Abstract
As the pathophysiology of acute myelogenous leukemia (AML) involves a block of myeloid maturation, a desirable therapeutic strategy is to induce leukemic cell maturation to increase the efficacy and to avoid the side effects of traditional chemotherapeutics. Through a compound library screen, 6-benzylthioinosine (6BT) was identified as a promising differentiation-inducing agent. 6BT induces monocytic differentiation of myeloid leukemia cell lines such as HL-60 and OCI-AML3, as well as primary patient samples as evidenced by morphology, immunophenotyping, and nitroblue tetrazolium reduction. Not only can 6BT induce differentiation but a subset of AML cell lines such as MV4-11 and HNT34 instead undergo 6BT-mediated cell death. Despite inducing cell death in some leukemic cells, 6BT exhibits extremely low toxicity on several nonmalignant cells such as fibroblasts, normal bone marrow, and endothelial cells. This toxicity profile may relate to the function of 6BT as an inhibitor of the nucleoside transporter, ent1, which is thought to prevent it from entering many cell types. In contrast, 6BT likely enters at least some leukemic cell lines as shown by its requirement for phosphorylation for its differentiation activity. 6BT is also able to synergize with currently used myeloid differentiation agents such as ATRA and decitabine. Early studies indicate that the mechanism of action of this compound may involve ATP depletion that leads to growth inhibition and subsequent differentiation. Besides in vitro activity, 6BT also shows the ability to impair HL-60 and MV4-11 tumor growth in nude mice. 6BT is a promising new monocytic differentiation agent with apparent leukemic cell–specific activity.
Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults with >13,000 cases expected in the United States this year (1).7 Despite advances in treatment, the 5-year survival is still only 33% in patients under the age of 65 years and 4% in patients >65 years (2).7 The poor efficacy of the current AML therapeutics may be due to the pathophysiology of AML as it is characterized by the arrest of differentiation of immature myeloid cells and rapidly dividing cells. Unfortunately, the current AML chemotherapeutics such as cytarabine and idarubicin primarily target the highly proliferative cells.
In contrast to the poor prognosis for the majority of patients with AML, the use of differentiation therapy for a rare subtype of AML, acute promyelocytic leukemia (APL; 5–10% of AML), illustrates the promise of differentiation therapy for AML. In APL, the use of the differentiation–inducing agent all-trans retinoic acid (ATRA) has revolutionized the treatment of APL and leads to the long-term survival and presumed cure of 75% to 85% of patients (3). By inducing myeloid leukemia cells to terminally differentiate, ATRA is able to successfully treat APL in combination with other chemotherapeutic agents without additional cytotoxicity. The use of ATRA is especially advantageous in the elderly as this population often cannot tolerate induction chemotherapy. By using therapies that include ATRA and low-dose chemotherapy, the elderly can achieve long-term survival. ATRA illustrates the great potential of differentiation-inducing agents for other subtypes of AML as they may provide comparable or greater efficacy and less toxicity than the currently used chemotherapeutics.
Although a wide range of myeloid differentiation-inducing compounds have been reported, few of them have shown clinical efficacy in treating AML. The most commonly used agents, ATRA and arsenic trioxide, have been found to be clinically useful only for AML patients with the rare APL subtype. In an attempt to discover potential clinically useful differentiation-inducing compounds, a cell-based, small compound library screen was performed that was intentionally biased to select relatively nontoxic compounds with high activity. We identified one particularly promising novel compound, 6-benzylthioinosine (6BT) through this screen. In this article, we show that not only does 6BT exhibit high myeloid differentiation-inducing activity in at least a subset of leukemic cells, but its putative mechanisms of action also highlight specific pathways that may be exploited in developing future leukemia therapeutics. Further the identification of differentiation-inducing compounds such as 6BT illustrates the potential of the devised compound library screen in identifying novel differentiation compounds with potential clinical utility.
Materials and Methods
Chemicals
1,25 dihydroxyvitamin D3, ATRA, Nitroblue tetrazolium (NBT), Nitrobenzylthioinosine, iodotubericin, and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma. 6BT was kindly provided by the National Cancer Institute (NCI) Developmental Therapeutics Program.
Cell lines
HL-60 and MV-411 cells were obtained from American Type Culture Collection. OCI-AML3, HNT-34, and OCIM2 cells were obtained from DSMZ. Patient samples were obtained from the stem cell core facility at University Hospitals Case Medical Center. MEF and human umbilical vascular endothelial cells (HUVEC) were kindly provided from the laboratory of Dr. Mary Laughlin (Case Western Reserve University, Cleveland, OH). Cells were cultured in Iscove’s modified Dulbecco’s medium (Invitrogen) except for MEF (DMEM; Invitrogen) and HUVEC (EGM Lonza). Medium was supplemented with 10% fetal bovine serum (FBS; Invitrogen), penicillin G (100 μg/mL), and streptomycin (100 μg/mL). Mononuclear cells were separated by Ficoll-Hypaque (Sigma) density-gradient centrifugation, and for selected patients, leukemic myeloblasts were isolated by flow sorting after staining with anti-CD34 PE (Becton Dickinson).
Cell proliferation and viability
Cell proliferation was assessed by manual counting cells with a hematocytometer. Cell viability was assessed by the trypan blue dye exclusion method.
Compound library screen
For screening, 100 μL of HL-60 cells (5 × 105 cells/mL) were cultured in 96-well plates. Cells were treated with 10 μmol/L of compounds from the following libraries: Lopac (Sigma), Prestwick (Prestwick Chemical), and NCI diversity collection. DMSO (0.1%) and ATRA (1 μmol/L) were used as negative and positive controls, respectively. After 5 d, the differentiation of the cells was assessed by the NBT reduction assay. To perform the NBT assay, 20 μL of a solution of NBT (5 mg/mL) and PMA 100 ng/mL (as a stimulant of the respiratory burst) were added to the HL-60 cells. The cells were incubated at 37°C for 30 min and the color of the wells was assessed. Those wells that displayed a visual color change from the production of blue insoluble formazan greater than the negative control were examined microscopically to determine the percentage of blue cells. At least 200 cells were counted for each positive well.
