room temperature under subdued lighting. Stop solution was added to each well and the absorbance at 450 nm read within 30 minutes. Blanks, control pools and standards (0.001 to 2.5 μg/mL) were run with each set of samples. The intra- and inter-assay coefficients of variation were 9.8% and 11.4% respectively.
2.8 Assay of Oxysterols and Cholesterol
The details of the oxysterol assays used in this study were published previously19. Single tubes were thawed and used specifically for cholesterol and oxysterol assays. The antioxidant butylated hydroxytoluene (BHT) was added immediately after thawing the sample. Oxysterols (24-hydroxycholesterol and 27-hydroxycholesterol) were analyzed by isotope dilution using gas chromatography/mass spectroscopy (GC/MS) using well known and reproducible methods28. Reproducibility and reliability of oxysterol measures were determined using a random selection of 14 samples with measures repeated one month apart.
Total cholesterol, HDLc, and triglyceride concentrations were determined at the same time as oxysterols from the same stored plasma samples from 2008 by conventional enzymatic methods from fasting (12-hour) blood samples. LDLc levels were estimated by the Friedewald equation29. All oxysterol, apolipoprotein and lipid analyses were conducted at the Heinz Nutrition Laboratory in the Department of Epidemiology, University of Pittsburgh.
2.9 Statistical Analysis
The allelic frequency for each SNP was evaluated for Hardy-Weinberg Equilibrium (HWE), using chi-square tests. We examined the associations between AD-related SNPs and Aβ-status using exact chi-square tests. Individual p-values and odds ratios were calculated for each genotype using logistic regression holding the most common genotype as referent.
The distribution of each plasma biomarker was assessed using histograms. Markers with skewed distributions (e.g. plasma ApoE levels) were log transformed (logApoE). Correlations between continuous measures for biomarkers and Aβ deposition were assessed using Pearson correlation coefficients. Differences in plasma biomarker levels by Aβ-status were assessed: first, in unadjusted models, using t-test statistics; and second, as standardized correlation coefficients for the odds of being Aβ-positive derived from logistic regression models adjusted for age, gender and statins use. Thresholds and linearity of the relationships between Aβ status and plasma biomarkers were assessed by re-running logistic models after dividing biomarkers into quartiles of their individual distributions. Potential effect modification by statin use, cognitive status and gender was assessed using interaction terms between the individual biomarkers and potential effect modifiers. Models with potential interaction terms (defined as p<0.15) were then stratified.
This analysis has a large sample size for a PiB-PET study; however, it lacks the sufficient power to test multiple hypotheses and correct for multiple statistical comparisons. The study was intended to explore the associations between plasma lipids, genetics and amyloid deposition in the brain. Instead of relying on p-values that are intended for hypothesis testing, we present confidence intervals around mean difference.
3.0 Results
Of the 194 participants in the GEM Neuroimaging Sub-Study, 178 had plasma samples available at study close. More than half (55%, n=97) of this cohort of non-demented very elderly adults were Aβ-positive for amyloid deposition in the brain.Aβ-positivity was associated with high systolic BP, low global cognitive scores (mini-mental state exam <27), but not associated with having MCI (Table 1) as presented previously21. Approximately half (n=84) of all the participants in this sample took statins at during the GEM Study.
Table 1.
GEM study sample characteristics for participants with biomarkers and Aβ deposition (n=175).
