Abstract
POTS (postural tachycardia syndrome) is a chronic form of OI (orthostatic intolerance). Neuropathic POTS is characterized by decreased adrenergic vasoconstriction, whereas hyperadrenergic POTS exhibits increased adrenergic vasoconstriction. We hypothesized that midodrine, an α1-adrenergic receptor agonist, would increase CVR (calf vascular resistance), decrease Cv (calf venous capacitance) and decrease orthostatic tachycardia in neuropathic POTS, but not alter haemodynamics in hyperadrenergic POTS. A total of 20 POTS patients (12 neuropathic and 8 hyperadrenergic), ages 12–20 years, participated in this randomized placebo-controlled double-blind cross-over study. Of these subjects, 15 were female. POTS subjects received 2 weeks of treatment with midodrine or placebo, with increased dosing from 2.5 to 10 mg three times daily. Following a 7-day drug-washout period, subjects received the cross-over treatment. HR (heart rate), MAP (mean arterial pressure), Q̇calf (calf blood flow) and CVR were measured supine and during 35° HUT (head-up tilt). Cv was measured supine. In neuropathic POTS, midodrine decreased supine HR, Q̇calf, and Cv, while increasing MAP and CVR compared with placebo. During HUT, in neuropathic POTS, midodrine decreased HR, Q̇calf and Cv, while increasing MAP and CVR. In hyperadrenergic POTS, placebo and midodrine both decreased upright HR and increased supine CVR. Placebo also increased supine Cv, compared with midodrine in hyperadrenergic POTS. Therefore midodrine improved postural tachycardia in neuropathic POTS by increasing CVR and decreasing Q̇calf and Cv, whereas these effects were not seen in hyperadrenergic POTS patients who experienced a placebo effect. This suggests that midodrine is probably an effective treatment for neuropathic POTS, but not for hyperadrenergic POTS.
Keywords: α-adrenergic receptor, blood flow, midodrine, postural tachycardia syndrome (POTS), tachycardia
INTRODUCTION
POTS (postural tachycardia syndrome) is a chronic form of OI (orthostatic intolerance) defined in adolescents by the onset of symptoms within 5 min of 70° HUT (head-up tilt) and an increase in HR (heart rate) of at least 40 beats/min or a HR >120 beats/min [1]. Symptoms include light headedness, changes in vision, nausea, headache, fatigue and mental cloudiness [2]. On the basis of the available evidence it has been suggested that POTS may be grouped into two main subtypes: neuropathic and hyperadrenergic [2–6].
Neuropathic POTS involves localized decreased noradrenergic vasoconstriction in either the lower extremities or in the splanchnic vasculature [7]. While upright, individuals with neuropathic POTS redistribute an excessive amount of their central blood volume to the dilated vasculature, and this orthostatic challenge is compensated for by a resulting tachycardia [3]. Conversely, the term hyperadrenergic has been used to describe POTS patients with excessive noradrenergic vasoconstriction characterized by increased peripheral resistance and decreased (or ‘low’) arterial Q̇calf (calf blood flow) [2]. Studies of hyperadrenergic POTS have shown decreased NO-mediated vasodilation [8] and excessive increases in plasma noradrenaline levels when upright [5,6]. Patients with hyperadrenergic POTS experience supine tachycardia that is potentiated when upright. [4]. In the present study, we have chosen to use the term ‘neuropathic’ to describe POTS patients who have high or normal Q̇calf and ‘hyperadrenergic’ to describe POTS patients with low Q̇calf.
Midodrine, an α1-adrenergic receptor agonist, is a common treatment for POTS, but it is inconsistently effective [2,4,9,10]. Differences in noradrenergic activity between neuropathic and hyperadrenergic POTS may explain this inconsistency, and previous studies of efficacy of midodrine in POTS subjects did not stratify participants into POTS subtypes [10–13]. Furthermore, the use of an α1-adrenergic receptor agonist may be undesirable in hyperadrenergic POTS subjects who exhibit excessive peripheral vasoconstriction [4]. Therefore we investigated the effectiveness of midodrine in neuropathic compared with hyperadrenergic POTS using a randomized double-blind placebo-controlled cross-over design to evaluate drug efficacy in each group. We hypothesized that midodrine would preferentially improve haemodynamic abnormalities in the neuropathic POTS group by increasing peripheral vascular resistance, decreasing venous capacitance and decreasing orthostatic tachycardia, but that haemodynamic improvement in the hyperadrenergic POTS group would be limited.