Differentiation
NBT reduction was performed in a similar manner as described for the compound library screen. Immunophenotyping was performed by costaining cells with CD11b-PE and CD14-FITC (Becton Dickinson). The stained samples were run on a Beckman Coulter Cytomics FC 500 cytometer. For morphology assessment, cytospin preparations were made using a Shandon cytospin3 cytocentrifuge, and the slides were stained with a modified wright-giemsa stain.
Soft agar clonogenic assay
HL-60 cells (2 × 105 cells/mL) were treated with 6BT or vehicle for 3 d. The cells were washed twice with PBS, and 3,000 cells were dissolved in 3 mL of 0.35% Soft Agar (Noble Agar; Sigma) supplemented with 20% FBS. The cells were incubated for 10 d in 37°C in a 5% CO2 incubator. After 10 d, the colonies were counted under a light microscope.
Cell cycle analysis
HL-60 cells were treated with 6BT or vehicle for 3 d. Cells were fixed overnight at −20°C in methanol. Cells were washed in PBS, treated with RNase A (final concentration, 0.5 μg/mL; Sigma), and stained with propidium iodide (50 μg/mL). The cells were kept at 4°C for 30 to 60 min and analyzed by flow cytometry.
Real-time PCR
Total RNA was isolated from HL-60 cells treated with 6BT or vehicle at indicated time points, using TRIzol reagent (Invitrogen). RNA was transcribed into cDNA using the Enhanced Avian RT First Strand Synthesis kit (Sigma). Relative quantitative PCR was performed in triplicate using the FastStart SYBR Green Master (Roche Diagnostics) on an Applied Biosystems 7500 Fast Real-TimePCR System. The primers used were CEBP/β5′ primer, 5′-GAACAGCAACGAGTACCGGGTG-3′, and 3′ primer, 5′-CCCATGGCCTTGACCAAGGAG-3′; β-actin 5′primer, 5′-GGACTTCGAGCAAGAGATGG-3′, and 3′primer, 5′-AGCACTGTGTTGGCGTACAG-3′; c-myc 5′ primer, 5′-GCCACGTCTCCACACATCAG-3′, and 3′ primer, 5′-TCTTGGCAGCAGGATAGTCCTT-3′. Thermal conditions of the system were as follows: 95°C for 5 min, 40 cycles at 95°C for 15 s, 55°C for 45 s, and 72°C for 1 min.
Western blot analysis
Western blot analysis was performed with p21 (Cell Signaling), bcl-2 (Santa Cruz biotechnology), and β-actin antibodies (Sigma). Cells were treated with 6BT or vehicle for the indicated times and washed in PBS. Cells were centrifuged and lysed with a Triton containing lysis buffer. Protein lysates (80 μg per lane) were electrophoresed on 10% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Millipore) using a semidry transfer apparatus (Bio-Rad). The membranes were blocked, incubated with the indicated primary antibodies at 4°C overnight, and then the appropriate horseradish peroxidase–conjugated secondary antibody. Protein bands were visualized by autoradiography after incubation with enhanced chemiluminescence reagent (Pierce).
Mouse xenograft
Six-wk-old male nude mice were injected bilaterally s.c. with 5 × 106 HL-60 or MV-411 cells. Drug treatment was started 10 d or 1 d after tumor cell injection for the established tumor or tumor formation models, respectively. Palpable tumors were present for the established tumor model before initiating drug treatment. 6BT or vehicle (150 mg/kg) was injected i.p. daily for 5 d and then 3 d a week for 2 wk for both model systems. Vehicle consisted of 20 μL of DMSO and 80 μL of peanut oil. The Case Western Reserve University Animal Research Committee approved all of the animal protocols used in this study.
ATP measurement
Cells were treated with vehicle or 6BT for the indicated times, and the relative ATP concentration was measured using the ATPlite 1step kit (Perkin-Elmer) according to the manufacturers recommendations. Briefly, after the indicated treatment time cells were washed in PBS and resuspended in RPMI at a concentration of 3 × 103 viable (trypan blue negative) cells per mL. Cells (100 μL) were mixed with 100 μL of ATPlite 1step reagent in a 96-well plate, and the luminescence was measured using a Spectramax L luminometer (Molecular Devices).
Results
Discovery of novel compounds
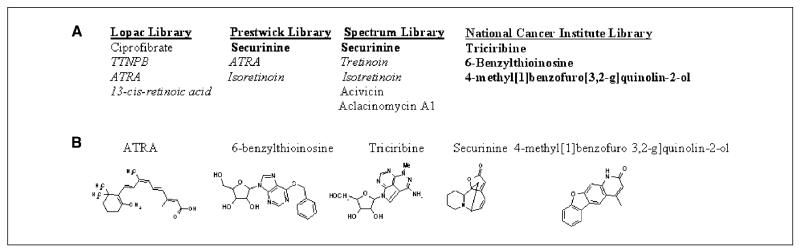
To discover clinically useful differentiation-inducing agents, we screened ~6,000 compounds taken from several small compound collections. The phenotypic screen assessed differentiation by measuring the functional maturation of HL-60 leukemic cells by the NBT reduction assay, a test that is highly specific to and has been used extensively as a measure of functional myelomonocytic differentiation (4-8). The NBT reduction test measures the ability of cells to generate a respiratory burst, a function that is only present in differentiated cells. From this screen, 4 novel compounds (as well as 8 known compounds), with at least 75% of the NBT reduction activity of the positive control, ATRA, were identified (Fig. 1A). All four novel compounds exhibit chemical structures unrelated to ATRA (Fig. 1B).