|
Aβ Deposition
|
|||||
|---|---|---|---|---|---|
|
Aβ-positive (n = 97) |
Aβ-negative (n = 78) |
||||
| n | % | n | % | p-value | |
| Age | |||||
| <85 | 25 | 26% | 24 | 31% | 0.598 |
| 85-86 | 30 | 31% | 23 | 29% | 0.989 |
| 86-87 | 21 | 22% | 15 | 19% | 0.892 |
| >87 | 21 | 22% | 16 | 21% | reference |
| Women | |||||
| Men | 54 | 56% | 50 | 64% | 0.259 |
| Women | 43 | 44% | 28 | 36% | reference |
| SBP (mmHg) | |||||
| <120 | 14 | 14% | 21 | 27% | reference |
| 120-140 | 49 | 51% | 37 | 47% | 0.093 |
| >140 | 34 | 35% | 20 | 26% | 0.036 |
| MMSE | |||||
| <27 | 29 | 30% | 14 | 18% | 0.041 |
| ≥27 | 64 | 66% | 60 | 77% | reference |
| missing | 4 | 4% | 4 | 5% | -- |
| Diagnosis | |||||
| Normal | 71 | 73% | 66 | 85% | reference |
| MCI | 26 | 27% | 12 | 15% | 0.090 |
| Statins | |||||
| No | 49 | 51% | 42 | 54% | 0.694 |
| Yes | 48 | 49% | 36 | 46% | reference |
3.1 Lipids, lipoproteins and brain Aβ deposition
The mean concentrations of plasma biomarkers were not significantly different by Aβ-imaging status in the total group (Supplemental Table). However, being in the highest quartile of plasma ApoE levels resulted in a significantly lower odds of being Aβ-positive [OR(95%CI) = 0.47(0.23-0.96), p=0.03] after adjustment for age, gender and statin use. Individual levels of lipid biomarkers including cholesterol, clusterin and oxysterols were not associated with Aβ-status (data not shown).
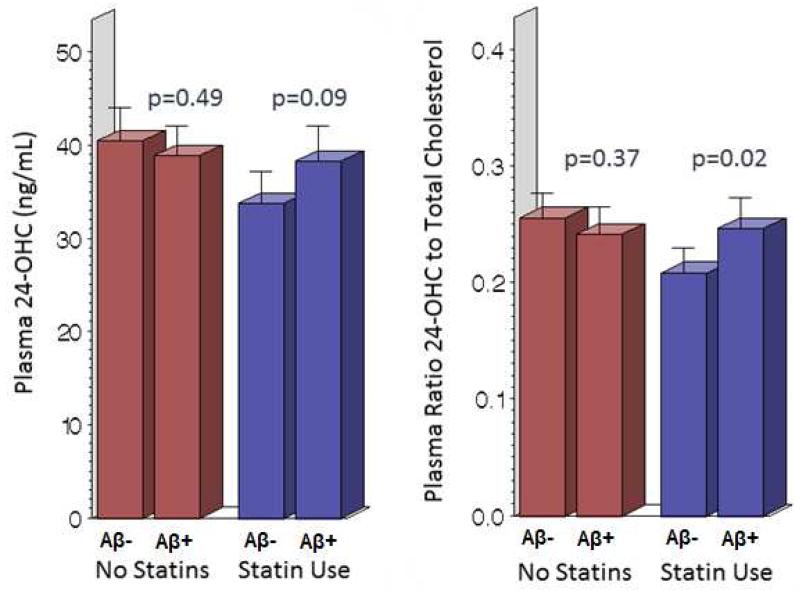
Statin use showed potential interactions with the ratio of 24OHC/Cholesterol (pinteraction=0.05), the ratio 27OHC/Chol (pinteraction=0.07) and plasma ApoE levels (pinteraction=0.09). In models stratified by statin use, the mean ratio 24OHC/Cholesterol (p=0.03) was significantly higher in Aβ-positive individuals relative to Aβ-negative individuals, among statin users. Differences in this ratio were likely a result of higher levels of 24OHC (figure 1) as cholesterol levels did not differ between the treatment groups. In models stratified by cognitive status (Table 2), Aβ-positive individuals with MCI only had significantly higher ratio of 24OHC/27OHC (p=0.04) and a slightly, yet not significantly higher ratio of 24OHC/Cholesterol (p=0.07).
Figure 1.
Plasma 24-hydroxycholesterol levels and Aβ-status stratified by statin user in the GEM Neuroimaging Sub-Study Participants (n=175). (Color figure not necessary; black and white is acceptable.)
Table 2.