MATERIALS AND METHODS
Subjects
A total of 20 POTS subjects, aged between 12 and 20 year, of which 15 were females, participated. Of these 12 subjects were diagnosed previously with neuropathic POTS, whereas 8 subjects were diagnosed previously with hyperadrenergic POTS. A total of 14 healthy control subjects participated in the study, but were not treated with midodrine or placebo. The diagnosis of POTS was verified in all subjects by a screening HUT test performed on a separate day during which we stratified POTS subjects based on supine Q̇calf as described previously [6,14]. Briefly, for this study, hyperadrenergic POTS had Q̇calf less than 1.2 ml/100 ml per min (low flow), whereas neuropathic POTS had Q̇calf greater than 1.2 ml/100 ml per min [7].
All POTS subjects had normal ECG and echocardiograms. All control subjects had normal ECG, but did not undergo echocardiogram evaluations. All subjects were free of evidence of cardiovascular, pulmonary, renal, neurological, endocrine or systemic illnesses. Subjects were not on any other medications while participating in the study. If they were on medications prior to enrolment, the medications were stopped for at least 2 weeks prior to the beginning of the study. All subjects refrained from consuming caffeine-containing products for at least 2 days before enrolment and while participating in the study.
Protocol
The present study was designed to evaluate the effects of treatment on the physiological responses to orthostatic stress. Additionally, control subjects only underwent one day of testing and were not treated with midodrine or placebo. Control subjects were used to compare pre-treatment baseline haemodynamics to the POTS subgroups. This study, conducted from 2001 to 2006 was approved by the IRB (Institutional Review Board) of the New York Medical College. Written informed consent was obtained from subjects over 18 years of age. For subjects under 18 years of age, subjects signed an age-appropriate form of assent and written, informed consent was obtained from a parent.
POTS protocol
POTS subjects had three consecutive visits to the laboratory. Tests were performed in a temperature-controlled room (24–26°C) after an overnight fast. At each visit, subjects were instrumented (see below) while supine and rested awake for 30 min to allow for acclimation. Data were recorded during the last 5 min of supine rest as baseline pre-treatment measurements. Following supine recordings, subjects underwent HUT testing. To mitigate the effects of the orthostatic challenge imposed by a 70° HUT, subjects were tilted to 35° for 15 min. The 35° angle was chosen because it is the lowest angle that produced increases in both HR and peripheral resistance in previous studies [16]. None of the study subjects, either POTS of controls, fainted during the 35° HUT.
Next, POTS subjects were randomized to treatments with either midodrine or placebo for 2 weeks. Midodrine and placebo were each placed within identical gelatin capsules with no identifiers. A research pharmacist prepared these capsules, randomized the subjects and dispensed all medications. Following this, subjects were monitored by a research nurse for compliance and side effects. Each treatment was taken for 14 days at which participants returned for testing as described above while on the last dose. Next, a 7-day drug washout period occurred followed by the cross-over treatment for 14 days. The protocol ended with the final testing on day 14 of the cross-over treatment while on the last dosage.
Midodrine and placebo dosages were increased based on the following: days 1–4: 2.5 mg three times daily; days 5–7: 5 mg three times daily; days 8–10; 7.5 mg three times daily, days 11–14: 10 mg three times daily. Neither the subjects nor study staff knew whether midodrine or placebo had been administered during each 2-week period; however, both groups knew of the dosage being taken throughout each 2-week period. This was allowed for safety monitoring of any side effects. If side effects occurred, the dose of midodrine or placebo was reduced to the last tolerated dose. All POTS subjects tolerated at least 7.5 mg of midodrine three times daily. Attempts were made to collect information on symptoms experienced during treatment; however, this data was incomplete and did not warrant analysis.
Control protocol
Control subjects only visited the laboratory once. The protocol for control subjects was similar to the first day of the POTS protocol. Briefly, subjects were fitted with the instruments (see below) while supine and rested awake for 30 min. Data were recorded during the last 5 min of supine rest. Next, subjects underwent HUT at 35° for 15 min. The last 5 min of tilt was analysed. None of the subjects fainted or experienced symptoms of pre-syncope while at 35°. Control subjects did not participate in the treatment part of this experiment.
