Summary
A growing body of genomic data on human cancers poses the critical question of how genomic variations translate to cancer phenotypes. We employed standardized shotgun proteomics and targeted protein quantitation platforms to analyze a panel of 10 colon cancer cell lines differing by mutations in DNA mismatch repair (MMR) genes. In addition, we performed transcriptome sequencing (RNA-seq) to enable detection of protein sequence variants from the proteomic data. Biological replicate cultures yielded highly consistent proteomic inventories with a cumulative total of 6,513 protein groups with a protein FDR of 3.17% across all cell lines. Networks of co-expressed proteins with differential expression based on MMR status revealed impact on protein folding, turnover and transport, on cellular metabolism and on DNA and RNA synthesis and repair. Analysis of variant amino acid sequences suggested higher stability of proteins affected by naturally occurring germline polymorphisms than of proteins affected by somatic protein sequence changes. The data provide evidence for multi-system adaptation to MMR deficiency with a stress response that targets misfolded proteins for degradation through the ubiquitin-dependent proteasome pathway. Enrichment analysis suggested epithelial-to-mesenchymal transition (EMT) in RKO cells, as evidenced by increased mobility and invasion properties compared to SW480. The observed proteomic profiles demonstrate previously unknown consequences of altered DNA repair and provide an expanded basis for mechanistic interpretation of MMR phenotypes.
Introduction
Colon cancer development is characterized by a well-documented series of genetic changes that drive the progression from early adenomas to metastatic carcinomas (1). These include a chromosomal instability (CIN), microsatellite instability (MIN), and CpG island methylation (CIMP) (1–3). In addition to these global genetic and epigenetic characteristics, a relatively small number of oncogenes and tumor suppressor genes are frequently altered in colorectal carcinoma, including, APC (~90%), p53 (~50%) and K-ras (~40%) (1, 2). More recent global sequencing approaches have described somatic mutations in several human tumor types (4, 5) and larger scale network studies, such as The Cancer Genome Atlas initiative have characterized mutations in hundreds of tumors, profiled tumor transcriptomes and cataloged cancer-related gene amplification and epigenetic silencing in colon and rectal carcinoma (6). The resulting wave of data poses the critical question of how genomic variations translate to cancer phenotypes. Genes and transcripts execute most of their functions through the proteins they encode. Systematic characterization of cancer proteomes thus provides a means to understand the translation of genomic variation to cancer phenotypes.
Here we address the largely unexplored problem of how specific cancer-related mutations translate to functional alterations through proteomes. A recent study demonstrated proteomic changes driven by gene copy number changes in cancer cells (7), but the proteomic consequences of gene mutations and gene silencing events remain unknown. We compared a panel of 10 colorectal carcinoma cell lines which display different mutations in DNA mismatch repair genes, as well as other colon cancer-associated genes. We employed shotgun proteomics by liquid chromatography-tandem mass spectrometry (LC-MS/MS), which enables global proteome surveys that can identify thousands of proteins from milligram quantities of cells or tissue (8, 9). Shotgun analyses provide a unbiased, global inventory of proteomes, together with quantitative estimates of protein abundances that translate to biological phenotypes (10).
We previously described methods to enhance global proteomic analyses using mutational and gene expression data obtained by transcriptome sequencing (RNA-seq) (11, 12). With these approaches, proteomic analysis yields higher numbers of identified proteins and detects specific sequence mutations and variants. In addition, RNA-seq data also provides transcript expression information, which can be combined with protein expression levels to identify regulatory changes in biological systems (13). Here we applied a combined proteogenomic analysis to explore the impact of mismatch repair deficiency due to several distinct mutations and epigenetic silencing events. The data broaden our understanding of phenotypes associated with mismatch repair and provide a template for future studies of how genomic and proteomic changes generate important cell phenotypes in cancer.
Methods
Cell lines and proteomic analysis by LC-MS/MS
All cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA) and grown as described previously (13). A summary of genetic features of the cell lines is provided in Table S1. Three separate replicate cultures for each cell line were analyzed by shotgun proteomics as described by Liu et al. (13). Spectral files were searched against the Human ENSEMBL protein database (version 36, release 52) using Myrimatch (version 1.5.6) (14). IDpicker version 3.0 was used to assign protein identifications to the identified peptides. The resulting dataset consisted of 6,094 protein groups with a 7.8% protein FDR (Tables S2 and S3).
Proteome analysis using RNA-seq data
Knowledge on transcriptome data can greatly enhance protein identification and expression level analyses, including that of variant peptide sequences (12). We generated whole transcriptome analysis for 9 of the 10 cell lines as described by Wang et al. (12). Since DLD1 and HCT15 were derived from the same colon cancer (15), we only generated the HCT15 RNA-seq data and used this dataset for both HCT15 and DLD1 analyses. FPKM (Fragments Per Kilobase of exon per Million fragments mapped) was extracted from cufflinks reports for genes for which expression levels could be determined, otherwise the gene FPKM values were set to 0. After removing genes with total FPKM less than 5 across 9 cell lines, we identified 14,846 expressed genes.
We used RNA-Seq data from 9 cell lines to generate customized protein databases that included all sequence variations found in the transcriptome. The HCT15 customized protein database was used for DLD1. Putative SNVs were called one library at a time with SAMtools mpileup and varFilter scripts (16). Then an R package customProDB was used to annotate the SNVs and generate a database by keeping nonsynonymous protein coding SNVs for each cell line. Protein searches were performed using the customized FASTA databases for each cell line resulting in a dataset containing 6,513 protein groups with a protein FDR of 3.17%. (Tables S4 and S5). Given the improvement of this dataset over the one generated using the standard database, all analyses were performed using the results based on the customized FASTA database.
Proteome analysis using microsatellite mutation data from SelTarBase
The SelTarBase database (www.seltarbase.org) provides a compilation of all published microsatellite mutations in expressed sequences. Single-nucleotide shifts in microsatellites can lead to truncated proteins that theoretically should contain novel sequences at the carboxy-terminal end, depending on where protein translation termination signals (stop-codons) are located within the alternative reading frame. If such shifted protein sequences are detectable, they would be unique to the mutant cell and could potentially be used as specific markers. We created a FASTA protein search database containing all possible frame shifts in the 167 genes listed in SelTarBase that would lead to novel predicted sequences of at least 5 amino acids for a total of 358 new database entries. A complete list of these genes is provided in Table S6. To account for read-through events, the first 3 stop codons were replaced by tryptophan for a sub-set of proteins.