Plasma biomarkers and Aβ-status by cognitive status.
| MCI |
Normal |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aβ-positive (n=25) |
Aβ-negative (n=12) |
Aβ-positive (n=71) |
Aβ-negative (n=66) |
|||||||
| mean | std | mean | std |
mean
difference (95% CL) |
mean | std | mean | std |
mean
difference (95% CL) |
|
| 24-OHC (ng/mL) | 40 | 11 | 35 | 12 | −5.53 (−13.57, 2.50) | 37 | 11 | 38 | 11 | 0.66 (−3.05, 4.38) |
| 27-OHC (ng/mL) | 168 | 42 | 175 | 42 | 7.43 (−22.68, 37.55) | 166 | 52 | 172 | 47 | 5.27 (−11.48, 22.02) |
| Cholesterol (mg/dL) | 153 | 27 | 163 | 38 | 9.81 (−12.12, 31.74) | 165 | 32 | 163 | 32 | −2.41 (−13.36, 8.54) |
| LDL (mg/dL) | 82 | 20 | 86 | 32 | 4.28 (−17.13, 25.68) | 88 | 28 | 89 | 29 | 0.43 (−9.23, 10.10) |
| HDL (mg/dL) | 46 | 10 | 44 | 12 | −1.87 (−9.52, 5.79) | 52 | 15 | 48 | 13 | −1.87 (−9.53, 5.79) |
| Triglycerides (mg/dL) | 129 | 56 | 166 | 62 | 36.99 (−4.44, 78.42) | 128 | 68 | 129 | 59 | 1.53 (−19.97, 23.04) |
| Clusterin (mg/dL) | 39 | 11 | 34 | 8 | −4.97 (−12.41, 2.47) | 38 | 10 | 38 | 8 | −0.32 (−3.41, 2.78) |
| Plasma ApoE (mg/dL) | 3.23 | 0.98 | 3.53 | 0.93 | 0.09 (−0.10, 0.27) | 3.29 | 0.86 | 3.42 | 0.84 | 0.04 (−0.04, 0.12) |
| ratio 24OHC/Chol | 0.27 | 0.09 | 0.22 | 0.07 | −0.05 (−0.11, 0.01) | 0.23 | 0.08 | 0.24 | 0.07 | 0.01 (−0.02, 0.03) |
| ratio 27OHC/Chol | 1.12 | 0.32 | 1.09 | 0.20 | −0.03 (−0.23, 0.18) | 1.04 | 0.38 | 1.09 | 0.37 | 0.05 (−0.08, 0.18) |
| ratio 24OHC/27OHC | 0.25 | 0.10 | 0.20 | 0.06 | −0.05 (−0.11, 0.00) | 0.24 | 0.09 | 0.23 | 0.09 | −0.01 (−0.04, 0.02) |
mean, standard deviation (std), between group mean differences and 95% confidence limits (CL) presented from t-tests.
3.2 Linear relationships between lipid biomarkers and brain Aβ deposition
The total amount of Aβ deposition in the brain was neither correlated with continuous measures of logApoE, nor with plasma lipids in the total group (all p>0.05). Among MCI participants, Aβ deposition was correlated with the ratio for 24OHC/27OHC (rho=0.38, p=0.01). The were no correlations between plasma biomarkers and Aβ deposition among MCI participants for 24OHC (rho=0.26, p=0.12) and 27OHC (rho=-0.24, p=0.15); Correlations were not significant for all plasma markers among cognitively normal individuals (p>0.43). Plasma ApoE levels did not differ by APOE genotype (mean±std: E4 carriers = 3.4±1.1 and non-E4 carriers = 3.3±0.8, p=0.88).
