Abstract
Altered peripheral haemodynamics, decreased cardiac output, decreased blood volume and increased AngII (angiotensin II) have been reported in POTS (postural tachycardia syndrome). Recent findings indicate that BMI (body mass index) may be reduced. In the present study, we investigated the hypothesis that reduced BMI is associated with haemodynamic abnormalities in POTS and that this is related to AngII. We studied 52 patients with POTS, aged 14–29 years, compared with 36 control subjects, aged 14–27 years. BMI was not significantly reduced on average in the POTS patients, but was reduced in patients with decreased peripheral blood flow. POTS patients were then subdivided on the basis of BMI, and supine haemodynamics were measured. There was no difference in blood volume or cardiac output once BMI or body mass were accounted for. When POTS patients with BMI < 50th percentile were compared with controls, calf blood flow [1.63 ± 0.31 compared with 3.58 ± 0.67 ml−1 · min−1 · (100 ml of tissue)−1] and maximum venous capacity (3.87 ± 0.32 compared with 4.98 ± 0.36 ml/100 ml of tissue) were decreased, whereas arterial resistance [56 ± 0.5 compared with 30 ± 4 mmHg · ml−1 · min−1 · (100 ml of tissue)−1] and venous resistance [1.23 ± 0.17 compared with 0.79 ± 0.11 mmHg · ml−1 · min−1 · (100 ml of tissue)−1] were increased. Similar findings were also observed when POTS patients with BMI < 50th percentile were compared with POTS patients with BMI > 50th percentile. There was no relationship between blood flow, resistance or maximum venous capacity with BMI in control subjects. BMI was inversely related to plasma AngII concentrations in those POTS patients with decreased peripheral blood flow, consistent with cachectic properties of the octapeptide. Patients with low-flow POTS had decreased body mass, but decreased body mass alone cannot account for findings of peripheral vasoconstriction. In conclusion, the findings suggest that reduced body mass relates to increased plasma AngII.
Keywords: angiotensin II, body mass index (BMI), haemodynamics, peripheral blood flow, postural tachycardia syndrome (POTS), vasoconstriction, young women
INTRODUCTION
POTS (postural tachycardia syndrome) is defined by excessive changes in HR (heart rate) and symptoms of orthostatic intolerance [1] (see the Materials and methods section for the complete criteria). Observed haemodynamic abnormalities are diverse [2–11]. Common final pathophysiological pathways include abnormal regional blood flows and blood volumes that produce excessive postural thoracic hypovolaemia and reflex tachycardia, or excessive sympatho-excitation and vagal withdrawal during upright posture, or both.
Differences in peripheral blood flow, especially in calf blood flow, allowed for a consistent classification scheme [10] that enabled us to partition patients into three groups. One group, which we denoted ‘low-flow POTS’, has globally reduced blood flow in association with absolute hypovolaemia, supine tachycardia, reduced stroke volume, sympatho-excitation, blunted orthostatic vascular responses, increased plasma Ang (angiotensin) II [12] and decreased bioavailability of cutaneous NO (nitric oxide) [13]. Interestingly, all patients were female. A second group, which we denoted ‘normal-flow POTS’, comprised patients of both genders with normal blood flow and blood volume while supine, which excessively redistributes to the splanchnic circulation during upright posture because of inadequate splanchnic vasoconstriction. Normal-flow patients developed thoracic hypovolaemia, intense peripheral vasoconstriction and acrocyanosis [14]. The third group, which we denoted ‘high-flow POTS’, comprised normovolaemic patients of both genders with increased blood flow in the lower extremities when both supine and upright. There is markedly increased microvascular filtration accounting for postural tachycardia in high-flow POTS [15]; the illness tends to be short-lived and they are not well-represented among the most chronic patients in recent research studies.
In previous studies, POTS patients had body masses and BMI [body mass index; body mass (in kg)/height (in m2)] averaged across groups, which tended to be decreased compared with healthy control subjects, but did not reach significance. The literature indicates that AngII can mediate anorexia and cachexia in model animal systems and in humans [16–18]. Studies have demonstrated the potential for increased AngII within the CNS (central nervous system) producing both sympatho-excitation [19] and cachexia [20].
In the present study, we investigated the hypothesis that BMI is significantly reduced in the low-flow POTS group compared with female normal-flow POTS patients and female healthy control subjects. We examined the relationship between BMI and abnormalities in blood flow and blood volume in all POTS patients, and examined whether BMI correlates with circulating AngII concentration in patients and controls.
MATERIALS AND METHODS
Subjects
POTS patients were recruited from patients referred to the Hypotension Center for investigation of chronic orthostatic intolerance lasting at least 3 months. Orthostatic intolerance was defined by the presence of dizziness, fatigue, exercise intolerance, headache, memory problems, palpitations, nausea, blurred vision, pallor and abnormal sweating while upright relieved by recumbence, which had no other medical explanation. The diagnosis of POTS was made in these patients during an upright tilt table test (see below). POTS was diagnosed by symptoms of orthostatic intolerance during tilting associated with an increase in sinus HR of > 30 beats/min or to a rate consistently > 120 beats/min during 10 min of tilting [5,21]. For the purposes of the present study, we enrolled only female subjects, because our previous studies have shown that all low-flow POTS patients are female. Using these methods, we recruited 52 female POTS patients (aged, 14.1–29 years; median age, 19.4 years). A total of 36 volunteer subjects were also recruited (all female; aged, 13.6–27.1 years; median age, 21.2 years) and were studied after a screening upright tilt at 70° demonstrated normal orthostatic response. Volunteer subjects served as a control group and were recruited from among adolescents referred for innocent heart murmurs. Subjects with a history of syncope or orthostatic intolerance were specifically excluded. Patients and control subjects were excluded if they used any chronic medication, except for birth control pills. Exclusion criteria also included smoking, pregnancy, or significant physical or mental illnesses; specific exclusions were diabetes, known dysautonomia or localized neuropathy, skin diseases, cardiac disease, cerebrovascular disease, peripheral vascular disease, neoplastic processes, inflammatory illnesses, collagen vascular disease, chronic angio-oedema or lymphoedema, uncontrolled hyperthyroidism, substance abuse or conditions, which precluded following the protocol.
Informed consent was obtained from the subjects according to the standards of the Committee for the Protection of Human Subjects (the IRB) at New York Medical College.
BMI
We measured BMI as a function of age in low-flow POTS patients compared with female normal-flow POTS patients and female control subjects. These data were plotted on a standardized chart from NHANES-II (Second National Health and Nutrition Examination Survey) of the Centers for Disease Control [22].