Protein expression comparisons based on spectral count data
Statistically significant differences in protein spectral counts between different groups were calculated using a quasi-likelihood Poisson distribution implemented Quasitel (17). Complete results from Quasitel analyses are presented in Tables S7 (MMR+ cell lines vs MMR− cell lines) and S8 (RKO cells vs all other cell lines).
WebGestalt functional class enrichment analysis
We used WebGestalt (18) at http://bioinfo.vanderbilt.edu/webgestalt for the functional interpretation of shotgun proteomics data. Our reference set consisted of all proteins identified across all cell lines. Differential proteins had at least a 2-fold difference in spectral counts and a FDR-corrected quasi-likelihood p-value of less than 0.05.
MRM analyses
Cell samples for MRM were prepared as for LC-MS/MS proteomics, except that peptide extracts were not subjected to IEF, and analyzed using our Labeled Reference Peptide method (19). Four optimized transitions for each peptide of corresponding proteins were selected using Skyline (20) (Table S9). A stable isotope labeled β-actin peptide [U-13C6, U-15N4-Arg]-GYSFTTTAER was used as an internal standard (60 fmol/injection) for relative quantification of target proteins. An unpaired t-test was used to test for significant differences between samples (n=3).
Western Blotting
Cell pellets were resuspended in ice-cold RIPA buffer (150 mM NaCl, 50 mM Tris, 0.1%SDS, 1% NP-40, 0.5% sodium deoxycholate and protease inhibitors), sonicated for 10 seconds, incubated on ice for 10 minutes and clarified by centrifugation at 13,000 × g. Protein concentrations were measured with the BCA assay. Lysates from each cell line (40 µg protein) were combined with 4× SDS loading buffer (Invitrogen, Carlsbad, CA), incubated at 70 °C for 10 min, and proteins were separated on 10% SDS-PAGE mini-gels. Proteins were transferred to PVDF membranes, which were probed with primary antibodies for MLH1 or MSH2 (Santa Cruz Biotechnology, Santa Cruz, CA)) and -actin (Abcam, Cambridge, MA). Membranes were probed with fluorophore-conjugated secondary antibodies (Invitrogen) and proteins were visualized on a fluorescent scanner (LI-COR Odyssey, LIC-COR, Lincoln, NE).
Cell proliferation and invasion assays
Cell mobility was measured in triplicate using a model wound healing assay in which scraped ‘wounds’ were created in confluent monolayers of cells with a pipette tip. Cell migration was observed at 0, 24, and 48 hours by cell growth within the scrape line and representative scrape lines for each cell line were photographed. Cell invasion capacity was measured on Transwell™ filters in serum-free medium. Serum containing medium (0.5%) was utilized as the chemoattractant in the lower chamber. After 72 hours of incubation, cells that had invaded to the lower surface membrane were fixed, stained and counted under a light microscope. Significance was evaluated with Student’s t-test.
Hierarchical Clustering
For unsupervised cluster analysis of the ten cell lines, the dataset was limited to the 300 proteins with the highest variation in expression in the dataset. Normalized spectral counts were log2-transformed and the 300 proteins with the highest variance were chosen for hierarchical cluster analyses and visualization in heat maps. For supervised cluster analysis, proteins were selected based on differential expression between the MMR+ and MMR− groups (3-fold difference, adjusted quasi p-value ≤ 0.05) and processed as described for unsupervised clustering.
Co-expression network construction and co-expression module identification
Multiple group comparison based on quasi-likelihood modeling was used to identify proteins that are differentially expressed across groups but consistent within replicates. To reduce data redundancy and facilitate downstream functional analysis, expression data was summarized to the gene level. We used a modified version of our previously published Iterative Clique Enumeration (ICE) algorithm (21) to identify 167 co-expression modules from the co-expression network. We identified 9 modules that showed differential expression using t-test with a corrected p-value of less than 0.1 between MMR+ and MMR− cell lines (Table S10).
Results
Proteomic analysis of 10 colorectal cancer cell lines
We analyzed a collection of 10 colorectal carcinoma cell lines that differ in their DNA mismatch repair (MMR) status (Table S1). Shotgun proteomic analyses performed on triplicate cultures of each of the cell lines yielded a total of 6,094 proteins at a protein FDR of 4.63% with the use of a generic ENSEMBL protein database. More than half of these proteins were observed across all cell lines (3,459 proteins total), while the average number of protein identifications per cell line was 4,373 (range: 3,183–4,953).
Using RNA-seq transcriptome data to extend global proteomic analyses
Protein identification in global proteomic surveys depends on prior knowledge of the protein sequences present in a given sample. Until recently, the typical shotgun proteomic identification step involved matching the observed MS/MS spectra sequences in standard protein databases, which generally do not contain variant sequences. We developed a novel strategy to use RNA-seq transcriptome data to create cell line-specific, custom databases (12) that reduce the search space of the database search and allow the identification of sequence variants. This analysis yielded a total of 6,513 protein groups (3.17% protein FDR) identified by 59,803 distinct peptides. Because of this improvement of 6.9% in protein identifications over standard database searches, the customized database results were used for all subsequent analyses (Table S5).
Identification of variant sequence peptides
A total of 59,803 distinct peptides were identified in the dataset, but only 763 distinct peptides representing 564 unique variant sequences compared to the standard ENSEMBL protein database (Table S11). Of these 571 variant peptide sequences, 329 (58%) had known genomic counterparts due to single nucleotide polymorphisms (SNPs). Of the remaining 242 peptides, 235 peptides harbored a single amino acid change and 7 peptides were the result of larger sequence changes. RNA-seq analysis identified 19,613 non-synonymous sequence variants in coding regions across all cell lines, which gives an overall ratio of (detected sequence variants)/(all possible variants from RNA-seq)= 2.9%. The reason that not all possible variants were detectable include 1) protein expression levels of many variants were too low for detection by our analysis platform; 2) peptide sequences surrounding the variant were not favorable for ionization and detection by MS; and 3) possible effects of variant sequence on protein stability. To further evaluate the last possibility, we separated variant peptides into those listed in the dbSNP database (22) and those that were newly detected in our study. On average, variant sequences representing germline polymorphisms were twice as likely of being detected in the proteome than non-dbSNP, and presumably somatic, variant sequences (4.0% versus 2.2%, respectively; p<0.0001, Chi2 test). This suggests that newly acquired sequence variants have a negative effect on protein stability.