Figure 1.
Identification of novel myeloid-differentiation inducing agents. A, list of novel differentiation-inducing compounds identified. Note novel differentiation-inducing compounds are in bold and retinoic acid related compounds are in italics. Securinine was identified from two libraries. B, novel differentiation-inducing compounds exhibit structures unrelated to ATRA.
6BT can induce myeloid differentiation
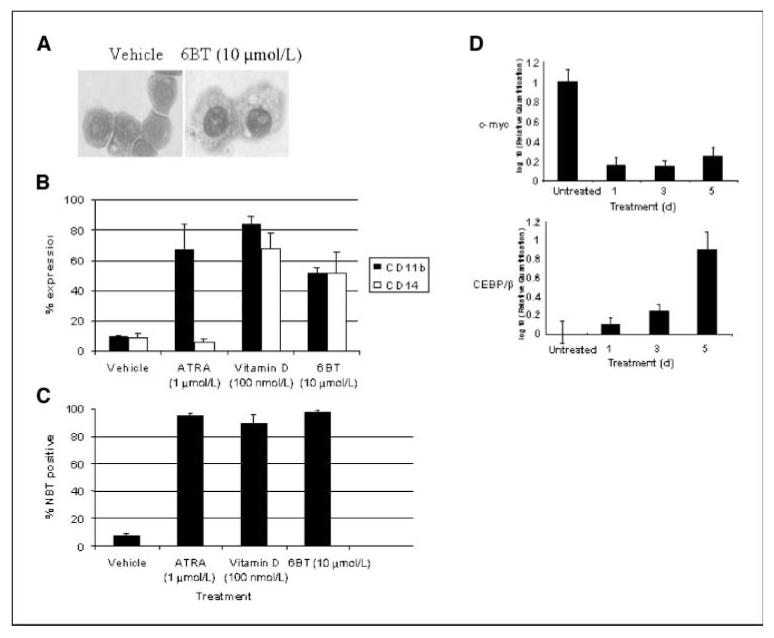
One compound in particular, 6BT, exhibits clinical potential. 6BT induces differentiation of HL-60 myeloid leukemia cells as shown by morphology, immunophenotyping, and NBT reduction (Fig. 2). Clear morphologic monocyte/macrophage differentiation is shown by such features as condensed nuclei, abundant cytoplasm, and vacuoles (Fig. 2A). In addition, 6BT induces cells to adhere to the tissue culture plate. Immunophenotyping with CD11b, which is upregulated during myelomonocytic differentiation, and CD14, which is up-regulated primarily during monocytic differentiation, further confirms the differentiation pathway. ATRA, which induces granulocytic differentiation, induces primarily CD11b expression, whereas 6BT and vitamin D3, which induce monocytic differentiation, lead to up-regulation of both CD11b and CD14 expression (Fig. 2B). 6BT exhibits similar differentiation-inducing activity to the highly active differentiation agents ATRA and vitamin D3 as measured by NBT reduction with 6BT differentiating 97% ± 1.9%, ATRA differentiating 95% ± 1.7%, and Vitamin D3 differentiating 91% ± 4.6% of HL-60 cells (Fig. 2C).
Figure 2.
6BT induces monocyte/macophage differentiation of HL-60 cells. A, 6BT induces morphologic changes consistent with monocyte/macrophage differentiation. After treatment for 4 d with 6BT, cytospin preparations were prepared and the cells were stained with wright-giemsa stain. Magnification, ×40. B, 6BT induces immunophenotypic changes consistent with monocytic differentiation. After treatment for 4 d, HL-60 cells were stained with CD11b-PE and CD14-FITC, and flow cytometric analysis was performed. Results are an average of three independent experiments. C, 6BT induces NBT reduction activity consistent with myelomonocytic differentiation. HL-60 cells were treated with the indicated compounds for 4 d and then the NBT reduction assay was performed. The percentage of NBT-positive cells was calculated by counting at least 200 cells under a light microscope. Results are an average of three independent experiments. D, 6BT induces the monocytic transcription factor CEBP/β and down-regulates the transcription factor c-myc. HL-60 cells were treated for the indicated number of days with vehicle or 10 μmol/L 6BT. The relative expression of CEBP/β and c-myc was determined by real-time PCR.
To further examine the ability of 6BT to induce myeloid differentiation, its effects on the transcription factors CEBP/β and c-myc that are regulated during myeloid differentiation were examined (Fig. 2D). Consistent with monocytic differentiation, the expression of the monocytic transcription factor CEBP/β was upregulated almost 10-fold compared with vehicle-treated cells at 5 days after treatment with 6BT. Also supportive of the induction of myeloid differentiation, the expression of the proliferation-related transcription factor c-myc is rapidly down-regulated after 6BT treatment.