Instrumentation and methods for haemodynamic measurements
Subjects were fitted with the instruments while supine on an electrically driven tilt table (Colin Instruments) with a footboard. A Finometer with height sensor was placed on the right index finger to continuously monitor AP (arterial pressure) (FMS). It was calibrated to the right brachial AP, which was measured via an automatic oscillometric BP (blood pressure) cuff (Colin Instruments). Similarly, lower limb AP at the popliteal artery was measured via a leg-sized automatic oscillometric BP cuff (Colin Instruments). An ECG was placed to monitor cardiac rhythm. An occlusion cuff (Hokanson) was placed around the thigh approximately 10 cm above a strain gauge (Hokanson), which was placed at the calf, to allow for measurement of Q̇calf via venous occlusion strain gauge plethysmography as described previously [6,14]. Lower extremity Q̇calf was measured at least three times, the results of which were averaged. Q̇calf was reported in units of ml/100 ml per min and estimated the rate of change of the increase in limb flow across the cross-sectional area of the limb. Lower extremity Pv (venous pressure) was measured by gradually increasing the occlusion cuff pressure until an increase in limb volume occurred. Pv measured by this method closely approximates invasive catheter-based measurements in humans [17].
Cv (calf venous capacitance) was measured using previously described techniques [15]. Briefly, while supine, the lower extremity was raised above heart level until no further decrease in volume was detected by the strain gauge, at which point the leg veins were considered empty (minus a small residual volume). After recovery from the leg lift, leg cuffs were inflated in incremental 10 mmHg steps, starting with the first pressure being 10 mmHg greater than Pv to a maximum of 60 mmHg above Pv. This produced progressive limb enlargement by inhibiting venous return and allowing for venous dilation. Pressure was maintained for 4 min at each pressure level to achieve a steady state. To calculate Cv, the two components of dilation (microvascular filtration compared with filling of capacitance vessels) were separated by extracting the linear component, which represents microvascular filtration, by the least squares methods [18]. Cv was calculated from the sum of filling of capacitance vessels added to the supine venous volume obtained from raising the limb. Thus, for each subject, a complete capacitance curve (percent change in calf volume in response to the change in pressure for each step) was generated.
Data analysis
MAP (mean AP) was calculated as the sum of SBP (systolic BP) plus 2×DBP (diastolic BP) all divided by 3 [MAP = (SBP + 2×DBP)/3]. CVR (calf vascular resistance) was calculated from the formula (MAPleg – Pv)/Q̇calf.
Data were interfaced to a personal computer through an A/D converter (DataQ) and sampled at 200 Hz. Analysis was done offline via custom proprietary software.
All measured values were reported as means ± S.E.M (standard error mean). Statistical analyses were completed using NCSS 8 software. Demographics data was compared between groups via one-factor ANOVA. Supine and upright comparison between control subjects and both POTS groups were done using two-factor repeated measures ANOVA. The factors were experimental group (control, neuropathic POTS and hyperadrenergic POTS) and angle (0° and 35°). Supine and upright measurements between neuropathic and hyperadrenergic POTS subjects were analysed using a three-factor repeated measures ANOVA. The factors were experimental group (neuropathic and hyperadrenergic), angle (0° and 35°) and treatment (pre-treatment, midodrine and placebo). For all ANOVA tests, post-hoc Student–Newman–Keul's multiple comparison's tests were applied when appropriate to determine differences. Significance was set at P < 0.05 for all tests.
RESULTS
Demographics
As shown in Table 1, age, height, weight and BMI (body mass index) did not vary between healthy control, neuropathic POTS and hyperadrenergic POTS subjects.
Table 1.
Demographic (A) and pre-treatment (B) values in control, neuropathic POTS and hyperadrenergic POTS subjects while supine and during HUT
| (A) | ||||
|---|---|---|---|---|
| Parameter | Controls (n = 14) | Neuropathic POTS (n = 12) | Hyperadrenergic POTS (n = 8) | P values |
| Age (years) | 17.2 ± 0.6 | 16.1 ± 0.5 | 16.8 ± 0.85 | 0.4 |
| Height (m) | 1.68 ± 0.02 | 1.69 ± 0.02 | 1.67 ± 0.04 | 0.8 |
| Weight (kg) | 63.4 ± 2.6 | 65.0 ± 5.0 | 58.0 ± 5.0 | 0.5 |
| BMI (kg/m2) | 23.0 ± 0.9 | 22.7 ± 1.5 | 20.5 ± 1.3 | 0.4 |
| (B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Controls (n = 14) |
Neuropathic POTS (n = 12) |
Hyperadrenergic POTS (n = 8) |
||||||
| Parameter | Tilt angle... | 0° | 35° | 0° | 35° | 0° | 35° | P values |
| HR (beats/min) | 68 ± 3 | 80 ± 3 | 71 ± 4 | 97 ± 6‡ | 77 ± 5* | 103 ± 9‡ | 0.02, 0.0001 | |
| MAP (mmHg) | 78 ± 2 | 80 ± 2 | 74 ± 3 | 79 ± 3 | 80 ± 3 | 80 ± 4 | 0.6 | |
| Q̇calf (ml/100ml per min) | 3.02 ± 0.4 | 2.42 ± 0.3 | 3.15 ± 0.4 | 3.69 ± 0.9 | 1.06 ± 0.1‡ | 1.69 ± 0.3 | 0.03 | |
| Cv (ml/100ml) | 5.00 ± 0.3 | 4.54 ± 0.3 | 2.99 ± 0.3‡ | 0.0004 | ||||
Values are means ± S.E.M.