Several known cancer-associated genetic mutations were observed at the proteome level, including the KRAS codon 12 G12V variant in SW480, a D140G mutation in the RAS homologue RAB1B in HCT15 and a TP53 P309S variant in SW480. Of the remaining variant peptides, a subset was chosen based on high spectral counts and distribution for verification by manual inspection and comparison with spectra obtained with synthetic peptides of the same sequence. Variant peptides derived from GPATCH4, CCT8, ANXA11, SRFS9, CEACAM1 and EPB41L1 were confirmed using this strategy (Figures S1A–F). Interestingly, the EPB41L1 sequence alteration changes a known serine phosphorylation site at codon 75 to leucine. EPB41L1 is a competitive inhibitor of AGAP2 (also called PIKE for PI3Kinase Enhancer), a protein that binds and activates PIK3CA (23). AGAP2 is a nuclear GTPase that is frequently overexpressed in cancer cells. Possible regulation of EPB41L1 activity by phosphorylation might be important for PI3-kinase activity in the cell.
Hierarchical clustering analysis and evidence for MMR-related protein signatures
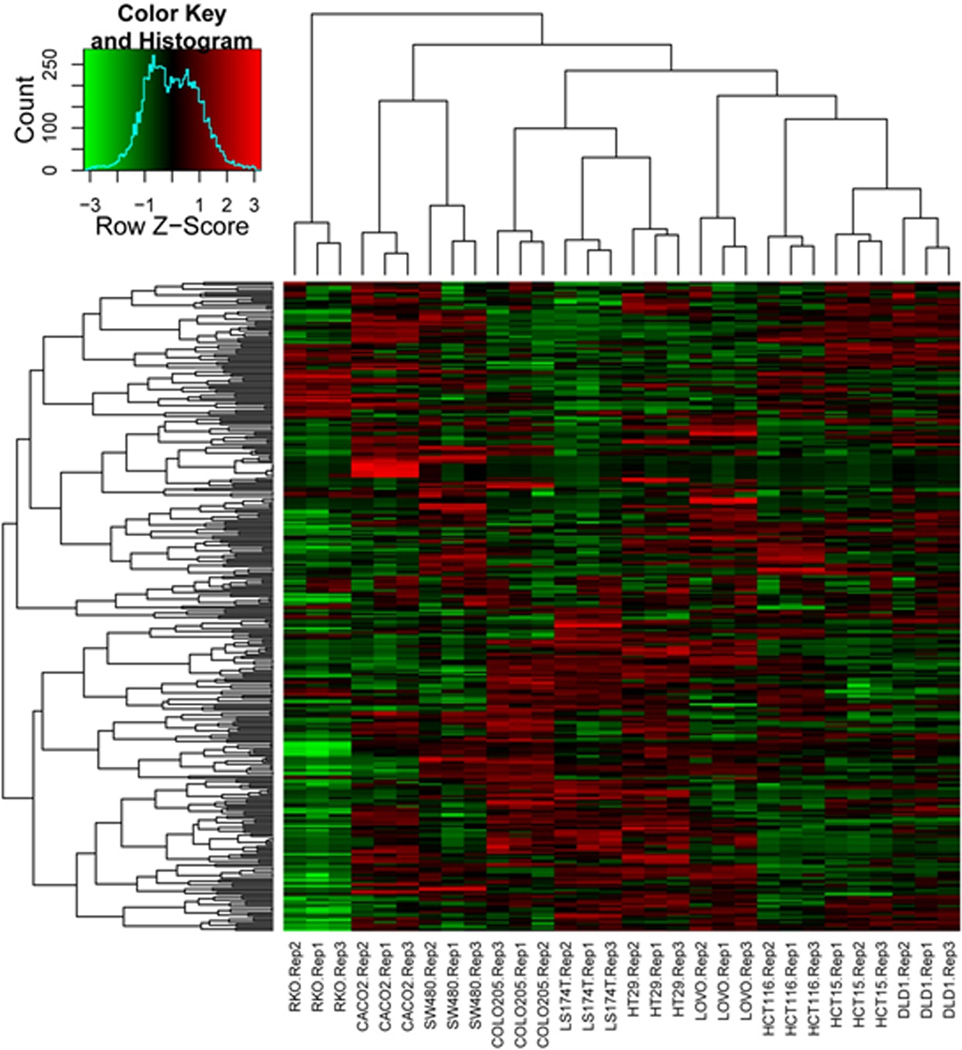
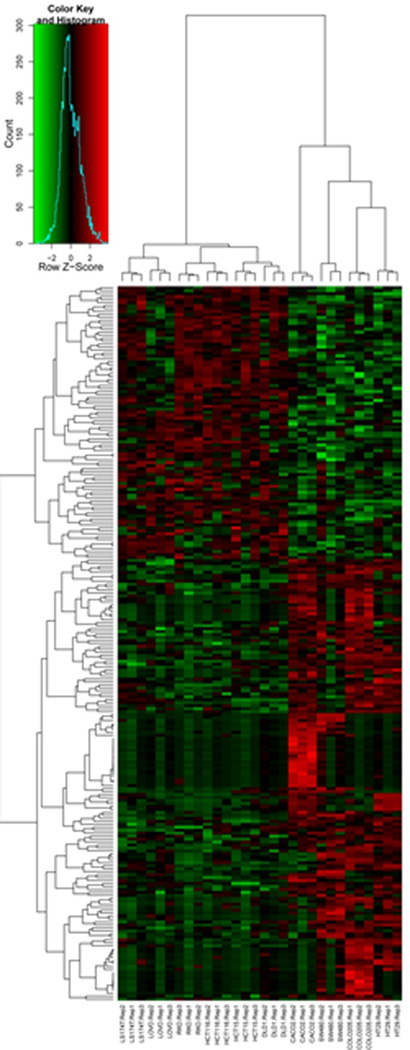
Unsupervised clustering of the 300 most variable proteins in the dataset grouped all biological replicates together (Figure 1). Of note, cell lines DLD-1 and HCT-15 grouped together; these cell lines were originally cultured by two investigators from different tissues harvested from the same patient and were subsequently shown to be genetically identical (15). Cell line RKO was most dissimilar to all the other cell lines, in large part due to the absence of keratins and extracellular matrix proteins in this cell line. In addition to these specific features, each of the 10 cell lines displayed distinct protein expression patterns, reflecting their unique proteomic characteristics.
Figure 1.