3.3 AD associated SNPs and brain Aβ deposition
We obtained genetic data on 11 SNPs previously identified with late onset AD from n=186 (n=96%) individuals with PiB-PET: APOE, EPHA, ABCA7, ARIB5B, BIN1, CD33, CD2AP, CLU, CR1, MS4A4A and PICALM. The CG genotype of ABCA7 (rs3752246) was present in 22% (n=38) of individuals in this study and was associated with more than a two-fold increase in the odds of being Aβ-positive [OR(95%CI) = 2.24(1.30-4.89)] relative to the CC genotype; however, no individuals with the GG genotype (total n=3) were Aβ-positive. Having one or more copies of C allele of EPHA1 (rs11767557) was associated with significantly lower odds of being Aβ-positive (Table 4). Having the APOE*4 allele was associated with a significantly higher odds of being Aβ-positive [OR(95%CI) = 7.47(2.75-20.26)]. In this study, only two participants were homozygous for the APOE*4 allele and one was Aβ-positive (Table 4). ARIB5B, BIN1, CD33, CD2AP, CLU, CR1, MS4A4A and PICALM were not associated with Aβ-status in this study.
Table 4.
Genes involved in cholesterol homeostasis with Aβ deposition.
|
Aβ Deposition
|
Unadjusted Odds of being Aβ-positive |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Aβ-positive (n = 99) |
Aβ-negative (n = 77) |
p-value | ||||||
| n | % | n | % | OR | 95% CI | |||
|
ABCA1 (rs2230806) |
||||||||
| CC | 58 | 59% | 37 | 48% | ref | ref | ||
| CT | 33 | 33% | 33 | 43% | 0.165 | 0.64 | 0.34 | 1.20 |
| TT | 8 | 8% | 7 | 9% | 0.572 | 0.73 | 0.24 | 2.18 |
|
ABCA7 (rs3752246) |
||||||||
| CC | 70 | 71% | 64 | 83% | ref | ref | ||
| CG | 27 | 27% | 11 | 14% | 0.042 | 2.24 | 1.03 | 4.89 |
| GG | 0 | 0% | 3 | 4% | -- | -- | -- | -- |
| EPHA1 (rs11767557 ) |
||||||||
| CC | 2 | 2% | 5 | 6% | 0.098 | 0.24 | 0.05 | 1.30 |
| TC | 24 | 24% | 31 | 40% | 0.023 | 0.47 | 0.24 | 0.90 |
| TT | 71 | 72% | 43 | 56% | ref | ref | ||
|
CLU (rs1532278) |
||||||||
| CC | 49 | 49% | 39 | 51% | ref | ref | ||
| CT | 35 | 35% | 28 | 36% | 0.988 | 1.00 | 0.52 | 1.91 |
| TT | 14 | 14% | 14 | 18% | 0.600 | 0.80 | 0.34 | 1.87 |
| APOE | ||||||||
| non- APOE*4 |
66 | 67% | 77 | 94% | ref | ref | ||
| APOE*4 | 32 | 33% | 5 | 6% | <0.001 | 7.47 | 2.75 | 20.26 |
4.0 Discussion
Aβ deposition is detected frequently among cognitively normal elderly adults. More than 55% of this very elderly sample was Aβ-positive. Our findings showed that Aβ-status differed little by demographic factors, but was significantly associated with blood pressure,global measures of cognition, and genetic and blood markers of cholesterol transport. The highest levels of apolipoprotein E were associated with Aβ-status in this cohort. The higher absolute levels of oxysterols and significantly higher ratios of 24OHC relative to (peripherally derived) 27OHC and cholesterol were associated with Aβ deposition among individuals with MCI and those taking statins; however, blood lipid levels were not associated with Aβ-status in either group. Genetic markers of cholesterol transport (e.g. ABCA7 and APOE4), as well as variation in EPHA1 were associated with Aβ-status; however other genetic markers previously associated with AD in GWAS studies were not associated with Aβ-status.