Physiological protocol
To compare physiological data, we divided patients on the basis of BMI (see the Data analysis and statistics subsection). Tests began in a temperature-controlled room after an overnight fast. An intravenous catheter was placed in the right antecubital fossa. A single ECG lead was recorded for heart rhythm and HR. Upper extremity BP (blood pressure) was monitored continuously with a finger arterial plethysmograph (Finometer; FMS) placed on the right middle or index finger. HR was derived from arterial pressure data. Finometer data were calibrated to a brachial cuff oscillographic pressure. MAP (mean arterial pressure) was calculated.
Following a 30 min acclimatization period, venous blood was withdrawn for measurement of AngII. Supine cardiac output was then estimated, and blood volume was determined by the ICG (Indocyanine Green) dyedilution technique [23]. Subsequently, forearm and calf blood flows were measured by venous occlusion SGP (strain gauge plethysmography), and Pv (venous pressure) was estimated. A stepwise increase in occlusion cuff pressure around the upper arm and thigh were used to generate steady-state volume–pressure relationships, venous capacitance and an assessment of maximum venous capacity. Measurements of supine haemodynamics made prior to the defining tilt table test were used for all comparisons.
HR, BP and strain gauge data were multiplexed through an A/D converter (DI-720; DataQ Ind) and were thereby synchronized.
Dye-dilution measurement of blood volume and cardiac output
The ICG dye-dilution technique was used to measure blood volume and cardiac output [23]. A spectrophotometric finger photosensor (DDG2000; Nihon–Kohden) was used, which has been validated by previous clinical studies [24]. The dye-decay curve fits a mono-exponential V0exp−[Kt], where K represents clearance by the liver divided by blood volume. The haematocrit was measured, and we extrapolated the dye-decay curve to the time of dye injection (t = 0), yielding estimated blood volume.
AngII assay
Following a 30 min equilibration period, venous blood for assay purposes was collected into an EDTA tube from the catheter in the antecubital vein with subjects supine. The EDTA tube also contained protease inhibitors, including 0.44 mmol/l 1,10 o-phenanthroline monohydrate, 0.12 mmol/l pepstatin and 1 mmol/l sodium p-hydroxymercuribenzoate. The sample was centrifuged and the plasma was removed. Plasma was extracted using Sep-Pak columns activated with 5 ml of sequential washes of a mixture of ethanol/water/4% acetic acid, methanol and distilled water. The sample was washed with acetone and water and then eluted with washes of a mixture of ethanol/water/4% acetic acid. The eluted sample was reconstituted and measured by RIA (Quest Diagnostics Laboratory, Nichols Institute, San Juan Capistrano, CA, U.S.A.). The minimum detectable level of the assay is 3.8 pg/ml. Intra-assay coefficient of variation was 4% at a mean value of 42 pg/ml; inter assay variation was 9.3% at a mean value of 96 pg/ml. The antibody used in this kit shows 100%cross-reactivity with AngII and 70% cross-reactivity to AngIII-(2–8) and Ang-(3–8), but no cross-reactivity with AngI. Therefore the values reported do not distinguish between AngII, AngIII and Ang-(3–8).
Tilt table testing
Recordings of supine BP and HR were obtained close to the end of the supine measurements. All subjects were then tilted upright to 70° for a maximum of 10 min. An electrically driven tilt table (Colin Medical) with a footboard was used. The test was curtailed on patient request due to intolerable symptoms.
SGP measurements
Venous occlusion SGP was used in all subjects to measure peripheral blood flow and volume parameters. Methods were adapted from the studies by Gamble et al. [25,26].
Measurement of blood flow
Occlusion cuffs were placed around the middle of the biceps and mid-thigh. Blood flow was measured by rapidly inflating occlusion cuffs to a pressure just below diastolic pressure to prevent venous egress. Diastolic pressure was verified in the supine position by oscillometry in the arm and calf contralateral to the strain gauges. We used a secondary cuff to prevent wrist and ankle flow. Arterial inflow [in ml · min−1 · (100 ml of tissue)−1] was estimated as the rate of change of the rapid increase in limb cross-sectional area.
Measurement of Pv
After returning to baseline, occlusion pressure was increased gradually until a limb volume change was just detected. This represents ambient venous pressure Pv and has been verified by invasive testing [25].
Calculation of arterial resistance
MAP, calculated as 0.33 ×(systolic BP) + 0.67 × (diastolic BP), and Pv were used to calculate arterial resistance to blood flow [in mmHg · ml−1 · min−1 · (100 ml of tissue)−1] using (MAP − Pv)/flow.
Volume–pressure relationship at venous pressure ≤ Pv
With cuffs deflated and the subject supine, the limb was elevated progressively, the elevation was measured at the level of the strain gauge, and the simultaneous decrease in limb volume with each elevation was recorded. Venous pressure at the strain gauge was estimated from the hydraulic formula P = Pv_rest − 0.776 × Δh, where the constant 0.776 is the pressure conversion factor from cm of blood to mmHg and Δh is the height of the strain gauge above the table. By fixing pressure with limb elevation, the portion of the capacitance vessel volume–pressure relationship less than Pv was obtained.
Volume–pressure relationship at venous pressure ≥ Pv
After returning the leg to its original position and after strain gauge limb size had returned to baseline, we increased the pressure in the occlusion cuff by 10 mmHg steps to 60 mmHg, starting at the first multiple of 10 exceeding Pv. By fixing the pressure with the congestion cuff, the portion of the capacitance vessel volume–pressure relationship exceeding Pv was obtained.
After step-wise increases in the pressure were complete, the occlusion cuff was rapidly deflated. The instantaneous pressure gradient at the transducer between the venous bed and central veins was estimated by the difference between 60 mmHg and the central venous pressure (assumed to be close to 0 mmHg). Venous resistance was estimated from the initial downward slope using the formula Rv=P(60 mmHg)/efflux [27].
Vascular filling
Increasing pressure steps are associated with plethysmographic changes in limb volume, which include contributions from both capacitance vessel filling and microvascular filtration [8]. Limb volume increases are at first curvilinear, but then increase linearly with time due to filtration. We used a modified least-squares analysis and curve stripping to separate venous filling from filtration.
Computation of the volume–pressure relationship (compliance relationship)
The overall volume–pressure relationship was constructed after curve stripping resolved the capacitance contribution to limb enlargement. Capacitance is assumed to reside primarily in veins and venules. The maximum venous capacity was taken as the difference between asymptotic maximum volume obtained during increasing occlusion cuff pressure steps and the minimum volume obtained by leg lifting.