P < 0.05
† P < 0.01
P < 0.001 between the experimental groups
Treatment dose at end point
All POTS subjects took at least 7.5 mg of midodrine or placebo three times daily. In the neuropathic POTS groups, two subjects tolerated 7.5 mg three times daily, whereas ten subjects tolerated 10 mg three times daily. In the hyperadrenergic POTS group, one subject tolerated 7.5 mg three times daily, whereas seven subjects tolerated 10 mg three times daily. There was no significant difference between mean dose taken three times daily in neuropathic (9.6 ± 0.3 mg) compared with hyperadren ergic (9.7 ± 0.3 mg) POTS (P = 0.8), nor the number of subjects taking each dose in each group (P = not significant), and the mean total amount of treatment taken daily did not differ between groups (28.8 ± 0.8 mg in neuropathic POTS compared with: 29.1 ± 0.9 mg in hyperadrenergic POTS; P = 0.8). All of the neuropathic subjects took the maximum number of placebo capsules, four capsules three times daily, the same number of capsules equivalent to 10 mg of midodrine. In the hyperadrenergic group, one subject took two capsules three times daily, whereas the remaining subjects took four capsules three times daily. There was no significant difference between the mean numbers of capsules taken three times daily between the two groups (P = 0.4).
Pre-treatment differences
HR and AP
HR and BP measurements recorded before drug treatment (pre-treatment) are shown in Table 1. Supine HR was higher in the hyperadrenergic group compared with controls (P = 0.02), but did not vary between the neuropathic group and controls (P = 0.4). HR increased in all groups during HUT (P = 0.03). Following HUT, HR was higher in both POTS groups compared with controls (P < 0001) but did not vary between POTS groups (P = 0.6). MAP did not vary between groups (P = 0.6) and was not affected by HUT (P = 0.2).
Vascular responses
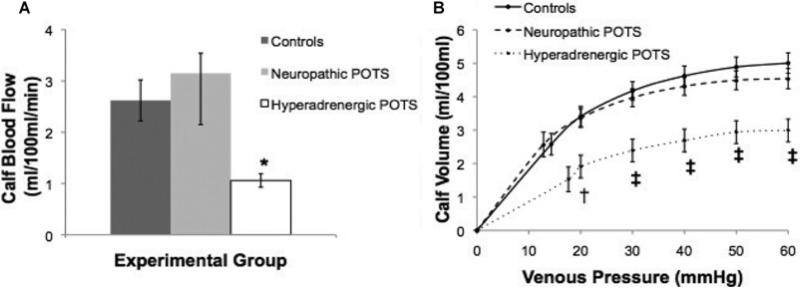
Figure 1 shows the comparison of Q̇calf and Cv in POTS and control subjects while supine. As shown in Figure 1(A), supine Q̇calf was lower in the hyperadrenergic POTS group compared with neuropathic POTS and the control subjects (P = 0.03). Table 1 shows that, although there was an increase in Q̇calf with increasing angle in the neuropathic POTS group, there was a decrease in Q̇calf with increasing angle in the control group, yet these differences did not achieve significance (P = 0.09). There was no change in Q̇calf with tilt in the hyperadrenergic POTS group (P = 0.7). However, as shown in Figure 1(B), while supine, hyperadrenergic POTS subjects had a significantly lower Cv than neuropathic POTS and control subjects (P = 0.0004).
Figure 1. Supine Q̇calf (A) and Cv (B) between the groups.
(A) Hyperadrenergic POTS had significantly less Q̇calf than control subjects and neuropathic POTS subjects. (B) The volume–pressure capacitance curve was blunted in hyperadrenergic compared with controls and neuropathic POTS. Values are means ± S.E.M. *P < 0.05, †P < 0.01 and ‡P < 0.001 between the groups.