4.1 Plasma markers and amyloid pathology
Associations between Aβ deposition and markers of cholesterol transport extended to blood apolipoprotein levels. Plasma levels of apolipoproteins E and clusterin have previously shown associations with AD. Plasma clusterin shows associations with prevalent AD and severity of AD11; atrophy of the entorhinal cortex, baseline disease severity, and rapid clinical progression in AD12, but not with incident AD11. Plasma clusterin is also associated with longitudinal brain atrophy during mild cognitive impairment (MCI)13. However, higher circulating levels of clusterin were not significantly associated with the odds of being Aβ-positive in this study. In contrast, the highest quartile of plasma ApoE levels was significantly associated with lower odds of being Aβ-positive, suggesting that higher levels of blood ApoE may be protective for Aβ deposition in the brain. Previous studies suggest that plasma ApoE levels are correlated with APOE-410. In particular, ApoE levels were significantly lower among ε4 homozygous individuals30. In this study, we had only two homozygous individuals which limited our ability to directly confirm this relationship; however, we did not find correlations between plasma ApoE levels and APOE*4 carrier status, suggesting that factors other than genotype are important modulators of plasma ApoE levels in the circulation. Blood cholesterol levels appear to have little relevance to brain Aβ deposition.
Plasma levels of 24OHC have been associated with various neurodegenerative diseases14, including AD15, 16, VaD17 and MCI as well as brain volume in cognitively normal adults18. Cross-sectional studies of plasma 24OHC and AD are inconsistent; the direction of these associations may be dependent on stage of disease when 24OHC is measured 31. Recently, our group observed that plasma oxysterols concentrations are higher in cognitively normal individuals with evidence of cerebrovascular disease on MRI and also in individuals who went on to develop AD and MCI over 8 years of follow-up19. It is uncertain whether high plasma 24OHC levels is directly related to AD pathology, as part of normal physiological process of compensation to CNS injury, is a reflection of the BBB disruption, or both. Until now, the associations between 24OHC and AD pathology have not been directly assessed.
Statin use and cognitive status appear to modify the several relationships between Aβ deposition and markers of cholesterol homeostasis. In this group of no-demented elderly adults, statin use modified associations between Aβ deposition in the brain and blood levels of apolipoproteins, 24OHC, and the ratios of oxysterols to cholesterol. Cognitive status appears to be an important factor in the association between the brain-derived oxysterol, 24OHC and Aβ deposition in the brain. Specifically, weak positive correlations with Aβ deposition were evident for 24OHC and the ratio of 24OHC/27OHC among participants with MCI, while weak negative correlations suggested for 27OHC and ApoE levels. Yet, these correlations with Aβ deposition were only significant for the ratio of 24OHC/27OHC among participants with MCI. These results suggest that the levels of brain-derived cholesterol metabolites may be altered relative to peripherally-derived oxysterol and cholesterol levels in older adults with cognitive dysfunction and AD-like Aβ deposition. Further, a higher ratio of 24OHC/27OHC among individuals with MCI may represent changes in cholesterol metabolism occurring early in the cognitive impairment process and may be informative for individuals experiencing cognitive declines; however, it is uncertain if all of these individuals with MCI will progress to AD.
4.2 Genetic markers and amyloid pathology
As previously reported, the APOE*4 allele showed strong associations with Aβ deposition in the brain in this study9. We also examined several SNPs that were associated with cholesterol homeostasis, including: ABCA1, ABCA7 and CLU. ABCA1 and ABCA7 are membrane-associated proteins, encoded by this gene, which are members of the superfamily of ATP-binding cassette (ABC) transporters. With cholesterol as its substrate, these ATP-associated proteins function as a cholesterol efflux pump in the cellular lipid removal pathway. ABCA1 is the cholesterol efflux regulatory protein (CERP) transporter primarily, which acts a as a major regulator of cellular cholesterol and phospholipid homeostasis. We found that genetic variations in ABCA7 and EPHA1 were associated with Aβ deposition in the brain. The associations between Aβ deposition and ABCA7 were similar for MCI and cognitively normal individuals; while those for EPHA1 were only apparent for among cognitively normal individuals. The EPHA1 gene belongs to the ephrin receptor subfamily of the protein-tyrosine kinase family and encodes for the ephrin type-A receptor 1 protein which has been implicated in mediating nervous system developmental events. Having one or more copies of the minor C allele at SNP (rs11767557) on EPHA1 was associated with a significantly lowers odds of being Aβ-positive. This result was consistent with the findings from two consortium GWAS studies that showed the minor C allele for this SNP (rs11767557) to be protective for late onset AD7 and having the minor A allele in another SNP (rs11771145) on EPHA1 was also associated with a lower odds of AD8.