Unsupervised heatmap of the top 300 most variable proteins across the 10 cell line dataset. Proteins were selected based on their variability between all cell lines. Spectral counts were log-transformed, normalized and used for clustering based on similarity in expression patterns. The three biological replicate preparations of cell lines clustered together in all cases, with the related cell lines DLD1 and HCT15 grouped closer together compared to the other cell lines (6 right-most lanes). RKO contains the most distinguishing features, mainly due to the absence of a large number of keratins expressed in the other cell lines.
Proteomic characteristics of epithelial-to-mesenchymal transition (EMT) in RKO cells
Protein expression data from RKO was characterized by dramatic decreases in expression of keratins, catenins, cadherins and related proteins involved in cytoskeletal structures and cell adhesion (Table S8). RKO cells differentially expressed 202 proteins as compared to the 9 other cell lines. The 105 proteins downregulated in RKO were significantly enriched in GO cellular component classes of cell-cell junction (GO:0005911, p<0.0001), cell periphery (GO:0071944, p<0.0001), and other classes related to extracellular matrix and cell-cell interactions. Similarly, KEGG categories for cell adhesion molecules (KEGG:04514, p=0.007), adherence junctions (KEGG:04520, p=0.007), and tight junctions (KEGG:04530, p=0.007) were downregulated in RKO. These differences suggested loss of epithelial characteristics in RKO cells, a hallmark characteristic of the EMT phenotype. Transcription factor target enrichment analysis of proteins with lower expression in RKO also were consistent with EMT. A large protein cluster was significantly enriched for targets of transcription factor TCF3 (also known as E2A; p=0.0008). This analysis also identified significant enrichment for downregulated proteins associated with the transcription factor ZEB1 (also known as AREB6 and TCF8), which is implicated in EMT, suppresses CDH1, and is known to be highly expressed in RKO (p=0.008) (24).
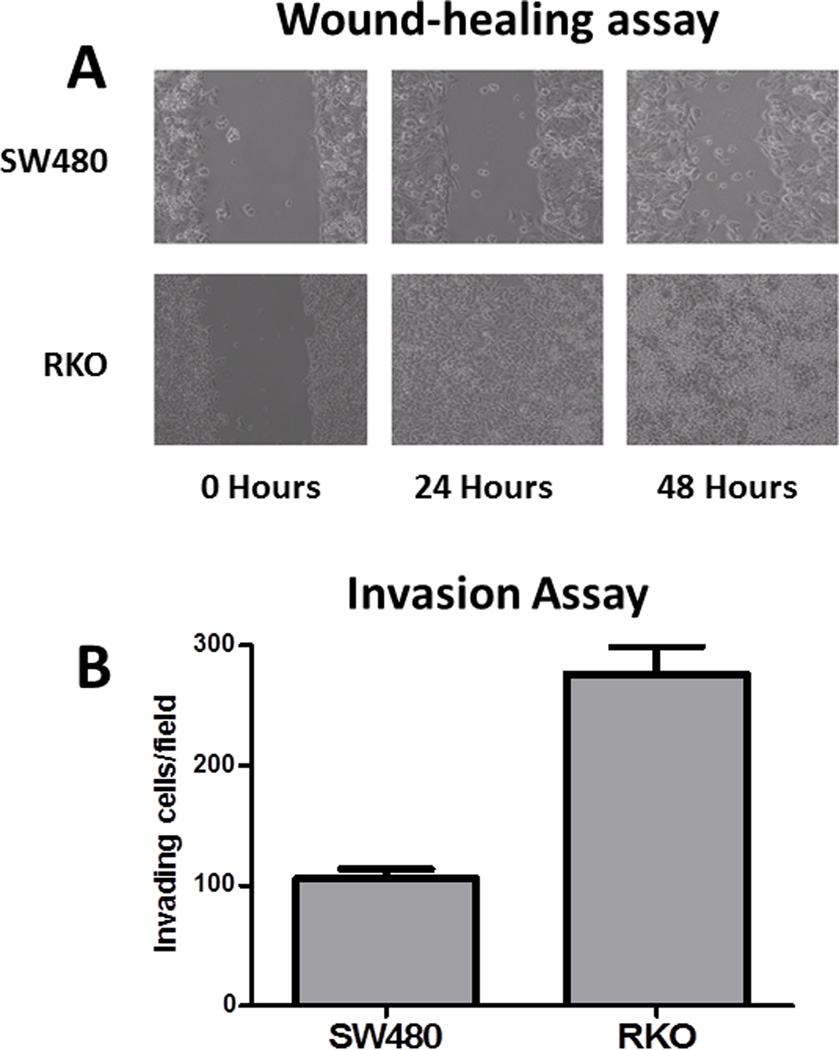
The combined results provide additional evidence that RKO cells display an EMT phenotype, a characteristic of invasive and metastatic cancers characterized by loss of intercellular contacts, loss of baso-apical polarity, gain of mesenchymal markers and increased invasive properties (25). To determine whether the EMT-like proteomic features of RKO cells conferred a functional EMT phenotype, we compared RKO and SW480 (non-EMT) phenotypes using wound-healing and invasion assays. In the wound-healing assay, RKO cells completely covered a scratched surface within 24 hours, whereas open areas remained with SW480 cells (Figure 2A). In a cell invasion assay (growth through TransWell™ filters), RKO cells were approximately 2.5-fold more invasive than SW480 (Figure 2B), thus confirming growth characteristics consistent with an EMT phenotype.
Figure 2.
Comparison of RKO and SW480 cell lines for motility and invasive properties associated with the EMT phenotype. A. RKO cells displayed faster migration across the scraped region of a cell monolayer in a model wound-healing assay compared to SW480 cells. B. RKO cells displayed approximately 2.5-times greater migration than SW480 cells over 72 hr through Transwell™ filters from serum-free medium in the upper chamber toward serum containing (0.5%) medium in the lower chamber.