Data analysis and statistics
Data were digitized, stored on a computer and were analysed off-line with customized software. HR and BP were determined at each stage expressed as means over 60 s intervals. Blood flows and peripheral resistances were calculated as outlined above.
For BMI measurements, following separation into percentiles, differences in the means among low-flow POTS, normal-flow POTS and control subjects were compared by one-way ANOVA. Differences in the variances among these groups were compared by F test.
Patients and control subjects were divided on the basis of size using the 50th percentile of the BMI to group subjects into those < 50th percentile (smaller BMI; BMI < 50%) and those > 50th percentile (larger BMI; BMI > 50%), regardless of flow status. Comparisons were made between (i) POTS patients with smaller BMI (BMI < 50%) and larger BMI (BMI > 50%); (ii) control subjects with smaller and larger BMI; (iii) POTS patients with smaller BMI and control subjects with smaller BMI; (iv) POTS patients with larger BMI and control subjects with larger BMI; and (v) all POTS patients and all controls. Comparisons were designed to determine whether BMI accounts for vascular changes. Tabular data were analysed by two-way ANOVA (POTS compared with control, and smaller compared with larger BMI). Results were calculated using SPSS software (version 14.0) and were drawn using GraphPad prism software (version 4). All results are reported as means ± S.E.M. Significance was defined as a P value < 0.05.
RESULTS
BMI and patient size
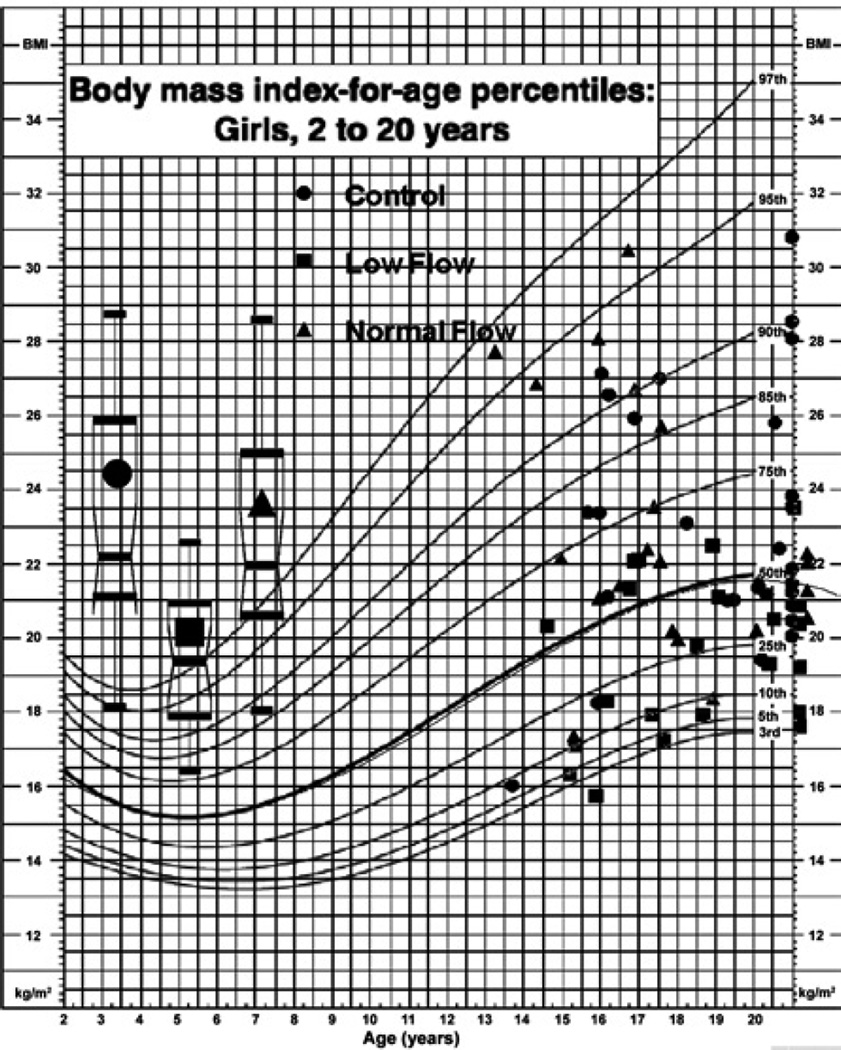
Figure 1 shows the results of BMI measurements stratified into low-POTS patients, normal-flow POTS patients and control subjects using the age-standard chart for each subject studied. Group differences were striking, with significantly lower mean BMI (P < 0.001) and variance (P < 0.025) in the low-flow POTS patients.
Figure 1. BMI of POTS patients and control subjects using an age-standard chart.
Ages > 20 years are placed on the right-hand axis (21 year mark). Age-dependent standards at > 21 years of age are equal to centiles at 20 years of age. Box–Whisker plots showing group medians, 5 and 95th percentiles (whiskers), and 25 and 75th percentiles (box) are shown on the left for each group. The distribution is similar for control and normal-flow POTS. Low-flow POTS patients have significantly lower BMIs, all falling < 50th percentile.
Table 1 indicates that, overall, far more POTS patients than control subjects fell within the smaller BMI range. This occurred because approx. half of the POTS patients were originally classified as low-flow, and most of the low-flow patients fell within the smaller BMI group. Of those originally classified as normal-flow POTS, approx. half are in the smaller BMI group and half in the larger BMI group. Thus a greater percentage of POTS patients than control subjects were in the low-flow group. Heights were similar in each group and, thus, smaller BMI was a consequence of lower body mass rather than increased height.
Table 1.
Characteristics of the POTS patients and control subjects
| POTS patients | Control subjects | |||||
|---|---|---|---|---|---|---|
| Smaller BMI | All BMI | Larger BMI | Smaller BMI | All BMI | Larger BMI | |
| n | 40 | 52 | 12 | 20 | 36 | 16 |
| Height (cm) | 170 ± 1 | 170 ± 1 | 171 ± 2 | 169 ± 2 | 168 ± 2 | 168 ± 3 |
| Body weight (kg) | 58 ± 1 | 63 ± 2 | 75 ± 4* | 61 ± 2 | 66 ± 2 | 75 ± 3* |
| BMI (kg/m2) | 20.0 ± 0.3 | 22.0 ± 0.5 | 26.5 ± 1.1* | 21.5 ± 0.5 | 23.8 ± 0.6 | 26.5 ± 0.7* |
| HR (beats/min) | 76 ± 2† | 76 ± 2 | 74 ± 2† | 64 ± 3 | 64 ± 2 | 63 ± 2 |
| Systolic BP (mmHg) | 121 ± 2 | 121 ± 2 | 122 ± 3 | 116 ± 3 | 118 ± 2 | 122 ± 2 |
| Diastolic BP (mmHg) | 68 ± 2 | 67 ± 2 | 63 ± 3 | 63 ± 2 | 64 ± 1 | 65 ± 2 |
Values are means ± S.E.M. Smaller BMI (BMI < 50 %), BMI < 50th percentile; Larger BMI (BMI > 50 %), BMI > 50th percentile.