Effects of treatment
Effects of treatment in neuropathic POTS
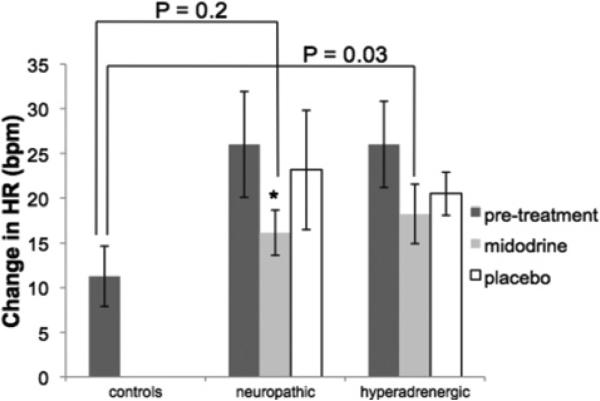
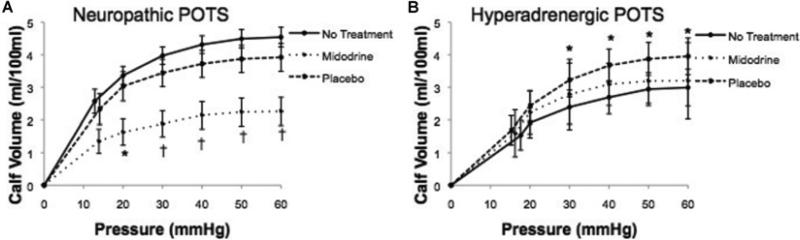
The effects of treatment on measured cardiovascular parameters are shown in Table 2. In neuropathic POTS, midodrine decreased HR (P = 0.0002) and Q̇calf (P = 0.008) compared with placebo at 0° and 35° HUT. Midodrine increased MAP (P = 0.006) and CVR (P = 0.001) compared with placebo at 0° and 35° in neuropathic POTS. As shown in Figure 2, midodrine, but not placebo, blunted the increase in HR from 0° to 35° HUT (P = 0.04). As shown in Figure 3(A), in neuropathic POTS, midodrine reduced Cv (P = 0.002) and the volume–pressure capacitance relationship compared with baseline and placebo (P < 0.01).
Table 2.
Cardiovascular responses to treatment in neuropathic and hyperadrenergic POTS
| Placebo |
Midodrine |
|||||
|---|---|---|---|---|---|---|
| Parameter | Tilt angle... | 0° | 35° | 0° | 35° | P value |
| HR (beats/min) | ||||||
| Neuropathic POTS | 69 ± 4 | 93 ± 6 | 63 ± 3‡ | 79 ± 3‡ | 0.0002 | |
| Hyperadrenergic POTS | 70 ± 4 | 91 ± 6 | 69 ± 7 | 87 ± 4 | 0.3 | |
| MAP (mmHg) | ||||||
| Neuropathic POTS | 77 ± 3 | 77 ± 2 | 79 ± 2† | 84 ± 3† | 0.006 | |
| Hyperadrenergic POTS | 81 ± 2 | 82 ± 2 | 77 ± 3 | 77 ± 5 | 0.6 | |
| Q̇calf (ml/100 ml per min) | ||||||
| Neuropathic POTS | 2.31 ± 0.4 | 3.56 ± 0.9 | 1.89 ± 0.2† | 1.09 ± 0.2† | 0.008 | |
| Hyperadrenergic POTS | 1.90 ± 0.3 | 1.62 ± 0.2 | 1.82 ± 0.2 | 1.28 ± 0.2 | 0.08 | |
| CVR [mmHg/(ml/100 ml per min)] | ||||||
| Neuropathic POTS | 27 ± 3 | 57 ± 12 | 33 ± 4‡ | 93 ± 15‡ | 0.001 | |
| Hyperadrenergic POTS | 34 ± 4 | 52 ± 13 | 35 ± 6 | 59 ± 14 | 0.9 | |
| Cv (ml/100ml) | ||||||
| Neuropathic | 4.00 ± 0.4 | 2.26 ± 0.4† | 0.002 | |||
| Hyperadrenergic | 3.95 ± 0.6 | 3.20 ± 0.6* | 0.02 | |||
Values are means ± S.E.M.
P < 0.05
P < 0.01
P < 0.001 compared with the cross-over treatment within group.
Figure 2. Changes in HR in response to HUT in controls, neuropathic POTS and hyperadrenergic POTS subjects.
Midodrine decreased the HR response to HUT in neuropathic but not in hyperadrenergic POTS. Following midodrine treatment, the HR responses of neuropathic POTS were not significantly different than the HR responses of healthy controls, whereas the hyperadrenergic group maintained augmented HR responses with midodrine treatment. *P < 0.05 compared with pre-treatment.