Identification of proteomic changes associated with MMR deficiency
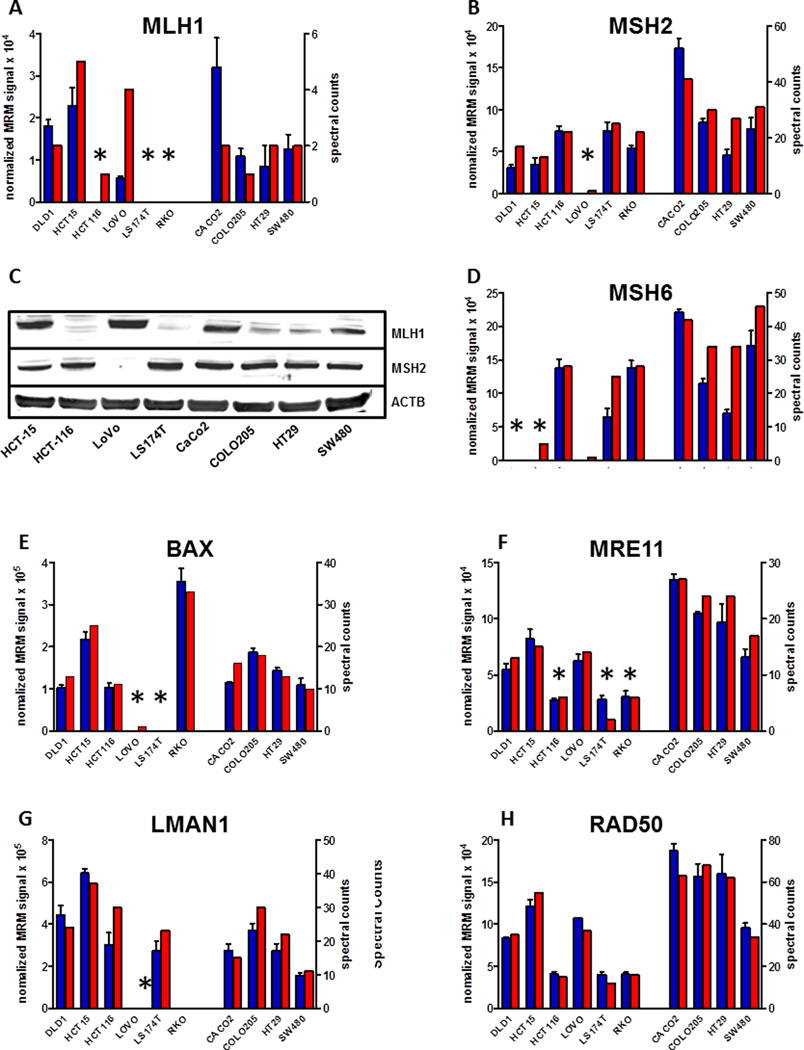
The genetic status of MMR genes in all of the cell lines in this study is known. This allowed us to study the proteomic correlates with known genetic and epigenetic alterations involving the MMR proteins MLH1, MSH2 and MSH6. Figure 3 summarizes the combined shotgun proteomic and MRM data for MLH1, MSH2 and MSH6, which indicate that the proteins corresponding to the mutated or silenced genes all are either absent or significantly downregulated. We also examined the proteins RAD50, LMAN1, BAX, and MRE11A, which contain mononucleotide repeats within their coding sequences and are prone to frameshift mutations secondary to loss of MMR, thus leading to loss of protein expression. Accordingly, BAX expression was lost in LoVo and LS174T cells, LMAN1 expression was lost in LoVo while RAD50 levels were lowered, but not lost in all of the MMR− cell lines, as none have homozygous inactivation of the gene. We also observed low levels of MRE11A protein expression in LS174T, HCT116 and LoVo, due to intronic microsatellite mutations, which lead to exon-skipping in these cell lines. MRM analyses confirmed all of the differences observed in shotgun MS analyses. Western blot analyses of MLH1 and MSH6 in several of the cell lines confirmed the shotgun and MRM data. MRM analyses of additional proteins found to be differentially expressed in MMR phenotypes verified the expression differences detected by shotgun proteomics (Figure S2A–T). These analyses demonstrate the validity of protein expression assessment through shotgun proteomic analyses and confirm expression changes in this group of MMR-associated proteins in the panel of colorectal carcinoma cell lines.
Figure 3.
Shotgun and MRM analyses provide consistent measurements of selective loss in expression of MMR proteins (MLH1, MSH2 and MSH6, panels A–D), selected protein products of MMR-sensitive target genes (BAX, MRE11, LMAN1, and RAD50, panels E–H). Shotgun proteomics data are plotted as spectral counts for triplicate analyses, whereas MRM data are plotted as summed signal intensity for measured transitions normalized to summed intensity for transitions measured for a reference peptide. Symbols above each bar indicate genotype for the corresponding gene, as summarized in Table S1 and in the cited literature. Panel C shows protein blot-analysis for MLH1 and MSH2 confirming protein levels observed by mass-spectrometry.
Proteomic consequences of MMR-associated slippage events in MMR− cell lines
Several hundred human genes harbor mononucleotide tracks in their coding sequences, tracks that are prone to DNA replication slippage in MMR− cells (Table S6). Because such slippage events alter the reading frame, they lead to novel C-terminal amino acid sequences in the affected proteins. We generated a customized FASTA database containing 167 human genes with known mononucleotide elements from SelTarBase that predicted potentially detectable peptides and used this FASTA database to search the proteomic data for all 10 cell lines. The results from this search included several low-quality matches to shifted peptide sequences (approximately 1–2 matches per cell line), but none of these matches could be validated through manual inspection of the assigned MS/MS spectra (data not shown). The conclusion from this proteomic search is that cells either remove mRNAs containing premature stop codons or that the resulting altered proteins are unstable and do not reach levels that allow detection in survey-type shotgun proteomics experiments.