6BT induces terminal differentiation
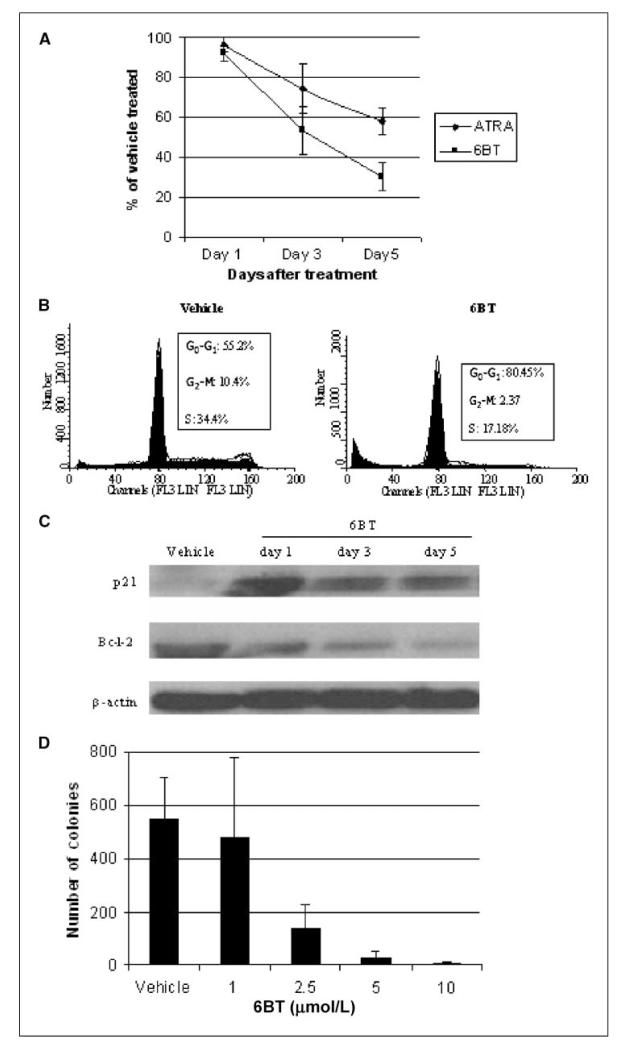
To assess the ability of 6BT to affect leukemic cell growth, its effects on HL-60 cell proliferation were measured. 6BT led to a significant inhibition of HL-60 cell growth compared with the vehicle control. At the doses used, 6BT induces even more growth inhibition than ATRA (30% ± 7.1% versus 58% ± 6.5% of vehicle-treated cells; Fig. 3A). To determine the mechanism of growth inhibition, cell cycle analysis was performed. 6BT growth inhibition involves accumulation of cells in the G0-G1 phase of the cell cycle with >80% of cells present in G0-G1 compared with 55% in the vehicle control at 72 hours after treatment (Fig. 3B). Consistent with accumulation in the G0-G1 phase, 6BT induces p21 that plays a critical role in preventing the G1 to S transition by inhibiting the cyclinD–cyclin-dependent kinase 4/6 complex and down-regulates bcl-2 that among other functions is involved in leukemic cell proliferation (Fig. 3C). To assess whether the observed 6BT-mediated growth inhibition and differentiation results in terminal differentiation, colony forming assays were performed in soft agar. Limited exposure (72 hours) of HL-60 cells to 6BT at doses of 5 and 10 μmol/L almost completely prevented colony growth in soft agar, demonstrating that terminal differentiation can be induced at low noncytotoxic concentrations (Fig. 3D).
Figure 3.
6BT has growth inhibitory effects on HL-60 cells. A, 6BT inhibits the proliferation of HL-60 cells. Cells were treated with the indicated compounds for 5 d, and the number of cells present at specific time points was assessed by counting at least 200 cells with a hematocytometer. Results shown represent the number of 6BT-treated cells present divided by the number of vehicle-treated cells at specific time points and are an average of three independent experiments. B, 6BT induces alterations of the cell cycle. HL-60 cells were treated with the indicated compounds for 3 d, and the cells were stained with propidium iodide and analyzed by flow cytometry. Results are representative of three independent experiments. C, 6BT leads to the up-regulation of p21 and down-regulation of Bcl-2. HL-60 cells were treated with the indicated compounds for 1, 3, or 5 d, and Western blot analysis was performed on the same membrane with p21, Bcl-2, and β-actin antibodies. D, 6BT inhibits colony formation in soft agar. HL-60 cells were incubated with 6BT or vehicle for 72 h, and the drug was washed off. An equal number of viable cells were added to soft agar, and colony formation was assessed after 10 d. Results are an average of two independent experiments performed in duplicate.
6BT induces differentiation or cell death in multiple leukemic cell lines and primary patient samples
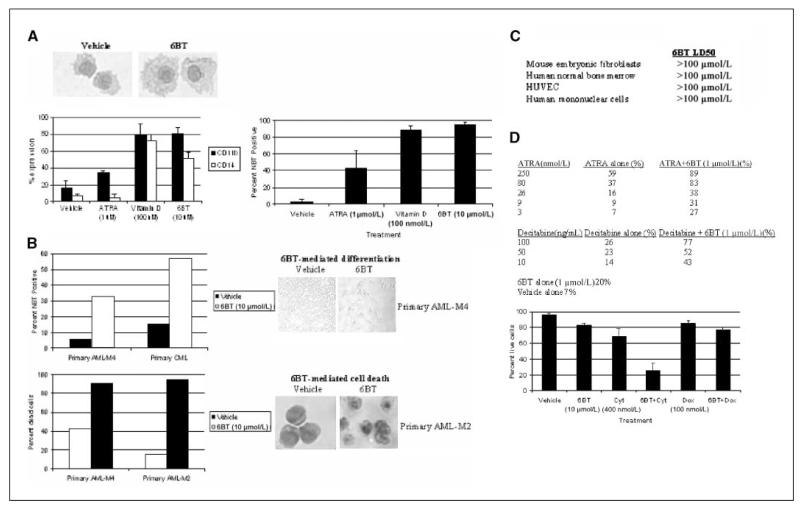
In addition to HL-60 cells (AML-M2), we assessed the ability of 6BT to induce myeloid differentiation in leukemic cells representing different AML subtypes including OCI-AML3 (AML-M4), HNT34 (AML-M4), MV-411 (AML-M5), and OCIM2 (AML-M6) cells as well as several primary leukemic patient samples. Similar to HL-60 cells, OCI-AML3 cells underwent monocyte/macrophage differentiation in response to 6BT as measured by morphology, NBT reduction, and immunophenotyping (Fig. 4A). Both CD11b and CD14 were significantly up-regulated in OCI-AML3 cells, suggesting monocytic differentiation. In contrast, ATRA treatment leads to granulocytic differentiation and only up-regulates CD11b, whereas Vitamin D3 also leads to monocytic differentiation.
Figure 4.