Figure 3. The effects of treatment on the volume–pressure capacitance relationship in neuropathic POTS (A) and hyperadrenergic POTS (B).
As shown in (A), midodrine blunted the capacitance curve in neuropathic POTS. Shown in (B), placebo increased capacitance in hyperadrenergic POTS. *P < 0.05 and †P < 0.01 compared with pre-treatment and cross-over treatment.
Effects of treatment in hyperadrenergic POTS
In hyperadrengic POTS, both midodrine and placebo lowered HR during 35° HUT (P = 0.05); however, there was no significant difference between midodrine and placebo (P = 0.3). Both midodrine and placebo significantly decreased supine CVR (P = 0.04) compared with pre-treatment, but there was no difference between the two treatments (P = 0.9). However, neither midodrine nor placebo effected HR at=0° (P = 0.2) or CVR at 35° HUT (P = 0.3). Neither treatment affected MAP (P = 0.6) or Q̇calf (P = 0.08) at 0° or 35° HUT. As shown in Figure 2, neither treatment decreased the HR response to tilt in hyperadrenergic POTS (P = 0.1). As shown in Figure 3(B), in hyperadrenergic POTS, placebo significantly increased Cv (P = 0.02) and the volume–pressure capacitance relationship (P < 0.05) compared with midodrine.
DISCUSSION
Main findings
The present study evaluated the effects of midodrine in patients with neuropathic and hyperadrenergic POTS which was categorized by Q̇calf, a measurement indicative of adrenergic vasoconstriction. There were two main findings in the present study. First, midodrine improved postural tachycardia compared with placebo in neuropathic but not in hyperadrenergic POTS by increasing CVR and decreasing Q̇calf and Cv. Secondly, there was a placebo effect in hyperadrenergic POTS. Thus this is the first study to show that treatment with midodrine has significant vascular effects in patients with neuropathic but not in those with hyperadrenergic POTS. These findings demonstrate the importance of stratifying POTS patients on the basis of Q̇calf.
Effects of midodrine on postural tachycardia
Midodrine improved postural tachycardia in neuropathic POTS. As shown in Figure 2, following the administration of midodrine, the increase in HR with HUT in neuropathic POTS was not different than the HR response of healthy controls. The decrease in upright tachycardia with midodrine in the neuropathic POTS group was likely related to an increase in measured CVR and the associated reduction in Q̇calf and Cv. Typically, neuropathic POTS subjects have excessive pooling in the extremities due to blunted peripheral vascular sympathetic activity [19]. When neuropathic POTS subjects are treated with midodrine, their vasoconstrictor response to orthostatic challenge is augmented. In these subjects with neuropathic POTS, the midodrine-induced increased vasoconstriction and venoconstriction in the legs may have facilitated central mobilization of blood from the legs that would normally be sequestered during HUT. The observed peripheral vascular changes likely improved cardiac output, venous return and thoracic hypovolemia, thus decreasing the orthostasis-related compensatory tachycardia.
However, in hyperadrenergic POTS, subjects exhibit exaggerated peripheral vasoconstriction pre-treatment. Both midodrine and placebo similarly decreased upright HR, yet did not alter the HR response to HUT in the hyperadrenergic group. Furthermore, midodrine did not further augment Q̇calf,, or Cv during 0° or 35° HUT. It is likely that there was only a minor change in cardiac output, venous return and thoracic hypovolemia in this group. Thus our data suggest that the changes in tachycardia while upright were not due to mobilization of peripheral blood volume in this group.
Placebo effect in hyperadrenergic POTS
Of additional interest was our finding that placebo decreased upright HR in hyperadrenergic POTS to a similar extent as midodrine. This placebo effect is difficult to explain, but may in part be due to the act of receiving a treatment, rather than being caused by a specific mechanism. In addition, both treatments also decreased CVR while supine, but had no effect on CVR during HUT. Furthermore, placebo increased Cv in hyperadrenergic POTS compared with midodrine treatment. The placebo effect has been verified in many disorders [20], and Raj et al. [21] have shown that placebo was effective at reducing HR in POTS subjects during a cross-over study of pyridostigmine. As in our present study, the reason behind their reported placebo effect was unclear. One possible explanation for the placebo effect seen in our study is that hyperadrenergic POTS subjects have decreased NO-dependent vasodilation [9] and increased plasma noradrenaline levels [5,6]. Stefano et al. [13] found that the placebo effect could be a vasodilation response controlled by NO signalling and its effects on noradrenaline synthesis and binding. Although speculative, receiving treatment in the form of placebo may reduce sympathetic activity and modulate its effect on HR and peripheral vasoconstriction.