Variation in CLU, the gene encoding for clusterin (apolipoprotein J) was not associated with Aβ deposition in this group. Taken together these SNPs associated with Aβ deposition may be involved in cholesterol transport, clearance and repair following damage to the nervous system. It is important to point out the genetic associations described here, except those for the APOE*4 allele, should be interpreted with caution due to the relatively small number cases assessed in this study.
5.0 Conclusions
A subset of plasma and genetic factors relating to cholesterol transport were associated with Aβ deposition in the brain of non-demented elderly adults. Concurrent lipid measures and oxysterol cholesterol metabolites do not appear to be related to Aβ deposition in cognitively normal individuals; however, the brain derived oxysterol, 24OHC may be a relevant biomarker for Aβ deposition for older adults with MCI and those taking lipid lowering medications. While late-life circulating blood cholesterol has little relevance to ongoing AD pathology, genetic and blood markers of cholesterol transport are associated with Aβ deposition in the brain. This may be because the lifelong effects of elevated cholesterol are governed by the genetic markers, and may not be reflected in cross-sectional studiesof late-life cholesterol levels which may be more related to the health status of the individual. Factors related to cholesterol transport in the brain may be potential targets for the prevention of Aβ deposition and dementia, and may cast light on the nature of brain responses to the neurodegenerative processes - responses that relate to endogenous neuroprotection and what we currently describe as brain reserve; they may also explain much of the highly variable course of cognitive decline.
Supplementary Material
Table 3.
Correlations between plasma biomarkers and Aβ deposition by cognitive status.
| MCI | Normal | |||
|---|---|---|---|---|
| Rho | p-value | Rho | p-value | |
| 24OHC | 0.256 | 0.116 | −0.066 | 0.432 |
| 27OHC | −0.237 | 0.146 | −0.033 | 0.693 |
| Ratio 24/27 | 0.388 | 0.015 | −0.030 | 0.719 |
| Cholesterol | −0.127 | 0.454 | −0.017 | 0.846 |
| ApoE | −0.254 | 0.130 | −0.069 | 0.426 |
| Clusterin | −0.079 | 0.640 | −0.006 | 0.942 |
ACKNOWLEDGEMENTS
We thank Ms. B. Hauth, Ms. A. Shaw and Ms. R. de la Vega for expert laboratory analyses.
SOURCES OF FUNDING
Supported by The National Institutes of Health grants: P50 AG005133, R37 AG025516, P01 AG025204, AG030653 and AG041718 and a NIA T32 postdoctoral training grant (T32 AG000181, recipient: TM Hughes).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENTS
T.M. Hughes, L.H. Kuller, R.W. Evans, S. DeKosky, J.D. Williamson, J.C. Price, B. Snitz, A.C. Cohen and I.M. Kamboh report no disclosures. W.E. Klunk is a coinventor of PiB, a technology described in this manuscript. As such, he has a financial interest in the license agreement, which GE Healthcare holds with the University of Pittsburgh. He has served as consultant to GE Healthcare, Janssen, Pfizer, Lilly, AstraZeneca, Wyeth, Roche, and Elan. C.A. Mathis is a coinventor of PiB, a technology described in this manuscript. As such, he has a financial interest in the license agreement, which GE Healthcare holds with the University of Pittsburgh. He has served as a consultant for GE Healthcare, Elan/Wyeth, Novartis, Janssen, Genzyme, Pfizer, Bristol Myers Squibb, IBA, and Baxter Bioscience. O.L. Lopez has served as a consultant for Lilly, Lundbeck, Merz, Lilly, and Baxter.