6BT has differentiation and specific cell death activity in multiple AML cell lines and patient samples, and can synergize with clinically used therapeutics. A, 6BT induces monocytic differentiation of OCI-AML3 cells. Cells were treated with 6BT for 4 d and assessed for differentiation by morphology, immunophenotyping, and NBT reduction as described in Fig. 2. B, 6BT can induce differentiation or cell death in primary patient samples. Primary leukemic blasts were purified by flow sorting after staining with CD34-PE. Cells were treated with 6BT (10 μmol/L) for up to 10 d and assessed for differentiation by morphology and NBT reduction and cell death by trypan blue dye exclusion as described in Fig. 2. An exception is that the photograph of the primary AML-M4 patient sample was unstained and taken directly from the tissue culture plate. C, 6BT exhibits low in vitro toxicity. The indicated cell lines were treated with up to 100 Am of 6BT for 5 d, and the LD50 was determined. Results represent three independent experiments. D, 6BT can synergize in HL-60 cells with ATRA, decitabine, and cytarabine. To assess ATRA and decitabine synergy, HL-60 cells were treated with the indicated compounds for 4 d, and the NBT reduction assay was performed as described in Fig. 2. Results are representative of three independent experiments. To assess cytarabine synergy, cells were treated with the indicated compounds for 72 h and viable cells were assessed by trypan blue staining. Results are an average of three independent experiments. Cyt, cytarabine; dox, doxorubicin.
Surprisingly, in contrast to OCI-AML3 cells, both HNT34 and MV-411 cells undergo cell death to low concentrations of 6BT with an LD50 at 5 days of 0.5 and 2 μmol/L for these cell lines, respectively. In contrast, the LD50 at 5 days for OCIAML3 and HL-60 cells is >100 and 30 μmol/L, respectively. Subtoxic concentrations of 6BT do not induce evidence of differentiation in HNT34 or MV-411 cells (data not shown). The OCIM2 cells did not respond to up to 30 μmol/L of 6BT by differentiation or cell death (data not shown). Besides leukemic cell lines, 6BT can also induce myeloid differentiation and, in some cases, cell death of primary leukemic patient samples. Of five leukemic samples tested, two showed evidence of differentiation (AML-M4 and chronic myelogenous leukemia), two different patient samples showed 6BT-mediated cell death (AML-M2 and AML-M4), and one sample seemed nonresponsive (AML-M5; Fig. 4B). One of the two patient samples that exhibited 6BT-mediated differentiation is from a patient with the closely related myeloid disorder, chronic myeloid leukemia in blast crisis, indicating the possible utility of 6BT in this disease as well. The other patient sample is derived from a patient with AML-M4 with relapsed leukemia. This patient sample let to dramatic morphologic changes characterized by attachment of the vast majority of leukemic cells to the tissue culture plate as well as an increase in NBT reduction activity (Fig. 4B). Both patient samples that exhibited cell death showed >90% dead cells as shown by morphology and trypan blue dye exclusion after 6BT treatment (Fig. 4B).
As 6BT induces differentiation activity and/or cell death against leukemic cell lines and patient samples at low micromolar concentrations, the potential toxicity of 6BT against nonmalignant cell types was assessed. Of particular note, the LD50 of 6BT on normal human bone marrow cells is >100 μmol/L, which was the highest dose tested. Similarly, the LD50 was >100 μmol/L for the other cell types examined including human umbilical vein endothelial cells, mouse embryonic fibroblasts, and human mononuclear cells (Fig. 4C).
6BT synergizes with other chemotherapy agents in treating leukemic cells
We also investigated if 6BT has synergistic effects on leukemic cells in combination with agents that have been clinically used in the treatment of AML. 6BT can synergize with decitabine (a hypomethylating agent used for myelodysplastic syndrome and AML) and low doses of ATRA to induce differentiation, indicating these compounds likely work through different pathways (Fig. 4D). Cotreatment of HL-60 cells with a low dose of 6BT (1 μmol/L) led to an increase in NBT reduction from 20% with 6BT alone to 77% with decitabine (100 ng/mL) and 89% with ATRA (250 nmol/L).
In addition, 6BT was tested for its ability to enhance cytarabine-mediated cell death. The currently used AML chemotherapeutic, cytarabine, is a nucleoside analogue known to primarily use the ent1 nucleoside transporter to enter and exit cells (9). As 6BT is a potent ent1 inhibitor, the ability of 6BT to inhibit or synergize with cytarabine-mediated leukemia cell killing was assessed. 6BT was found to significantly augment cytarabine-mediated HL-60 cell death. After 48 hours of treatment with cytarbine alone (400 nmol/L), 68.8% ± 9.9% of cells were alive compared with only 26.6% ± 8.8% after cotreatment with 6BT. Another chemotherapeutic, doxorubicin, which does not depend upon ent1, did not show synergy with 6BT (Fig. 4D). This result reveals the potential that ent1 may be a desirable drug target for combination therapies with cytarabine or other nucleoside analogues that primarily use ent1.