P < 0.05 compared with larger BMI for a given subject group;
P < 0.05 compared with control.
Haemodynamic data
HR and BP
Table 1 also shows that HR, measured when supine, was increased in all POTS patients compared with control subjects. Although HR in POTS patients exceeded HR in controls, there was no relationship with body mass in the present study. There were no differences in BP while supine.
Cardiac output and blood volume
Cardiac output and blood volume paralleled patient size, regardless of classification (Table 2). Blood volume and cardiac output averaged over all POTS patients were decreased (P < 0.05) compared with control subjects, because there were so many more POTS patients with decreased body mass and smaller BMI. There was no significant difference between POTS patient with smaller BMI and controls with smaller BMI, or POTS patient with larger BMI and controls with larger BMI. When normalized to body mass, blood volume and cardiac output were similar for POTS patients and control subjects and for the larger and smaller BMI groupings. Thus, for example, blood volume was 74 ± 5 ml/kg of body mass for POTS patients with smaller BMI, 72 ± 5 ml/kg of body mass for POTS patients with larger BMI, 70 ± 4 ml/kg of body mass for controls with smaller BMI and 73 ± 5 ml/kg of bodt mass controls with larger BMI.
Table 2.
Blood flow and blood volume in the POTS patients and control subjects
| POTS patients | Control subjects | |||||
|---|---|---|---|---|---|---|
| Smaller BMI | All BMI | Larger BMI | Smaller BMI | All BMI | Larger BMI | |
| n | 40 | 52 | 12 | 20 | 36 | 16 |
| Cardiac output (litres/min) | 5.1 ± 0.5 | 5.6 ± 0.4† | 7.5 ± 1.1 | 6.0 ± 0.5 | 6.9 ± 0.4 | 7.6 ± 1.0 |
| Blood volume (litres) | 4.0 ± 0.2 | 4.3 ± 0.2† | 5.5 ± 0.7 | 4.3 ± 0.2 | 4.9 ± 0.2 | 5.6 ± 0.6 |
| Forearm | ||||||
| Pv (mmHg) | 10.3 ± 0.6 | 10.6 ± 0.5 | 11.4 ± 1.1 | 10.1 ± 0.5 | 9.8 ± 0.6 | 9.9 ± 0.9 |
| Blood flow [ml−1 · min−1 · (100 ml of tissue)−1] | 2.31 ± 0.20 | 2.40 ± 0.16 | 2.55 ± 0.28 | 2.09 ± 0.38 | 2.49 ± 0.28 | 2.88 ± 0.40 |
| Maximum venous capacity (ml/100 ml of tissue) | 4.93 ± 0.25 | 4.92 ± 0.22 | 4.83 ± 0.45 | 3.97 ± 0.61 | 4.22 ± 0.32 | 4.26 ± 0.44 |
| Arterial resistance [mmHg · ml−1 · min−1 · (100 ml of tissue)−1] | 37 ± 0.6 | 35 ± 0.5 | 31 ± 6 | 38 ± 7 | 32 ± 4 | 28 ± 3 |
| Venous resistance [mmHg · ml−1 · min−1 · (100 ml of tissue)−1] | 0.60 ± 0.07 | 0.62 ± 0.08 | 0.65 ± 0.11 | 1.01 ± 0.37 | 0.82 ± 0.20 | 0.55 ± 0.10 |
| Calf | ||||||
| Pv (mmHg) | 17.3 ± 0.5† | 16.8 ± 0.5 | 15.8 ± 0.1.1 | 13.2 ± 0.5 | 14.0 ± 0.8 | 15.1 ± 1.2 |
| Blood flow [ml−1 · min−1 · (100 ml of tissue)−1] | 1.63 ± 0.31*† | 1.84 ± 0.41† | 2.66 ± 0.14 | 3.58 ± 0.67 | 3.22 ± 0.48 | 2.94 ± 0.30 |
| Maximum venous capacity (ml/100 ml of tissue) | 3.87 ± 0.32*† | 4.32 ± 0.20† | 4.83 ± 0.35 | 4.98 ± 0.36 | 5.21 ± 0.25 | 5.29 ± 0.35 |
| Arterial resistance [mmHg · ml−1 · min−1 · (100 ml of tissue)−1] | 56 ± 0.5*† | 48 ± 0.5† | 32 ± 3 | 30 ± 4 | 30 ± 3 | 27 ± 3 |
| Venous resistance [mmHg · ml−1 · min−1 · (100 ml of tissue)−1] | 1.23 ± 0.17† | 1.12 ± 0.08 | 1.0 ± 0.13 | 0.79 ± 0.11 | 0.89 ± 0.11 | 1.01 ± 0.19 |
Values are means ± S.E.M. Smaller BMI (BMI < 50 %), BMI < 50th percentile; Larger BMI (BMI > 50 %), BMI > 50th percentile.
P < 0.05 compared with larger BMI for a given subject group;
P < 0.05 compared with control.
Blood flow, Pv, resistance and venous capacity
Forearm
As shown in Table 2, there were no significant differences in forearm Pv, blood flow, maximum venous capacity or arterial or venous resistances across groups and subject BMI. Arterial resistance tended to be inversely related to BMI in both the POTS and control groups, but did not reach significance. The maximum forearm venous capacity also tended to be increased (P = 0.07) in POTS patients unrelated to size.
Calf
Calf results were different from forearm results. Table 2 shows that Pv was significantly greater for POTS patients with smaller BMI compared with controls with smaller BMI (P < 0.001).
