Abstract
Orthostasis means standing upright. One speaks of orthostatic intolerance (OI) when signs, such as hypotension, and symptoms, such as lightheadedness, occur when upright and are relieved by recumbence. The experience of transient mild OI is part of daily life. ‘Initial orthostatic hypotension’ on rapid standing is a normal form of OI. However, other people experience OI that seriously interferes with quality of life. These include episodic acute OI, in the form of postural vasovagal syncope, and chronic OI, in the form of postural tachycardia syndrome. Less common is neurogenic orthostatic hypotension, which is an aspect of autonomic failure. Normal orthostatic physiology and potential mechanisms for OI are discussed, including forms of sympathetic hypofunction, forms of sympathetic hyperfunction and OI that results from regional blood volume redistribution. General and specific treatment options are proposed.
Keywords: autonomic, hyperpnea, orthostatic hypotension, orthostatic tachycardia, postural tachycardia, syncope
Introduction: what is orthostatic intolerance?
Orthostasis means ‘standing up’. Thus, ortho-static intolerance (OI) is defined by the inability to remain upright. It is often erroneously designated ‘orthostasis’. More specifically, OI can be defined by the inability to remain upright relieved by recumbence [1]. The overall purpose of this article is to discuss OI in young people, and how it relates to the autonomic nervous system (ANS) and to the modulation of the ANS by vascular transmitters.
OI is related to deviations from optimum regulation of heart rate (HR), blood pressure (BP) and cerebral blood flow (CBF) that make remaining upright impossible. Environmental factors that promote OI (e.g., hot climate, dehydration) and the ubiquity of many forms of OI in the general population (simple faint, initial orthostatic hypotension [OH]) make some forms of OI the result of overtaxed control systems, rather than disease per se. Thus, for example, any person can be made to suffer a simple faint given sufficient provocation [2]; therefore, should vasovagal syncope (VVS) be regarded as an illness? The answer is perhaps yes if it importantly impairs the quality of life.
Signs & symptoms of OI: relation to reduced CBF, sympathoexcitation & parasympathetic withdrawal
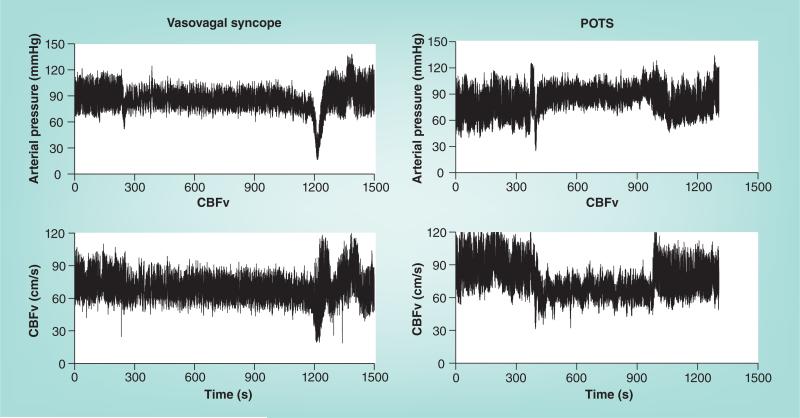
The inability to remain upright can be defined subjectively in terms of patient symptoms alone or more objectively by the combination of signs and medically associated symptoms. Typical signs and symptoms include loss of consciousness or less severe cognitive deficits, such as lightheadedness and dizziness, as well as vertigo, pallor, visual difficulties or scotomata, fatigue, tachycardia, bradycardia, hypotension and sometimes hypertension, headache, weakness, abdominal pain and vomiting, palpitations, anxiety, diaphoresis, tremor and exercise intolerance that is often delayed or may occur as part of a postural syndrome [3]. Among these, signs and symptoms referable to the CNS, such as loss of consciousness, severe dizziness and cognitive difficulties, are the most common reasons given for terminating an orthostatic stressor (upright posture) and seeking recumbence. Many of the remaining signs and symptoms are directly related to increased sympathoexcitation (e.g., pallor, hypertension, headache, palpitations, anxiety, diaphoresis, tremor), vagal withdrawal (e.g., tachycardia, abdominal pain and vomiting) or vagotonia (bradycardia during VVS). The capability of assessing CBF along with the measurements of beat-to-beat HR and BP facilitates the separation of spurious OI, or psychogenic syncope, from bona fide physiological impairments because reduced CBF is a common feature in all forms of physiological OI, but not in psychogenic syndromes. Representative-reduced perfusion of the brain [4,5] is shown in Figure 1 for two common forms of OI – VVS (simple faint) and postural tachycardia syndrome (POTS). Reduced CBF may be related directly to hypotension [6,7] and can be related to hypocapnia [8,9], but not always [5]. Because CBF is autoregulated [10,11], it ought to remain stable within a range of cerebral perfusion pressures. However, impaired autoregulation results in decreased CBF that becomes directly dependent on perfusion pressure [4,5]. Thus, swings in BP cause swings in CBF, and any fall in BP contributes excessively to decreased CBF and impaired brain blood flow. That being said, virtually all people experience some degree of episodic mild OI during their lives, although only transiently during intercurrent illnesses or with dehydration [12]; occasional VVS is extraordinarily common [13].