6BT shows high activity in mouse xenograft experiments
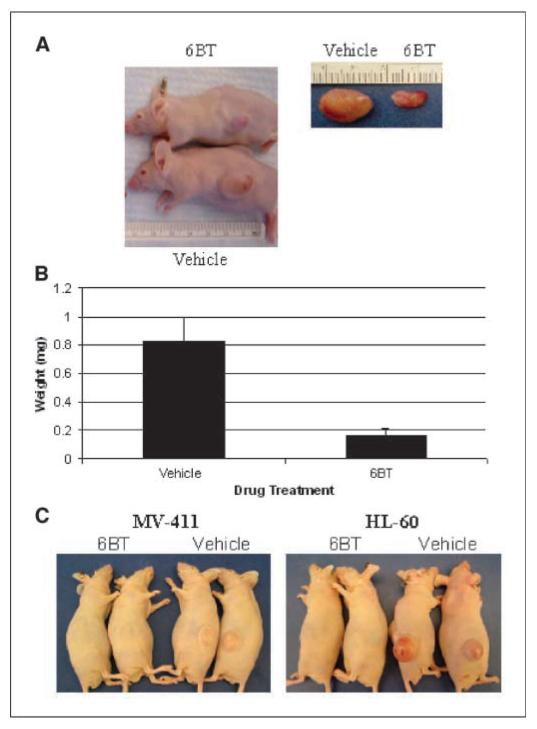
Mouse AML xenograft models were used to determine the in vivo activity of 6BT. First, 6BT was assessed for its ability to inhibit the growth of established HL-60 s.c. tumors in nude mice. In the established tumor model, tumors were significantly smaller after 6BT treatment (150 mg/kg) compared with vehicle-treated mice (0.16 grams ± 0.05 grams versus 0.73 grams ± 0.28 grams; Fig. 5A-B).
Figure 5.
6BT has in vivo activity in both tumor progression and tumor formation models. A, 6BT inhibits HL-60 cell tumor progression. Representative picture of the mice and dissected tumors at the end of the study period. B, 6BT-treated mice exhibit significantly smaller tumors in the tumor progression model. Results shown represent the average weight of tumors dissected from the mice at the end of the study period. C, 6BT completely prevents MV4-11 and HL-60 tumor formation. Representative pictures are shown after the mice were sacrificed at 4 wk after tumor cell inoculation.
Flow cytometric analysis of the dissected tumors (n = 3) at the end of the study period shows evidence of the induction of the mature myeloid marker CD11b (27.7% ± 1.5% in the vehicle and 63.3% ± 6.4% in the 6BT-treated tumors). Surprisingly, there was no difference in CD14 expression between the tumors in the 6BT and vehicle mice.
In the second model system, the ability of 6BT to prevent either HL-60 or MV-411 tumor formation in nude mice was assessed. In this model, 6BT treatment initiated 1 day after leukemic cell inoculation completely prevented tumor formation of both leukemic cell types in all five mice tested for each cell line with bilateral tumor cell inoculations (Fig. 5C). Therefore, as a single agent, 6BT exhibits in vivo activity.
Phosphorylation of 6BT is required for differentiation induction
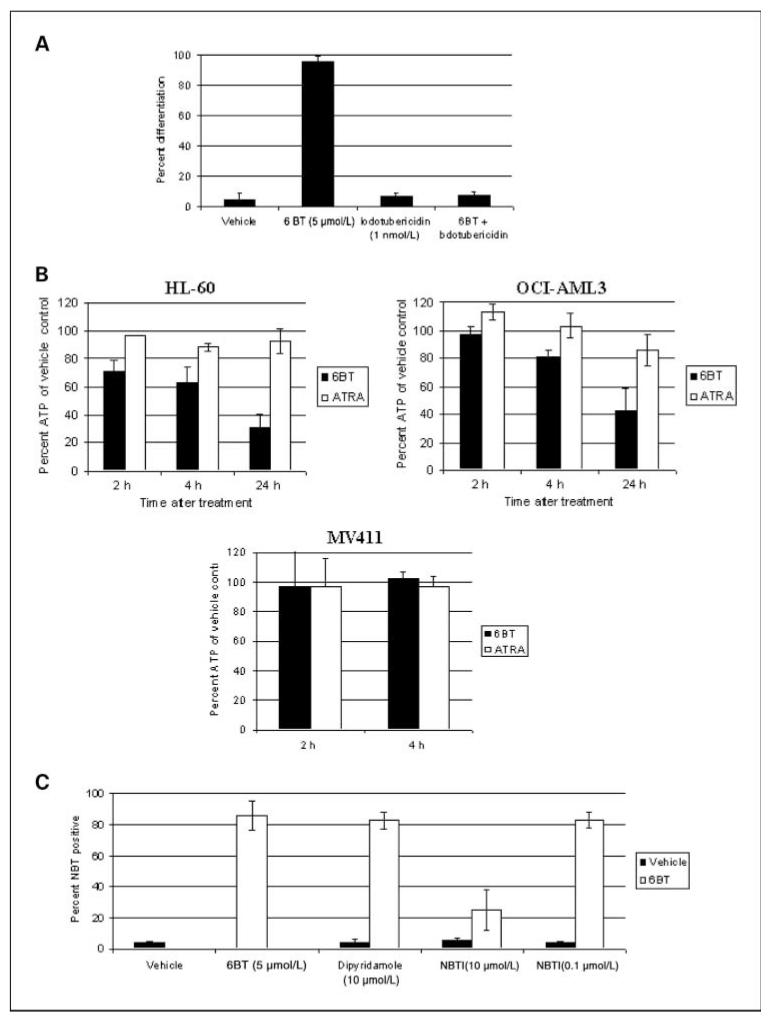
To begin to elucidate the mechanism of 6BT-mediated differentiation induction, a closely related compound 6-methyl-thioinosine (6MT) was used as a model. 6BT is nearly identical in chemical structure to 6MT except for substitution of a methyl group for the benzyl group. As 6MT is monophosphorylated by adenosine kinase, the role of phosphorylation in 6BT activity was assessed (10). Phosphorylation of 6BT is required for its differentiation activity as there is complete loss of 6BT-mediated differentiation induction of HL-60 cells with coincubation of 1 nmol/L of the specific adenosine kinase inhibitor, 5-iodotubericin (Fig. 6A). The requirement of 6BT phosphorylation for its activity also provides strong evidence that it enters leukemic cells.
Figure 6.