Co-expression network analysis reveals modular organization of proteomic adaptations to MMR deficiency
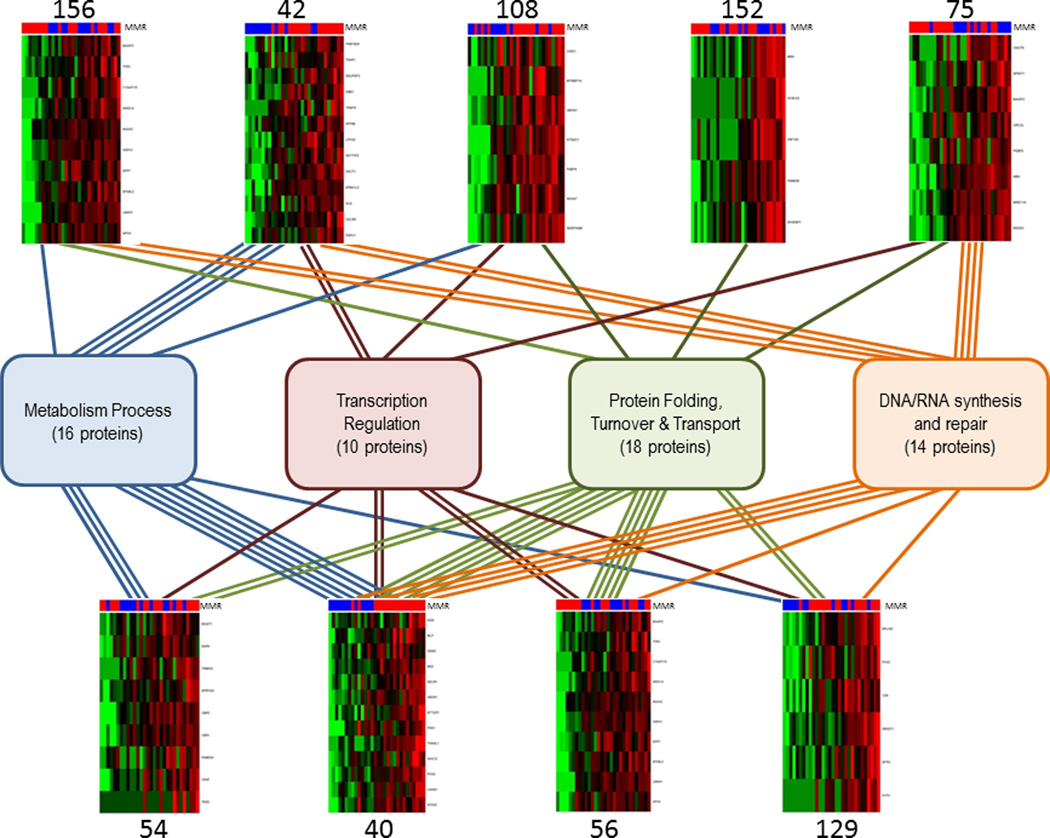
To detect coordinated proteomic adaptations to MMR deficiency, we performed co-expression network analysis and identified 167 co-expression modules. From these, we identified 9 modules that showed the largest differential expression between MMR+ and MMR− cell lines that represent coordinated proteomic changes (Table S10). We evaluated the 76 proteins comprising these modules to identify common functional classifications using Gene Ontology categories and identified four groups of related functions (Table S12), including a) protein turnover, transport and folding (18 proteins), b) metabolic processes (16 proteins), c) DNA/RNA synthesis and repair (14 proteins), and d) transcription regulation (10 proteins) (Figure 4).
Figure 4.
Protein co-expression modules represent shared proteomic characteristics of MMR− and MMR+ cell lines. Co-expression network analysis analyzed proteins with high variance across all 10 cell lines cell lines and yielded a protein co-expression network represented by 167 co-expression modules. Heatmaps visualizing expression patterns of nine of the co-expression modules are shown. Columns represent normalized spectral counts for proteins in a replicate culture, rows represent proteins. Indicator bars at the top of each heatmap indicate MMR status (green = MMR−; red = MMR+). Red shading in heatmaps indicates increased protein levels in MMR− cells; green shading indicates decreased protein levels in MMR− cells. Boxes in the middle represent functional classifications; numbers in parentheses indicate the total number of proteins in each classification from the nine co-expression modules.
The largest group (group a) contains proteins involved in regulation of protein folding (DECR1, TRAP1, HSPH1, LMAN1, FKBP2, FKBP4, WFS1 and ERLIN2), protein turnover (UBXN1, DPP7, TFRC, SH3KBP1, EFTUD1 and C10orf118) and protein transport (DNM2, NAPA, TIMM23 and ABHD11). The functions represented in these co-expression modules suggest a coordinated program of adaptation to the translation of misfolded, variant polypeptides in MMR− cells. Several of the proteins identified in this module are involved in HSP90-mediated cellular stress response (TRAP1, HSPH1, LMAN1); other proteins involved in this response that are significantly upregulated in MMR− cells, but were not identified through co-expression network analysis include STUB1, HSP90AA, HSP90AB1, CDC37 and ATXN2 (Table S7). These data suggest enhanced chaperone expression and activity of the cellular response to proteins with variant sequences that need to be removed from the cell.
The second co-expression module consisted of 16 metabolic proteins with higher levels in MMR− cell lines compared to MMR+ cell lines, most of which were located in the mitochondria. Several of these proteins, for example CISD1, NDUFAF3, ATP5B, MUT, OXCT1 and PDK1, play critical roles in glucose and fatty acid metabolism. The discovery of this network of proteins is unexpected and one could speculate that MMR deficiency places added metabolic demands on cells due to the need to maintain adaptive responses in protein turnover.
The third group, categorized as DNA/RNA synthesis and repair, included several DNA repair proteins from module 75 (RAD50 and MRE11A) and the related double-strand break (DSB) repair protein NBN, which are constituents of the MRN complex involved in Non-Homologous End Joining (NHEJ) (26). In addition, module 75 contains a component of the origin recognition complex, ORC3, essential for the initiation of DNA replication in eukaryotic cells and a subunit of the CCR4-NOT complex, CNOT4 that functions as a general transcription regulation complex. All these proteins are coordinately downregulated in MMR− cells, suggesting decreased DNA synthesis and repair in MMR− cells (Figure 4). Other proteins in this group are indicative of RNA surveillance (DIS3, UTP20) and RNA splicing (DHX15, ESRP1, and SFPQ) (Table S12).
Taken together, the functions represented in these co-expression modules suggest a coordinated program of adaptation to the translation of misfolded, variant polypeptides in MMR− cells. Although consistent with enhanced chaperone expression and protein translation, the co-expression of other proteins involved in metabolism, vesicle trafficking and transcription regulation suggest a previously unrecognized scope of adaptation to proteotoxic stress secondary to DNA MMR deficiency.
To study the proteomic consequences of MMR, we used quasi-likelihood modeling to identify proteins that were differentially expressed between the MMR+ and MMR− cell lines. A total of 245 protein entries were statistically significant at p<0.05 and these were visualized in a heatmap in Figure 5. This figure illustrates global common features that are affected in response to MMR status but also indicate proteomic characteristics that are unique for individual cell lines. For example, a cluster of proteins is highly expressed in Caco-2, including APOE, FN1, DCDC2, LAMA5, CD74, and MYL3; all proteins involved in cellular motility (GO:0048870). Of the proteins with higher expression in MMR− cell lines compared to MMR+ cell lines, HSP90AA1, HSP90AB1, CCT6B, CCT8, DNAJA2 and CDC37 bind to unfolded proteins (GO:0051082) and STUB1, HSP90AA1 and CCT6B are involved in chaperone-mediated protein complex assembly (GO:0051131). These results indicate a potential upregulation of proteins that manage degradation of aberrant proteins in the cell.