Figure 1. Arterial pressure (upper panels) and cerebral blood flow velocity measured by transcranial Doppler ultrasound (lower panels).
The left panels show data from a vasovagal syncope patient, while the right panels show data from a POTS patient. Arterial pressure and CBFv are initially stable, then decrease gradually and finally abruptly decrease by >50% with loss of consciousness in the syncope patient. The POTS patient has no decrease in arterial pressure, but has a >20% reduction in CBF throughout tilt.
CBFv: Cerebral blood flow velocity; POTS: Postural tachycardia syndrome.
Normal stressors & the autonomic regulatory framework
Next, we explain the phenomenology and physiology of ortho-static stress. Standing upright and exercise comprise the most common physical stressors of daily life and “demand the full capabilities of the reflexes that govern cardiovascular function” [14]. They, therefore, depend on intact arterial and venous vascular structure, intact vasomotor control and sufficient blood volume and oxygen-carrying capacity. However, foremost among compensatory mechanisms are the skeletal muscle pump and the respiratory–abdominal muscle pump [15,16]. These form the bases for a variety of physical orthostatic countermaneuvers that will be addressed below [17,18]. The compensatory responses to orthostasis are diverse and overlapping – thus, mildly reduced blood volume or reduced vasoconstrictive capabilities are often well tolerated.
Neurohumoral vascular mediators have importance, especially in a modulatory capacity; they are relatively slow to develop and, therefore, rarely directly determine initial responses to orthostatic stress [19]. Rapid cardiovascular adjustments depend on the ANS, although the myogenic response [20] and local flow-dependent mechanisms [21] may have a similar time course. Nevertheless, the ANS forms the framework for rapid circulatory adjustments resulting in changes in HR, arterial vasoconstriction, venoconstriction, adrenal secretion, renovascular adjustments and cardiac contractility to maintain BP. Within the systemic circulation, the parasympathetic nervous system via vagal nerve efferents contributes most to HR changes at rates less than the intrinsic rate [22], although recent work indicates strong vagal influences on sympatho excitation [23]. In addition, recent data suggest that parasympathetic ganglia have important effects on nitrergic (nitric oxide [NO] within nerves) vasodilation of the large cerebral arteries [24].
Rapid autonomic adjustments also depend on local environmental biochemistry produced by slower endocrine, and local regulatory mechanisms. These more slowly affect the vasculature in response to changes in posture. However, during more chronic changes, they can modulate autonomic function and vascular tone. Thus, tonically altered NO and angiotensin-II act at CNS [25] and peripheral vascular [26] levels to alter the response to adrenergic vasoconstriction. While parasympathetic control of HR plays an important role in the beat-to-beat maintenance of BP, the sympathetic nervous system and its primary vascular neuro-transmitter norepinephrine [27], and cotransmitter neuropeptides Y and ATP [26], are of paramount importance. Autonomic control of HR and BP during orthostasis is provided by regulatory subsystems designated ‘baroreflexes’ whose primary concern is the maintenance of BP under changing conditions. These are loosely grouped into arterial and cardiopulmonary baroreflexes, which interact with potent mechanoreceptor and chemoreceptor networks to maintain BP during orthostasis.