Clinical benefits of midodrine in neuropathic compared with hyperadrenergic POTS
Our experience during the conduct of the present study also suggests that midodrine was generally well-tolerated by all POTS subjects. Although only three subjects could not tolerate the highest dosage of 10 mg three times daily, all subjects tolerated 7.5 mg three times daily. The most commonly reported side effects were headache and cutis anserine (so-called ‘goose-bumps’), but the severity of these was not enough to deter treatment. Supine hypertension is a common side effect of midodrine in other forms of OI [22], but was not experienced by any of our POTS subjects of either subtype.
Previous evaluations of midodrine have shown its effectiveness to be inconsistent which is in contrast to our study that shows its effectiveness in at reducing HR in all 12 neuropathic POTS patients studied. Neuropathic POTS, which is the more common variant of POTS [5], may improve with midodrine since its effects likely mitigate the undesirable consequences of peripheral venous pooling. The exaggerated sympathetic activity in hyperadrenergic POTS may respond to different treatments, such as β-blockade [23].
Our results describing the utility of midodrine are somewhat similar to other trials in POTS patients, however, no previous study has stratified POTS patients according to our subtypes. Gordon et al. [14] and Hoeldtke et al. [13] found midodrine, among other medications, to be acutely effective at reducing orthostatic tachycardia in POTS. Lai et al. [10] found that midodrine was an effective treatment in 14 out of 47 adolescents with POTS and determined by subjectively reported symptom surveys. They did not compare the results of midodrine to placebo, and their study was retrospective. Chen et al. [11] found a once daily, 2.5 mg, dose of midodrine to be superior to β-blockade, and/or conventional treatment in a group of children with POTS who were significantly younger than those in the current study.
Limitations
Owing to individual differences, not all subjects tolerated the same midodrine dose during the study. However, as noted previously, all subjects tolerated at least 7.5 mg three times daily and there were no significant differences in dosing between experimental groups. Subjects may also have experienced side effects of midodrine thereby making the ‘blinding’ of the present study difficult. However, our subjects did not report difficulties while on midodrine for the short duration of the study. As discussed, subjects were classified as neuropathic or hyperadrenergic based on their supine leg blood flow. We did not test for nerve density or neuropathy to directly show denervation in the neuropathic subjects [5]. We also did not employ measurements of sympathetic outflow by either microneurography or by measuring plasma noradrenaline, which is the typical method of diagnosis of this subtype [4,5,8]. Instead, we evaluated efferent sympathetic activity via changes in vascular resistance. We also did not collect adequate information of symptoms from patients in this study and therefore cannot report on potential symptomatic improvement with midodrine treatment.
We found that in neuropathic POTS with placebo treatment, Q̇calf increased with HUT, presumably due to a gravitationally mediated increase in blood flow without adequate sympathetic mediated vasoconstriction. We also showed that in response to midodrine, Q̇calf decreased while supine due to drug-induced vasoconstriction. However, we cannot explain why, in the presence of midodrine, Q̇calf decreased further with HUT. Since we have no direct measure of sympathetic activity we cannot comment on whether this is due to a further increase in sympathetic tone. In addition, the amount of desglymidodrine, the active metabolite of midodrine presumably remained stable during this time, but direct measurement of this is lacking as well in the present study.
Summary and practical significance
The results of the present study have demonstrated the efficacy of midodrine at decreasing postural tachycardia in a group of subjects with neuropathic POTS using a double-blind, placebo-controlled, cross-over study design. Hyperadrenergic POTS subjects responded similarly to midodrine and placebo treatment. The data suggests the utility of subtyping individuals with POTS based on supine Q̇calf prior to initiation of drug therapy, and that only the neuropathic group may exhibit a physiological response to midodrine.
CLINICAL PERSPECTIVES.
● POTS is a chronic form of OI with few proven treatments, presumably due to its varying pathophysiology. The present study evaluated the effects of midodrine, an α-adrenergic receptor agonist, in patients with neuropathic and hyperadrenergic POTS subtypes distinguished by measuring Q̇calf.
● The main findings of the present study are that midodrine improved postural tachycardia compared with placebo in neuropathic POTS by increasing peripheral vasoconstriction and venoconstriction. However, hyperadrenergic POTS subjects experienced a placebo effect to treatment.
● Thus this is the first study to show that treatment with midodrine has significant vascular effects in patients with neuropathic but not in those with hyperadrenergic POTS. These findings demonstrate the importance of stratifying POTS patients on the basis of Q̇calf for determination of their treatment.