Mechanism by which 6BT can induce differentiation. A, phosphorylation of 6BT is essential for its activity. HL-60 cells were treated with 6BT alone or in combination with the specific adenosine kinase inhibitor, 5 iodotubericidin for 4 d, and the NBT reduction assay was performed as described in Fig. 2. Results are an average of three independent experiments. B, 6BT potently depletes ATP stores in leukemic cells. HL-60, OCI-AML3, and MV411 cells were treated for the indicated time points with 6BT, ATRA, or vehicle. An equal number of viable (as measured by trypan blue) cells was assessed for ATP using a luciferase-based assay system. The results shown represent the amount of ATP in the treated groups as a percentage of the ATP present in the vehicle-treated group. Results are an average of three independent experiments. C, inhibition of ent1 does not block 6BT-mediated uptake into leukemic cells. HL-60 cells were treated with the indicated compounds with or without a low dose of 6BT (5 μmol/L) for 3 d, and the NBT reduction assay was performed as detailed in Fig. 2. Results are an average of three independent experiments.
6BT depletes ATP stores in HL-60 cells
Because mono-phosphorylated 6MT is known to act as a potent inhibitor of the enzyme amidophosphoribosyltransferase, the first committed step in de novo purine synthesis, the ability of 6BT to deplete ATP, was assessed (11). 6BT was found to potently deplete ATP stores in HL-60 cells (Fig. 6B). Treatment of HL-60 cells with 6BT (5 μmol/L) leads to rapid depletion of ~70% of ATP stores within 24 hours. The depletion of ATP is not due to the differentiation process itself as, after 6BT treatment, HL-60 cells contain 30.3% ± 9.2% of the ATP levels of vehicle-treated cells compared with 93.0% ± 9.1% in ATRA-treated cells (1 μmol/L). Although 6BT does induce growth arrest of HL-60 cells and the cells ultimately die, the time course of significant cell death (~7 days) and ATP depletion (hours) is significantly different. 6BT was also found to deplete ATP in OCI-AML3 cells (50–60% at 24 hours) but did not lead to significant ATP depletion, at least at early time points, in MV411 cells that undergo 6BT-mediated cell death instead of differentiation. Due to the induction of cell death in MV411 cells, it was not possible to assess the ATP depletion at 24 hours.
6BT may use a non-ent1 transporter to enter leukemic cells
To begin to elucidate how 6BT may enter leukemic cells, well-characterized nucleoside transporter inhibitors were used. Neither nitrobenzylthioinsoine (NBTI) nor dipyridamole was able to block low-dose 6BT-mediated differentiation of HL-60 cells at optimal doses for the inhibition of ent1 (Fig. 6C). In contrast, when NBTI was used at an extremely high concentration (10 μmol/L) that is capable of inhibiting several other nucleoside transporters, a dramatic decrease in 6BT-mediated differentiation was observed (86% ± 9.6%–25% ± 13.20%). Therefore, 6BT is likely capable of entering leukemic cells through a nonent1-mediated pathway that is at least partially inhibited by NBTI. It is well-established that leukemic cells can express nucleoside transporters not widely expressed on other cells (12).
Discussion
We identified a novel myeloid differentiation-inducing compound, 6BT, through a small compound library screen. This compound induces monocyte/macrophage differentiation in a subset of leukemia cell lines and patient samples. The mechanism of action of this compound may involve the depletion of ATP stores that leads to growth inhibition and subsequent differentiation. Besides differentiation induction, a subset of leukemic cell lines and patient samples instead undergo 6BT-mediated cell death at significantly lower concentrations (>100-fold) than required to induce similar cell death in several nonmalignant cells including normal human bone marrow cells. It is possible that this cell death pathway is mediated through an unknown mechanism that does not involve ATP depletion. We have also shown that 6BT can inhibit leukemic tumor growth in mouse xenograft models.
The successful use of differentiation therapy for AML has been clearly shown by the remarkable success of ATRA in 5% to 10% of AML patients. As ATRA targets retinoic acid receptor a and induces granulocytic differentiation, the mechanism of action of 6BT is likely significantly different as it may target ATP depletion and induces monocytic differentiation. Although many other differentiation-inducing compounds have been described previously, the vast majority are not therapeutic candidates due to suboptimal differentiation activity, inability to induce terminal differentiation, poor drug-like properties, or unacceptable toxicities. One notable example is Vitamin D, which is a potent monocytic differentiation-inducing agent but leads to toxic hypercalcemia at the required dosages (13). Although 6BT is early in development, it can induce almost the complete differentiation of some leukemic cell lines as well as at least one of the limited number of patient samples tested, and also force leukemic cells to terminally mature to a point where they lose their proliferative capacity.
6BT has several intriguing properties that support its further preclinical development. The apparent favorable toxicity profile of 6BT may directly relate to its well-characterized activity as an inhibitor of ent1 (14). The ability to inhibit ent1 may also contribute to its capacity to synergize with cytarabine to induce leukemic cell death. Although not previously shown with cytarabine or leukemic cells, it has been shown for other chemotherapeutics such as 5-fluorouracil (5-FU) that inhibition of nucleoside transporters with dipyridamole can enhance cell killing by preventing the efflux of a 5-FU metabolite (15). This finding opens up the attractive possibility of using lower doses of cytarabine that may have increased efficacy and more tolerability. Future studies will address whether the ability of 6BT to inhibit ent1 is directly related to its ability to synergize with cytarabine to kill HL-60 cells. It will also be important to assess if ent1 inhibition leads to enhanced chemotherapeutic efficacy with unacceptable toxicities.
Interestingly, a portion of the leukemic cell lines tested that do not undergo 6BT-mediated differentiation undergo cell death in response to concentrations of 6BT that are at least 100 times lower than those required to induce equivalent death in nontumor-derived cell types such as fibroblasts, human endothelial cells, and normal human bone marrow cells (LD50 values of ~1 μmol/L versus >100 μmol/L). Despite the ability of 6BT to induce leukemic cell death or differentiation, the mouse models show that 6BT can be effective in vivo against leukemic cells that respond to 6BT by either differentiation (HL-60) or cell death (MV-411). Interestingly, 6BT led to the up-regulation of the mature myeloid marker CD11b but not CD14 in tumors. It is possible that that pathway of differentiation is different in vivo as it is likely influenced by various cytokines and growth factors.