Figure 5.
Supervised cluster analysis according to MMR status. The heatmap visualizes the 245 proteins with quasi-likelihood p-value of less than 0.05 across the 10 cell line dataset. Spectral counts were log-transformed, normalized and used for clustering based on similarity in expression patterns. As with unsupervised cluster analysis, the three biological replicate preparations of cell lines grouped together in all cases. General expression patterns separate MMR− from MMR+ cell lines although high levels of protein clusters are apparent for some cell lines.
Discussion
Colorectal tumors with MIN comprise a major subset of colorectal cancers and are notable for distinct clinical characteristics (27) and high frequency of mutations (6). MIN tumors have been distinguished previously from other colon cancer types by gene expression profiles (28–30), which suggest broad adaptations to MMR deficiency. In MIN tumors, increased mutation frequency increases levels of frame-shifted sequences, which generate premature stop-codons in the mRNA. These abnormal mRNAs are selectively removed by nonsense-mediated decay (NMD), a process that is active in MIN tumors (31). This process is highly effective in removing mRNAs on which translation has stalled, but not all nonsense-containing mRNAs are sensitive to NMD and the process does not remove mRNAs that harbor single nucleotide sequence variants. Thus, increased production of sequence variant proteins in MIN tumors may demand adaptation of protein quality control mechanisms, which could contribute to the MIN phenotype.
Using gene expression analysis, Banerjea et al. identified upregulation of immunomodulatory genes in MIN tumors, such as heat shock proteins, chaperone molecules and cytokines, and linked these findings to processing and presentation of antigenic peptides. Increased levels of somatically mutated peptides could result from increased mutational rates observed in MMR-defective tumors, and these peptides may be related to the strong immunogenic response triggered by MIN tumors (32, 33). However, the immunomodulary response described by Banerjea et al. was observed in primary carcinomas and it is unclear if the gene expression changes occurred in the cancer cells or in the inflammatory component of the specimens. In addition, the study was based on mRNA expression profiling and did not study functional significance at the protein level. Our study provides additional information by studying proteomic changes in tumor cell lines and to our knowledge provides the most detailed documentation of the extent to which defects in MMR translate into the proteome.
Our proteomic findings are in agreement with the notion that MMR− cells have upregulation of cellular processes that handle aberrant proteins, either through protein folding, degradation and unfolded protein binding, which could enable MMR− cells to counteract the deleterious effects of abnormal protein load. Evidence for increased protein folding activity came from the presence of FKBP2 and FKBP4, LMAN1, TRAP1 and WFS1, while a separate co-expression module indicated increased ubiquitin-dependent protein degradation: UBXN1, DPP7, TFRC, and ERLIN2. The involvement of ubiquitin-dependent proteasomal degradation is in agreement with a recent paper by Kim et al., demonstrating that aberrant mRNAs that escape NMD lead to mutant proteins that are degraded via the ubiquitin-proteasome system (34).
Apart from protein expression changes, our data provide a separate line of evidence for a cellular response to abnormal proteins. The acquisition of RNAseq data for all cell lines and the application of our newly developed pipeline for the detection of variant peptide sequences from shotgun proteomic data (12) allowed us to quantify the levels of variant peptides resulting from germline polymorphisms (SNPs) and from somatic mutations. This analysis clearly showed that SNP-encoded variant peptides were more likely to be detected in the shotgun proteomic datasets compared to somatically acquired variant peptides. These data suggest that proteins harboring new sequence variants are less stable in the cell than proteins with variant sequences encoded by germline SNPs. This observation is also consistent with our failure to detect novel peptides resulting from frame-shifted protein coding sequences in the limited number of genes that harbor repeated elements as targets for MMR. The unavailability of germline data precludes the analysis of individual sequence variants by comparing DNA from normal tissues to tumor DNA from the same patient. Nevertheless, for global comparisons, it seems reasonable to postulate that sequence variant peptides in the different SNP databases are more likely to be germline polymorphisms than new sequence variants.
Co-expression network analysis identified a large module of proteins that included proteins involved in DNA and RNA synthesis and repair. The driving proteins behind these modules are the MMR proteins responsible for the MMR phenotype (MLH1, MSH2, MSH6, etc) and proteins involved in repair of double-strand breaks (RAD50, MRE11A, NBN, etc). RAD50 and MRE11A have lower expression levels in MMR− cell lines because of mutations caused by defective MMR, but increased protein levels of SFPQ, PFAS, ANXA3 and ORC3L suggest increased DNA synthesis and repair in MMR+ cells compared to MMR− cells. Our proteomic data did not indicate any increase in proteins involved in NMD, although several of the key NMD proteins were detectable in the dataset (UPF1, 2 and 3B). Co-expression network analysis also indicated differences in transcription regulation and in the process of metabolism between MMR+ and MMR− cells. To our knowledge, an association between MMR status and changes in cellular energy metabolism has not been reported. Our findings suggest that such an association might exist and provide a rationale for further study to elucidate the possible relationship between these biological processes.
Our analyses also indicated an EMT phenotype of RKO cells based on proteomic analyses. The dramatic downregulation of CDH1 observed in RKO cells is a hallmark of EMT (25, 35). However, we observed 105 other downregulated proteins in RKO cells, which includes many components of cell adhesion and cytoskeleton and proteins that regulate these networks. Gene enrichment analysis of these proteins indicated activation of the EMT-associated transcription factor ZEB1. Although some EMT characteristics of RKO have been reported previously (24) and analyses of EMT-like models with other analysis platforms have been reported (36–38), our data provide the largest proteomic dataset to describe this phenomenon. Our detection of increased mobility and invasive properties of RKO compared to one of the other cell lines, SW480 provides preliminary confirmation of an EMT phenotype.
Here we demonstrate that standardized proteome analysis platforms can capture the protein expression characteristics of distinct phenotypes driven by cancer-related mutations. Our data provide the first insight into the proteomic consequences of DNA mismatch repair deficiency and indicate that wide-spread mutational load leads to an adaptive stress response that allows cells to remove mutant proteins. Sequence variations may produce misfolded proteins that are subsequently degraded through the ubiquitin-dependent proteasomal pathway. In addition, global proteomic analyses proved sensitive enough to detect an EMT phenotype in one of the cell lines and features of this phenotype were detectable using biological assays. This work demonstrates the potential of mass spectrometry-based global protein analyses and subsequent confirmation using targeted protein detection. Recent work by the NCI Clinical Proteomic Technology Assessment for Cancer (CPTAC) network has demonstrated the feasibility of implementing standardized proteome analyses across multiple laboratories (39–41) and we anticipate that these tools will dramatically expand our ability to understand the association between cancer-related genomic variation and cancer phenotypes.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grants U54CA126479 and U24159988 (to DCL) and R01GM088822 (to BZ). We thank Misti Martinez, Kristin Carpenter and Sarah Stuart for technical assistance. We thank Zhiao Shi for programming assistance.