ACKNOWLEDGEMENTS
We thank the research subjects for their participation. Special thanks to the Departments of Pediatrics and Physiology at New York Medical College.
FUNDING
This work was supported by the National Heart, Lung, and Blood Institute [grant numbersRO1-HL074873, RO1-HL087803, 1-F30-HL-097380], and the Chronic Fatigue and Immune Deficiency Syndrome (CFIDS) Association.
Abbreviations
- AP
arterial pressure
- BP
blood pressure
- Cv
calf venous capacitance
- CVR
calf vascular resistance
- DBP
diastolic BP
- HR
heart rate
- HUT
head-up tilt
- MAP
mean AP
- OI
orthostatic intolerance
- POTS
postural tachycardia syndrome
- Pv
venous pressure
- Q̇ calf
calf blood flow
- SBP
systolic BP
Footnotes
AUTHOR CONTRIBUTION
Julian Stewart designed and conducted the study with the assistance of Marvin Medow. Amanda Ross, Anthony Ocon, Marvin Medow and Julian Stewart took part in data analysis and interpretation. Amanda Ross and Anthony Ocon wrote the paper. Amanda Ross prepared the Tables and Figures. Amanda Ross, Julian Stewart and Marvin Medow revised the paper before submission.
REFERENCES
- 1.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J. Pediatr. 2012;160:222–226. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart JM. Update on the theory and management of orthostatic intolerance and related syndromes in adolescents and children. Expert Rev. Cardiovasc. Ther. 2012;10:1387–1399. doi: 10.1586/erc.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart JM. Mechanisms of sympathetic regulation in orthostatic intolerance. J. Appl. Physiol. 2012;113:1659–1668. doi: 10.1152/japplphysiol.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics. 2013;131:968–980. doi: 10.1542/peds.2012-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis, and management. Indian Pacing Electrophysiol. J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 6.Low PA, Sandrioni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J. Cardiovasc. Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am. J Physiol. Heart Circ. Physiol. 2003;285:H2749–H2756. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- 8.Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation. 2005;112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubb BP. Postural tachycardia syndrome. Circulation. 2008;117:2814–2817. doi: 10.1161/CIRCULATIONAHA.107.761643. [DOI] [PubMed] [Google Scholar]
- 10.Lai CC, Fischer PR, Brands CK, Fisher JL, Porter CB, Sriscoll SW, Graner KK. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and β-blockers. Pacing Clin. Electrophysiol. 2009;32:234–238. doi: 10.1111/j.1540-8159.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Wang L, Sun J, Qin J, Tang C, Jin H, Du J. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ. J. 2011;75:927–931. doi: 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- 12.Hoeldtke RD, Bryner KD, Hoeldtke ME, Hobbs G. Treatment of postural tachycardia syndrome: a comparison of octreotide and midodrine. Clin. Auton. Res. 2006;16:390–395. doi: 10.1007/s10286-006-0373-0. [DOI] [PubMed] [Google Scholar]
- 13.Stefano GB, Fricchione GL, Slingsby BT, Benson H. The placebo effect and relaxation response: neural processes and their coupling to constitutive nitric oxide. Brain Res. Rev. 2001;35:1–19. doi: 10.1016/s0165-0173(00)00047-3. [DOI] [PubMed] [Google Scholar]
- 14.Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin. Auton. Res. 2000;10:29–33. doi: 10.1007/BF02291387. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105:2274–2281. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute α-1 adrenergic agonist and a β-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. 2002;106:2964–2954. doi: 10.1161/01.cir.0000040999.00692.f3. [DOI] [PubMed] [Google Scholar]
- 17.Christ F, Gamble J, Baschnegger H, Gartside IB. Relationship between venous pressure and tissue volume during venous congestion plethysmography in man. J. Physiol. 1997;503:463–467. doi: 10.1111/j.1469-7793.1997.463bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press; Cambridge: 1992. pp. 59–70. [Google Scholar]
- 19.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, Mosqueda-Garcia R. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–2159. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 20.Benson H, Epstein MD. The placebo effect: a neglected asset in the care of patients. JAMA, J. Am. Med. Assoc. 1975;232:1225–1227. doi: 10.1001/jama.232.12.1225. [DOI] [PubMed] [Google Scholar]
- 21.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 22.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension: a randomized, double-blind multicenter study. JAMA, J. Am. Med. Assoc. 1997;277:1046–1051. [PubMed] [Google Scholar]
- 23.Raj SR, Black BK, Biaggioni I, Sachin PY, Ramirez M, Dupont WD, Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome (POTS): less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]