Previous studies by El Kouni et al. (16) have suggested that 6BT does not normally enter mammalian cells, and the lack of its uptake seems to be related to its potent activity as an inhibitor of the nucleoside transporter, ent1. Interestingly, this group has studied 6BT as a potential therapeutic for Toxoplasma gondii infection as 6BT can be selectively transported by Toxoplasma-infected mammalian cells by using the Toxoplasma adenosine/purine transporter. The lack of uptake of 6BT into many mammalian cells likely explains the apparent favorable toxicity profile of 6BT. Consistent with our results, El Kouni et al. reported that 6BT and related analogues exhibit low toxicity using human fibroblasts.
In contrast to many mammalian cells, 6BT likely can enter leukemic cells as evidenced by its dependence on phosphorylation for its activity. As it is well-known that leukemic cells express nucleoside transporters not widely expressed on other cell types, it is possible that 6BT is using one of these transporters to enter leukemic cells. The identification of the 6BT transport mechanism may help to explain the relative sensitivity of specific leukemic cells to 6BT. It is possible that 6BT nonresponsive leukemic cells do not express the particular transporter(s) required for 6BT to enter cells.
Overall, 6BT is a promising new AML differentiation–inducing agent. 6BT exhibits both in vitro and in vivo activity in at least a subset of leukemic cells. Due to the current poor prognosis of patients with AML, new agents and therapeutic strategies are greatly needed. Further elucidation of the mechanism of action of 6BT and its in vivo properties are necessary to determine its clinical potential.
Acknowledgments
Grant support: ASCO career development award and T’Kindt family memorial (W. Tse) and NIH grant CA98916 (M.L. Agarwal).
Footnotes
American Cancer Society. Detailed Guide: Leukemia-Acute Myeloid (AML). http://www.cancer.org/docroot/CRI/content/CRI_2_4_1×_What_Are_the_Key_Statistics_About_Acute_Myeloid_Leukemia_AML.asp?sitearea=.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Kell J. Emerging treatments in acute myeloid leukaemia. Expert Opin Emerg Drugs. 2004;9:55–71. doi: 10.1517/eoed.9.1.55.32955. [DOI] [PubMed] [Google Scholar]
- 3.Tallmann MS. Curative therapeutic approaches to APL. Ann Hematol. 2004;83:S81–2. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 4.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–74. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburger PE, Chovaniec ME, Greenberger JS, Cohen HJ. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979;82:315–22. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins SJ, Bodner A, Ting R, Gallo RC. Induction of morphological and functional differentiation of human promyelocytic leukemia cells (HL-60) by componuds which induce differentiation of murine leukemia cells. Int J Cancer. 1980;25:213–8. doi: 10.1002/ijc.2910250208. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmo P, Pagano MC, Giustolisi R. The nitroblue tetrazolium activated test in the study of granulocytic function. Proposed methodology. Boll Soc Ital Biol Sper. 1980;56:183–7. [PubMed] [Google Scholar]
- 8.Newburger PE, Speier C, Borregaard N, Walsh CE, Whitin JC, Simons ER. Development of the superoxide-generating system during differentiation of the HL-60 human promyelocytic leukemia cell line. J Biol Chem. 1984;259:3771–6. [PubMed] [Google Scholar]
- 9.Clarke ML, Damaraju VL, Zhang J, et al. The role of human nucleoside transporters in cellular uptake of 4′-thio-h-d-arabinofuranosylcytosine and h-d-arabino-sylcytosine. Mol Pharmacol. 2006;70:303–10. doi: 10.1124/mol.105.021543. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka H, Kamatani N, Nishida Y, Nishioka K, Mikanagi K. Relationship between phosphorylation and cytotoxicity of 2-chloroadenosine and 6-methylmercaptopurine riboside in human cells. Biochim Biophys Acta. 1984;798:291–4. doi: 10.1016/0304-4165(84)90318-0. [DOI] [PubMed] [Google Scholar]
- 11.Shantz GD, Smith CM, Fontenelle LJ, Lau HK, Henderson JF. Inhibition of purine nucleotide metabolism by 6-methylthiopurine ribonucleoside and structurally related compounds. Cancer Res. 1973;33:2867–71. [PubMed] [Google Scholar]
- 12.Flanagan SA, Meckling-Gill KA. Characterization of a novel Na+-dependent, guanosine-specific, nitrobenzylth-ioinosine-sensitive transporter in acute promyelocytic leukemia cells. J Biol Chem. 1997;272:18026–32. doi: 10.1074/jbc.272.29.18026. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava MD, Ambrus JL. Effect of 1,25(OH)2 Vitamin D3 analogs on differentiation induction and cytokine modulation in blasts from acute myeloid leukemia patients. Leuk Lymphoma. 2004;45:2119–26. doi: 10.1080/1042819032000159924. [DOI] [PubMed] [Google Scholar]
- 14.Gupte A, Buolamwini JK, Yadav V, Chu CK, Naguib FN, el Kouni MH. 6-Benzylthioinosine analogues: promising anti-toxoplasmic agents as inhibitors of the mammalian nucleoside transporter ENT1 (es) Biochem Pharmacol. 2005;71:69–73. doi: 10.1016/j.bcp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Grem JL. Biochemical modulation of fluorouracil by dipyridamole: preclinical and clinical experience. Semin Oncol. 1992;19:56–65. [PubMed] [Google Scholar]
- 16.el Kouni MH, Guarcello V, Al Safarjalani ON, Naguib FN. Metabolism and selective toxicity of 6-nitroben-zylthioinosine in Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:2437–43. doi: 10.1128/aac.43.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]