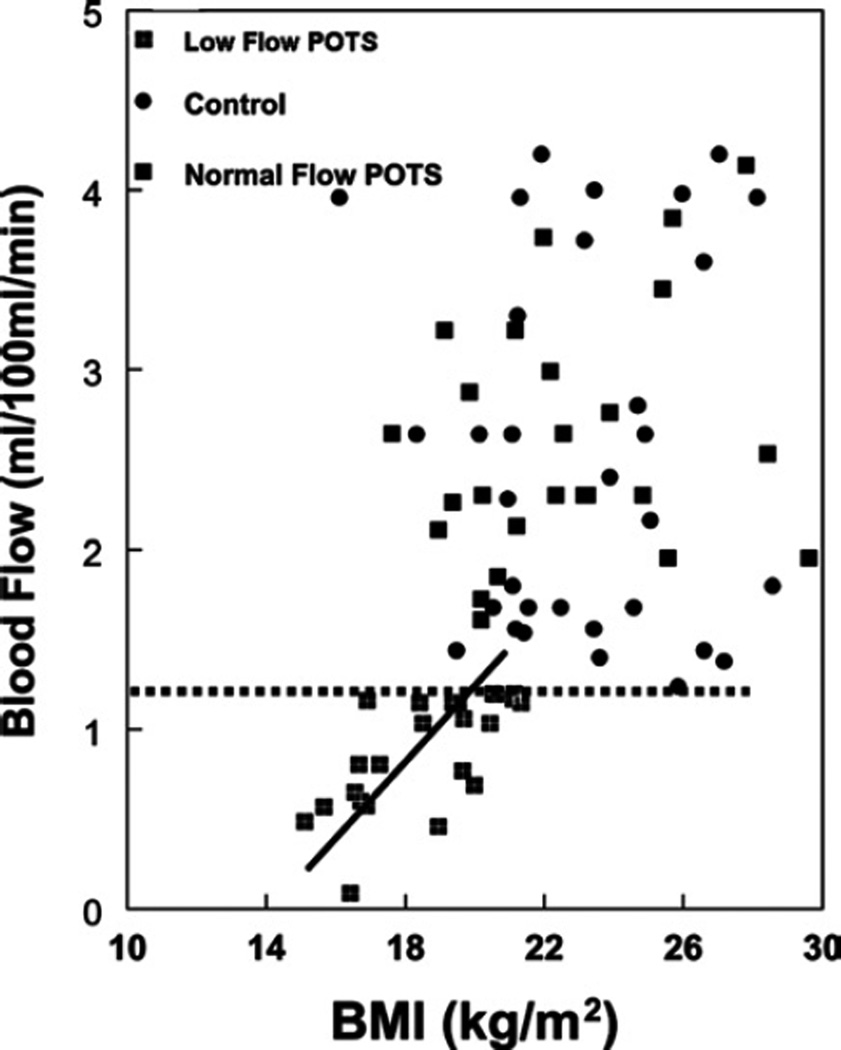
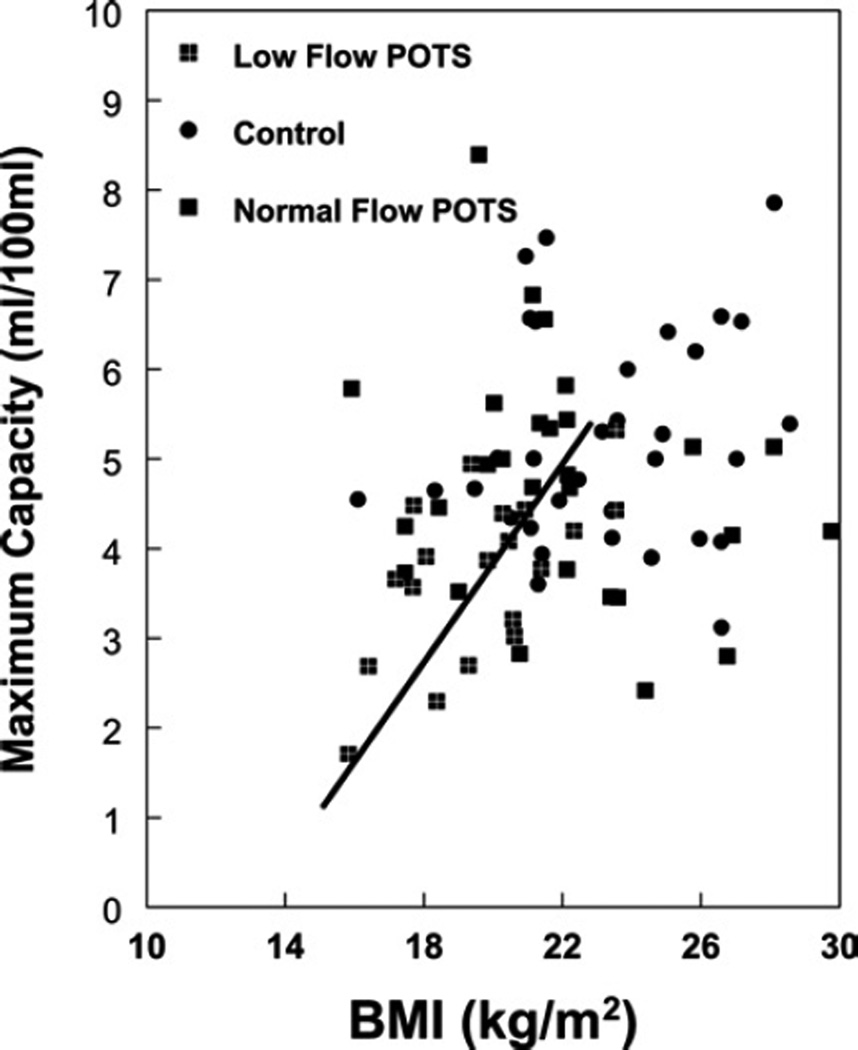
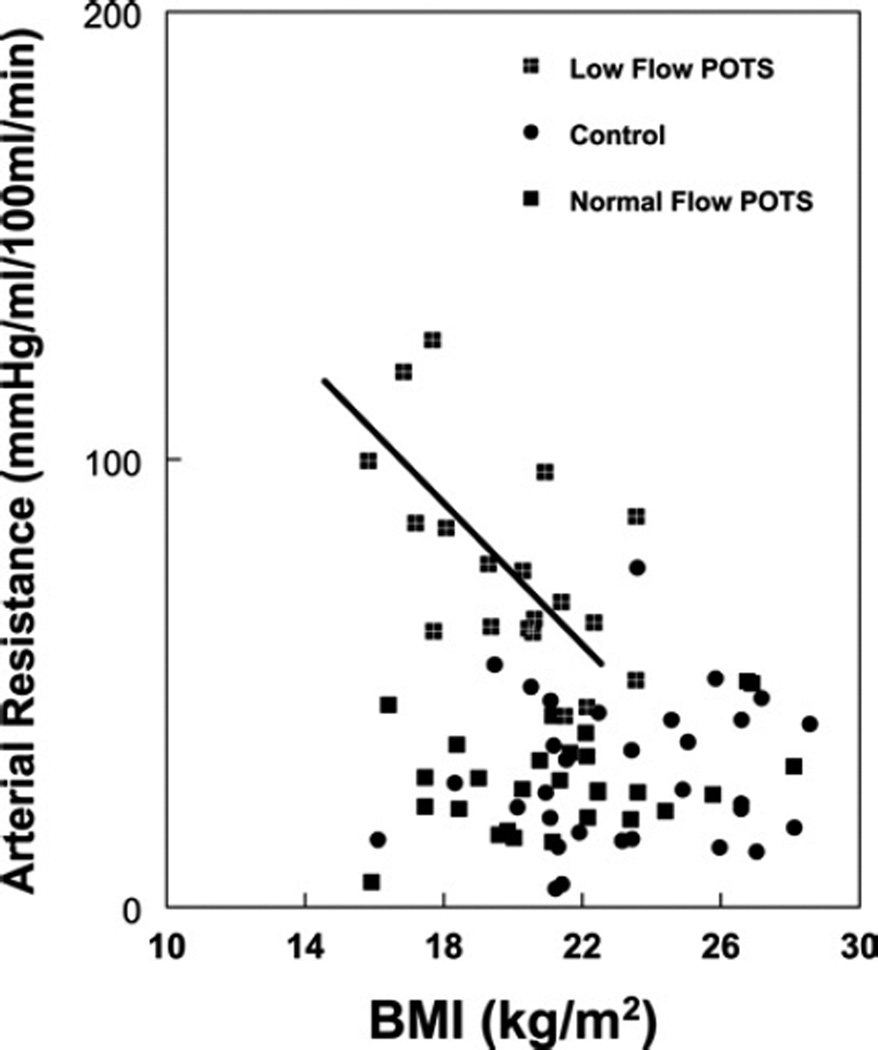
Figures 2–4 show calf blood flow, calf arterial resistance and calf maximum venous capacity respectively, for all POTS patients and control subjects as a function of BMI. These quantities were unrelated to BMI in control subjects, but distributed among two population groupings (P = 0.002) in POTS patients, as determined by cluster analysis using the SPSS k means clustering analysis: a smaller cluster with a BMI centred at 19.3 kg/m2, and a larger cluster with a BMI centred around 24.5 kg/m2.
Figure 2. Relationship between calf blood flow and BMI.
POTS patients cluster into two subgroups: one subgroup with blood flow significantly decreased compared with control, and a second subgroup with blood flow similar to control. These are separated by a dotted horizontal line. A linear regression is shown for the first subgroup with r2 = 0.50. Regression against the second group was not significant (r2 = 0.027).