References
- 1.Di Paolo G, Kim T-W. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuinness B, O’Hare J, Craig D, Bullock R, Malouf R, Passmore P. Statins for the treatment of dementia. Cochrane Database Syst Rev. 2010;8:CD007514. doi: 10.1002/14651858.CD007514.pub2. [DOI] [PubMed] [Google Scholar]
- 3.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2009;2:CD003160. doi: 10.1002/14651858.CD003160.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. Journal of internal medicine. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 5.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leoni V, Solomon A, Kivipelto M. Links between ApoE, brain cholesterol metabolism, tau and amyloid beta-peptide in patients with cognitive impairment. Biochem Soc Trans. 2010;38:1021–1025. doi: 10.1042/BST0381021. [DOI] [PubMed] [Google Scholar]
- 10.Kiddle SJ, Thambisetty M, Simmons A, et al. Plasma based markers of [11C] PiB-PET brain amyloid burden. PLoS One. 2012;7:e44260. doi: 10.1371/journal.pone.0044260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrijvers EM, Koudstaal PJ, Hofman A, Breteler MM. Plasma clusterin and the risk of Alzheimer disease. JAMA. 2011;305:1322–1326. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- 12.Thambisetty M, Simmons A, Velayudhan L, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thambisetty M, An Y, Kinsey A, et al. Plasma clusterin concentration is associated with longitudinal brain atrophy in mild cognitive impairment. Neuroimage. 2012;59:212–217. doi: 10.1016/j.neuroimage.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leoni V. Oxysterols as markers of neurological disease - a review. Scan J Clin Lab Investigation. 2009;69:22–25. doi: 10.1080/00365510802651858. [DOI] [PubMed] [Google Scholar]
- 15.Bretillon L, Siden A, Wahlund L, et al. Plasma levels of 24S-hydroxycholesterol in patients with neurological diseases. Neurosci Lett. 2000;293:87–90. doi: 10.1016/s0304-3940(00)01466-x. [DOI] [PubMed] [Google Scholar]
- 16.Papassotiropoulus A, Lutjohann D, Bagli M, et al. Plasma 24S-hydroxycholesterol: a peripheral indicator of neuronal degeneration and potential state marker for Alzheimer’s disease. NeuroReport. 2000;26:1959–1962. doi: 10.1097/00001756-200006260-00030. [DOI] [PubMed] [Google Scholar]
- 17.Lutjohann D, Papasotiropoulus A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular dementia patients. J Lipid Res. 2000;41:195–198. [PubMed] [Google Scholar]
- 18.Solomon A, Leoni V, Kivipelto M, et al. Plasma levels of 24S-hydroxycholesterol reflect brain volumes in patients without objective cognitive impairments but not with Alzheimer’s disease. Neurosci Lett. 2009;462:89–93. doi: 10.1016/j.neulet.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 19.Hughes T, Kuller L, Lopez O, et al. Markers of cholesterol metabolism in the brain show stronger associations with cerebrovascular disease than Alzheimer’s disease. J Alzheimers Dis. 2012;30:53–61. doi: 10.3233/JAD-2012-111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeKosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27:238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Mathis C, Kuller L, Klunk W, et al. In vivo assessment of amyloid-β deposition in non-demented very elderly subjects. Annals Neurology. 2013 doi: 10.1002/ana.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price J, Klunk W, Lopresti B, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 23.Aizenstein H, Nebes R, Saxton J, Price J, Mathis C, Tsopelas JA. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen AD, Mowrey W, Weissfeld LA, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–215. doi: 10.1016/j.neuroimage.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Archives of general psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 26.Snitz B, O’Meara E, Carlson M, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 28.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterols oxidation products in human plasma by isotope dilution-mass spectroscopy. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Gupta VB, Laws SM, Villemagne VL, et al. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011;76:1091–1098. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 31.Hughes TM, Rosano C, Evans RW, Kuller LH. Brain Cholesterol Metabolism, Oxysterols, and Dementia. J Alzheimers Dis. 2013;33:891–911. doi: 10.3233/JAD-2012-121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.