Footnotes
P. Halvey is currently employed by Momenta Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 4.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 5.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 6.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger T, Cox J, Mann M. Proteomic changes resulting from gene copy number variations in cancer cells. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox J, Mann M. Is proteomics the new genomics? Cell. 2007;130:395–398. doi: 10.1016/j.cell.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Yates JR, Ruse CI, Nakorchevsky A. Proteomics by mass spectrometry: approaches, advances, and applications. Annu Rev Biomed Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 10.Halvey P, Zhang B, Coffey RJ, Liebler DC, Slebos RJC. Proteomic Consequences of a Single Gene Mutation in a Colorectal Cancer Model. J Proteome Res. 2012;11:1184–1195. doi: 10.1021/pr2009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Su Z, Ma ZQ, Slebos RJC, Halvey P, Tabb DL, et al. A bioinformatics workflow for variant peptide detection in shotgun proteomics. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.006536. M110.006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Slebos RJ, Wang D, Halvey PJ, Tabb DL, Liebler DC, et al. Protein identification using customized protein sequence databases derived from RNA-Seq data. J Proteome Res. 2012;11:1009–1017. doi: 10.1021/pr200766z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Halvey PJ, Shyr Y, Slebos RJ, Liebler DC, Zhang B. Integrative Omics Analysis Reveals the Importance and Scope of Translational Repression in microRNA-mediated Regulation. Mol Cell Proteomics. 2013;12:1900–1911. doi: 10.1074/mcp.M112.025783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabb DL, Fernando CG, Chambers MC. MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res. 2007;6:654–661. doi: 10.1021/pr0604054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TR, Dorotinsky CS, McGuire LJ, Macy ML, Hay RJ. DLD-1 and HCT-15 cell lines derived separately from colorectal carcinomas have totally different chromosome changes but the same genetic origin. Cancer Genet Cytogenet. 1995;81:103–108. doi: 10.1016/0165-4608(94)00225-z. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Gray W, Zhang H, Chung CH, Billheimer D, Yarbrough WG, et al. Comparative shotgun proteomics using spectral count data and quasi-likelihood modeling. J Proteome Res. 2010;9:4295–4305. doi: 10.1021/pr100527g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Liu Q, Zimmerman LJ, Ham AJ, Slebos RJ, Rahman J, et al. Methods for peptide and protein quantitation by liquid chromatography-multiple reaction monitoring mass spectrometry. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.006593. M110 006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Z, Derow CK, Zhang B. Co-expression module analysis reveals biological processes, genomic gain, and regulatory mechanisms associated with breast cancer progression. BMC Syst Biol. 2010;4:74. doi: 10.1186/1752-0509-4-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, et al. Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell. 2000;103:919–930. doi: 10.1016/s0092-8674(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 24.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–541. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 25.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Zha S, Boboila C, Alt FW. Mre11: roles in DNA repair beyond homologous recombination. Nat Struct Mol Biol. 2009;16:798–800. doi: 10.1038/nsmb0809-798. [DOI] [PubMed] [Google Scholar]
- 27.Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunican DS, McWilliam P, Tighe O, Parle-McDermott A, Croke DT. Gene expression differences between the microsatellite instability (MIN) and chromosomal instability (CIN) phenotypes in colorectal cancer revealed by high-density cDNA array hybridization. Oncogene. 2002;21:3253–3257. doi: 10.1038/sj.onc.1205431. [DOI] [PubMed] [Google Scholar]
- 29.Mori Y, Selaru FM, Sato F, Yin J, Simms LA, Xu Y, et al. The impact of microsatellite instability on the molecular phenotype of colorectal tumors. Cancer Res. 2003;63:4577–4582. [PubMed] [Google Scholar]
- 30.Banerjea A, Ahmed S, Hands RE, Huang F, Han X, Shaw PM, et al. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer. 2004;3:21. doi: 10.1186/1476-4598-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Bchiri J, Buhard O, Penard-Lacronique V, Thomas G, Hamelin R, Duval A. Differential nonsense mediated decay of mutated mRNAs in mismatch repair deficient colorectal cancers. Hum Mol Genet. 2005;14:2435–2442. doi: 10.1093/hmg/ddi245. [DOI] [PubMed] [Google Scholar]
- 32.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, et al. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93:6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 33.Saeterdal I, Bjorheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim WK, Park M, Kim YJ, Shin N, Kim HK, You KT, et al. Identification and Selective Degradation of Neopeptide-Containing Truncated Mutant Proteins in the Tumors with High Microsatellite Instability. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0684. [DOI] [PubMed] [Google Scholar]
- 35.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 36.Keshamouni VG, Michailidis G, Grasso CS, Anthwal S, Strahler JR, Walker A, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. J Proteome Res. 2006;5:1143–1154. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- 37.Mathias RA, Wang B, Ji H, Kapp EA, Moritz RL, Zhu HJ, et al. Secretome-based proteomic profiling of Ras-transformed MDCK cells reveals extracellular modulators of epithelial-mesenchymal transition. J Proteome Res. 2009;8:2827–2837. doi: 10.1021/pr8010974. [DOI] [PubMed] [Google Scholar]
- 38.Larriba MJ, Casado-Vela J, Pendas-Franco N, Pena R, Garcia de Herreros A, Berciano MT, et al. Novel snail1 target proteins in human colon cancer identified by proteomic analysis. PLoS One. 2010;5:e10221. doi: 10.1371/journal.pone.0010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulovich AG, Billheimer D, Ham AJ, Vega-Montoto L, Rudnick PA, Tabb DL, et al. Interlaboratory study characterizing a yeast performance standard for benchmarking LC-MS platform performance. Mol Cell Proteomics. 2010;9:242–254. doi: 10.1074/mcp.M900222-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabb DL, Vega-Montoto L, Rudnick PA, Variyath AM, Ham AJ, Bunk DM, et al. Repeatability and reproducibility in proteomic identifications by liquid chromatography-tandem mass spectrometry. J Proteome Res. 2010;9:761–776. doi: 10.1021/pr9006365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.