Figure 4. Relationship between maximum calf venous capacity and BMI.
Patients cluster into two subgroups: one subgroup with blood flow significantly decreased compared with control, and a second subgroup with blood flow similar to control. A linear regression is shown for the first subgroup with r2 = 0.36.
Calf blood flow normalized to calf volume and maximum calf venous capacity normalized to calf volume were directly related to BMI in POTS patients (r2 = 0.50 for flow, and r2 = 0.28 for venous capacity), but not for control. Calf arterial resistance was inversely related to BMI for POTS patients (r2 = 0.36), but not for control.
AngII
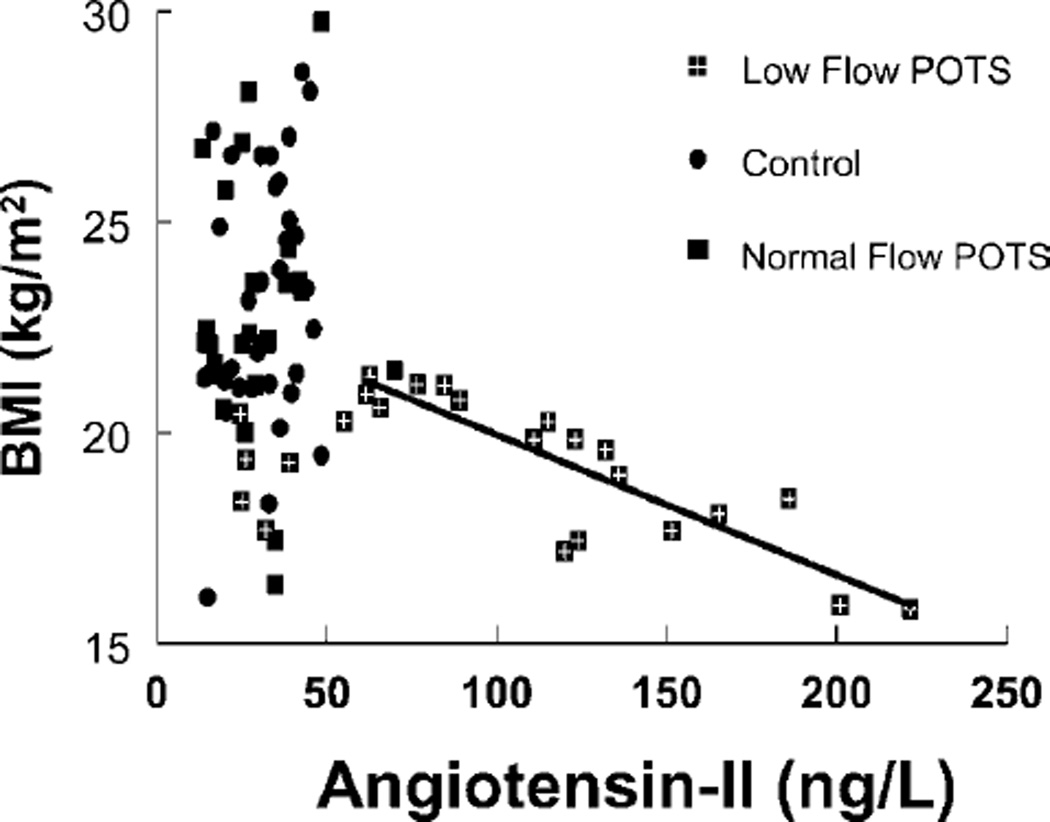
We investigated a potential relationship between BMI and plasma AngII concentration (Figure 5). Subjects were not subdivided on the basis of AngII plasma concentrations, but rather on the basis of BMI and leg blood flow criteria. The results indicate that > 50% of low-flow POTS patients have abnormally high AngII and that, in these patients, AngII is inversely correlated (r2 = 0.77) with BMI.
Figure 5. Relationship between BMI and plasma AngII.
There is an inverse relationship (r2 = 0.77) between BMI and AngII concentration in those low-flow POTS patients with increased AngII (> 50 ng/l).
DISCUSSION
The most interesting results in the present study are that female POTS patients with reduced peripheral blood flow (our low-flow POTS group) had BMIs (body mass) that were generally at or below the 50th percentile for age, and that increased AngII was associated with decreased BMI in these patients.
Is there a relationship between haemodynamics and BMI?
The remainder of the present study explored the possibility that BMI or body mass alone accounted for the haemodynamic findings related to POTS in young women. For the purposes of the present study, we therefore only included groups of female POTS patients who presented to our Center and stratified them on the basis of BMI. The results indicated that body mass alone cannot account for findings related to peripheral blood flow, maximum venous capacity and arterial and venous resistance in the calves, whereas body mass can account for differences in cardiac output and blood volume. The thinner the subject, the less the cardiac output and blood volume. The occurrence of reduced BMI compared with controls in the overall group of POTS patients may account for findings of reduced cardiac output and blood volume in POTS reported by us and others [28–30].
Peripheral blood flow, resistance and venous capacity measurements made by SGP are normalized to limb volume and are expressed in units of ml/100 ml of tissue. This may be one source of variation among heavier and lighter patients: there may be relatively more dense tissue, such as bone/unit limb volume, in subjects with lower BMIs compared with subjects with higher BMIs. This could bias flow data, since bone blood flow is less likely to be accurately measured using venous occlusion methods. However, such bias appears to have had no effect among the control subjects or the normal-flow POTS patients.
We measured peripheral maximum venous capacity, which we defined as the maximum blood volume/unit volume of tissue that can be stored within a given extremity. Although there was a trend towards increased maximum venous capacity in the forearms of all POTS patients compared with control subjects, this did not reach significance. However, we were able to show a distinct reduction in maximum venous capacity in the calves of POTS patients in the smaller BMI group. This is consistent with our previous observations [9], and with the study by Freeman et al [31].
The results with regard to blood volume are different from the results of Jacob et al. [28] and Raj et al. [29], who demonstrated a decreased average blood volume in POTS patients. However, their results (and our own previous results) were expressed as actual volume measurements, rather than measurements/unit of body mass. This may account for these differences.
Why are POTS patients thinner? The AngII connection
Why is BMI reduced in low-flow subjects? One possible answer derives from our findings of excessive concentrations of AngII in low-flow POTS patients [12], although this may have no causal relationship. AngII was measured using an extensively validated commercial assay performed by Quest Diagnostics Laboratory.
To investigate this potential explanation of our results, we plotted BMI as a function of AngII concentration (Figure 5). The results indicate that > 50% of these low-flow patients have abnormally high AngII and that, in these patients, AngII is inversely correlated with BMI.
AngII could mediate anorexia and cachexia; however, there are arguments on both side of this hypothesis. In model animal systems and humans, AngII is directly related to IGF-1 (insulin-like growth factor-1) and the regulation of oxygen consumption [16–18,32]. However, low BMI is not commonly associated with high-renin hypertension in which plasma AngII levels are commonly elevated. Inhibition of lipolysis by AngII may be important and has been demonstrated by Goossens et al. [33] in adipose and skeletal muscle tissue in both obese and normal-weight subjects using microdialysis techniques. Also, increased circulating AngII contributes to cachexia in heart failure [32], but here too results are easily confounded by cachectic pro-inflammatory cytokines [e.g. the old name of TNFα (tumour necrosis factor α) was cachexin]. Studies have demonstrated the potential for increased AngII within the CNS to produce both sympatho-excitation [19] and cachexia [20]. Sympatho-excitation is a major factor in hyperadrenergic POTS in which Furlan et al. [3] have demonstrated that resting sympathetic outflow is increased and there is a blunted response to sympathetic stimulation with orthostatic stress. Although we have not measured sympathetic nervous system activity in these patients, resting vasoconstriction and poor response to orthostatic stress were demonstrated during prior experiments [28,29,34], and are consistent with the sympatho-excitation observed by Furlan et al. [3]. Since low-flow POTS patients are also relatively NO-deficient [13], it is interesting to speculate whether central AngII excess and NO deficit could account for increased sympathetic activity. Further work will focus closely on such possibilities.
Limitations
The present study is an associative one and, therefore, it is difficult to assign causality. POTS patients with low BMI have less absolute cardiac output and blood volume. This could result in a greater compensatory increase in AngII and sympathetic nervous system activation, which might contribute to POTS; however, when normalized to body mass, these cardiac output and blood volume differences disappear. Conversely, reductions in cardiac output and blood volume could contribute to decreased BMI. It is therefore impossible in our present study to determine which is more important: BMI or Ang haemodynamics. It is the classic ‘chicken and egg’ difficulty of associative observations that precludes any definitive conclusion that AngII causes decreased BMI.
The assay for AngII also cross-reacts with other Ang species. Specifically, it will detect Ang-(1–7) equally well, which is a vasodilator produced in various tissue. However, our low-flow POTS patients are markedly vasoconstricted. Nevertheless, it may be safer to consider an increase in assayed Ang as a marker, rather than as an absolute measure, of AngII.
We do not know how plasma or local concentrations of AngII change over the age range investigated in the present study. This may be important to understanding how Ang produces and modulates vasoconstriction in low-flow POTS patients.
We used only BMI, which is a rather non-specific index of increased or decreased body mass, and it tells us nothing specific about the proportion of lean body mass or fat, or whether there are alterations in other tissues involved.
We investigated only young female subjects, as most POTS patients are female, with estimates ranging from 75–80% [35]. However, low-flow POTS patients are exclusively female, although the reason for this remains unexplained. The age range used required that we employ CDC (Centers for Disease Control) age-dependent criteria for subjects < 21 years old. These criteria are present in the standard graph contained within Figure 1.
We have not measured sympathetic activation directly nor correlated this with BMI, low-flow POTS or AngII. This will be remedied in future studies.
The majority of the subjects were all menstruating females, but we did not determine the menstrual phase in any of our subjects, either those with POTS or controls, and none were amenorrhoeic. Although not affecting BMI appreciably, menstrual cycle effects could change haemodynamic properties [36,37]. Previous studies have demonstrated that hormonal fluctuations that occur during the normal menstrual cycle may alter autonomic regulation of arterial pressure during various environmental stimuli [38], although there is no apparent effect on orthostatic tolerance [36].
There could be some relationship with deconditioning; however, many of our low-flow POTS patients were previously athletic and often maintained exercise regimens despite their illness.
Conclusions
In the present study, we have demonstrated that patients with low-flow POTS have decreased body mass, but that decreased body mass alone cannot account for the vasoconstrictive findings. Rather, the results suggest that the reduced BMI observed in these patients may be the result, rather than the cause, of their specific pathophysiology. We have also shown that low BMI is correlated with plasma AngII in a large subset of low-flow POTS patients, which could form a pathophysiological part of their illness. However, these observations are largely associative and, therefore, unable to determine causality. Previous findings in similar patients show reduced NO bioavailability and sympatho-excitation. We speculate that chronic sympatho-excitation related to increased AngII and decreased NO results in increased peripheral resistance and decreased body mass in low-flow POTS.
Figure 3. Relationship between calf arterial resistance and BMI.
POTS patients cluster into two subgroups: one subgroup with resistance significantly increased compared with control, and a second subgroup with resistance similar to control. A linear regression is shown for the first subgroup with r2 = 0.40.
ACKNOWLEDGMENTS
We thank the members of the Department of Pediatrics, especially its Chairman, Dr Leonard Newman, and the Division of Pediatric Cardiology, especially its Director, Michael H. Gewitz M.D., for their unflagging support.
Abbreviations
- Ang
angiotensin
- BMI
body mass index
- BP
blood pressure
- CNS
central nervous system
- HR
heart rate
- ICG
Indocyanine Green
- MAP
mean arterial pressure
- NO
nitric oxide
- POTS
postural tachycardia syndrome
- Pv
venous pressure
- SGP
strain gauge plethysmography
REFERENCES
- 1.Low PA, Schondorf R, Novak V, Sandroni P, Opfer-Gehrking TL, Novak P. Postural tachycardia syndrome. In: Low PA, editor. Clinical Autonomic Disorders: Evaluation and Management. Philadelphia: Lippincott-Raven; 1997. pp. 681–697. [Google Scholar]
- 2.Farquhar WB, Taylor JA, Darling SE, Chase KP, Freeman R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation. 2000;102:3086–3091. doi: 10.1161/01.cir.102.25.3086. [DOI] [PubMed] [Google Scholar]
- 3.Furlan R, Jacob G, Snell M, et al. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–2159. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Holmes C, Frank SM, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002;106:2358–2365. doi: 10.1161/01.cir.0000036015.54619.b6. [DOI] [PubMed] [Google Scholar]
- 5.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin. Proc. 1999;74:1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 6.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 7.Schondorf R, Benoit J, Stein R. Cerebral autoregulation in orthostatic intolerance. Ann. N.Y. Acad. Sci. 2001;940:514–526. doi: 10.1111/j.1749-6632.2001.tb03702.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart JM, Weldon A. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J. Appl. Physiol. 2001;90:2025–2032. doi: 10.1152/jappl.2001.90.6.2025. [DOI] [PubMed] [Google Scholar]
- 9.Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105:2274–2281. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N. Engl. J. Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin. Sci. 2006;110:255–263. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation. 2005;112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2749–H2756. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JM. Microvascular filtration is increased in postural tachycardia syndrome. Circulation. 2003;107:2816–2822. doi: 10.1161/01.CIR.0000070951.93566.FC. [DOI] [PubMed] [Google Scholar]
- 16.Cassis L, Helton M, English V, Burke G. Angiotensin II regulates oxygen consumption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R445–R453. doi: 10.1152/ajpregu.00261.2001. [DOI] [PubMed] [Google Scholar]
- 17.Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA. Mechanisms contributing to angiotensin II regulation of body weight. Am. J. Physiol. 1998;274:E867–E876. doi: 10.1152/ajpendo.1998.274.5.E867. [DOI] [PubMed] [Google Scholar]
- 18.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 20.Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R422–R428. doi: 10.1152/ajpregu.00537.2003. [DOI] [PubMed] [Google Scholar]
- 21.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 23.Bloomfield DA. Dye Curves: the Theory and Practice of Indicator Dye Dilution. Baltimore: University Park Press; 1974. [Google Scholar]
- 24.He YL, Tanigami H, Ueyama H, Mashimo T, Yoshiya I. Measurement of blood volume using indocyanine green measured with pulse-spectrophotometry: its reproducibility and reliability. Crit. Care Med. 1998;26:1446–1451. doi: 10.1097/00003246-199808000-00036. [DOI] [PubMed] [Google Scholar]
- 25.Gamble J, Christ F, Gartside IB. Mercury in silastic strain gauge plethysmography for the clinical assessment of the microcirculation. Postgrad. Med. J. 1992;68(Suppl. 2):S25–S33. [PubMed] [Google Scholar]
- 26.Gamble J, Gartside IB, Christ F. A reassessment of mercury in silastic strain gauge plethysmography for microvascular permeability assessment in man. J. Physiol. 1993;464:407–422. doi: 10.1113/jphysiol.1993.sp019642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolaides AN. Investigation of chronic venous insufficiency: a consensus statement. Circulation. 2000;102:E126–E163. doi: 10.1161/01.cir.102.20.e126. [DOI] [PubMed] [Google Scholar]
- 28.Jacob G, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Relation of blood volume and blood pressure in orthostatic intolerance. Am. J. Med. Sci. 1998;315:95–100. doi: 10.1097/00000441-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H665–H673. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman R, Lirofonis V, Farquhar WB, Risk M. Limb venous compliance in patients with idiopathic orthostatic intolerance and postural tachycardia. J. Appl. Physiol. 2002;93:636–644. doi: 10.1152/japplphysiol.00817.2001. [DOI] [PubMed] [Google Scholar]
- 32.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann. Med. 2004;36:518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 33.Goossens GH, Blaak EE, Saris WH, van Baak MA. Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J. Clin. Endocrinol. Metab. 2004;89:2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- 34.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am. J. Med. Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hirshoren N, Tzoran I, Makrienko I, et al. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J. Clin. Endocrinol. Metab. 2002;87:1569–1575. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- 37.Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am. J. Hypertens. 2004;17:994–1001. doi: 10.1016/j.amjhyper.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1091–R1097. doi: 10.1152/ajpregu.00162.2003. [DOI] [PubMed] [Google Scholar]