Why do we need these control mechanisms? The normal orthostatic response
Standing up decreases venous return to the heart and shifts a large fraction of central blood volume, in excess of 500 ml in the adult human, to the dependent body parts – that is, those below the hydrostatic indifference point located roughly at the diaphragm [28]. It is the gravitational, hydrostatic gradients established by the upright posture that cause dependent pooling of blood below the heart.
Initial OH
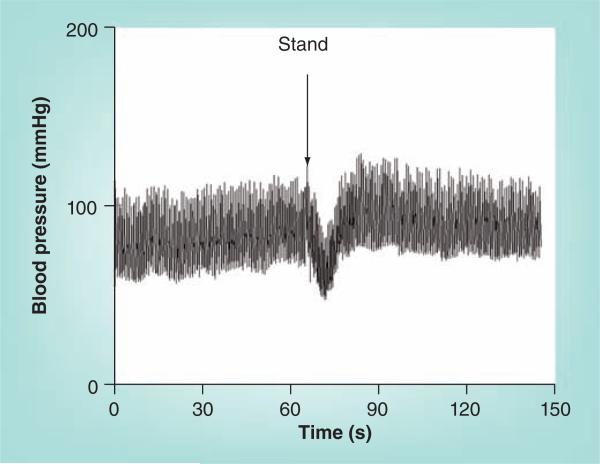
There is an initial transient fall in BP shortly after standing up, as shown in Figure 2. Initially, blood is very rapidly redistributed by gravity from the central thoracic circulation to the dependent body parts: predominantly into the venous vasculature of the lower limbs and splanchnic circulation [29]. The decrease in BP is inversely related to initial vascular tone [30]. While compensatory increases in HR begin within a heart beat, a delay of approximately 10–15 s occurs before the onset of active compensatory sympathetic responses and BP falls. This initial response, denoted ‘initial orthostatic hypotension’ [31], is completed within 30–60 s, and BP is restored, often while the typical adolescent balances precariously while holding onto furniture. Rarely, fainting can occur, particularly if the child rapidly (say within 15 s) engages in exercise, which further dilates leg vasculature. Orthostatic counter maneuvers (Table 1) can avoid or remediate the condition [31,32]. This normal state of transient mechanical dysequilibrium is by far the most commonly experienced form of OI [33] in the young and is normal. Sympathetic arterial tone contributes to resting vasoconstriction that affects BP recovery [34]. Arterial tone varies among individuals [35] and, therefore, alters the time to recovery across individuals.
Figure 2. Image showing initial orthostatic hypotension.
Arterial blood pressure is shown during a standing test. The blood pressure begins to decrease immediately upon standing, reaching its nadir in about 15 s and recovers spontaneously. The interbeat interval is quite decreased when hypotensive, corresponding to an increased heart rate.
Table 1.
Treatment methodologies.
| Orthostatic syndrome | Defect/pathophysiology | Treatment |
|---|---|---|
| Initial orthostatic hypotension | None /rapid redistribution of blood to dependent body | Physical countermaneuvers: sitting, isometric exercise (exercise pressor reflex) Medication is rarely used |
| Neurogenic orthostatic hypotension | Systemically defective or absent adrenergic vasoconstriction | Physical countermaneuvers: lie down, sit down, squat, clench buttocks, leg crossing, support garment |
| Autonomic failure may be present and often includes parasympathetic dysfunction | Droxidopa, salt and water loading, fludrocortisone, midodrine, atomoxetine + yohimbine. If secondary (e.g., diabetes) treat underlying disorder Rapid water ingestion palliation |
|
| Non-neurogenic orthostatic hypotension | Loss of blood volume, vasodilator drugs | Correct problem |
| Neuropathic POTS | Loss of regional vasoconstrictive ability | Physical countermaneuvers Droxidopa, midodrine, mestinon Exercise Rapid water ingestion palliation |
| Hyperadrenergic POTS | Adrenergic potentiation | Physical countermaneuvers β-blockers, fludrocortisone, exercise |
| Postural vasovagal syncope | ? Loss of regional vasoconstrictive ability ? Acute reversible baroreflex dysfunction |
Physical countermaneuvers Salt and water Acute water ingestion Selective serotonin re-uptake inhibitors, midodrine Rapid water ingestion palliation Pacemaker for asystolic vasovagal faint |
POTS: Postural tachycardia syndrome.
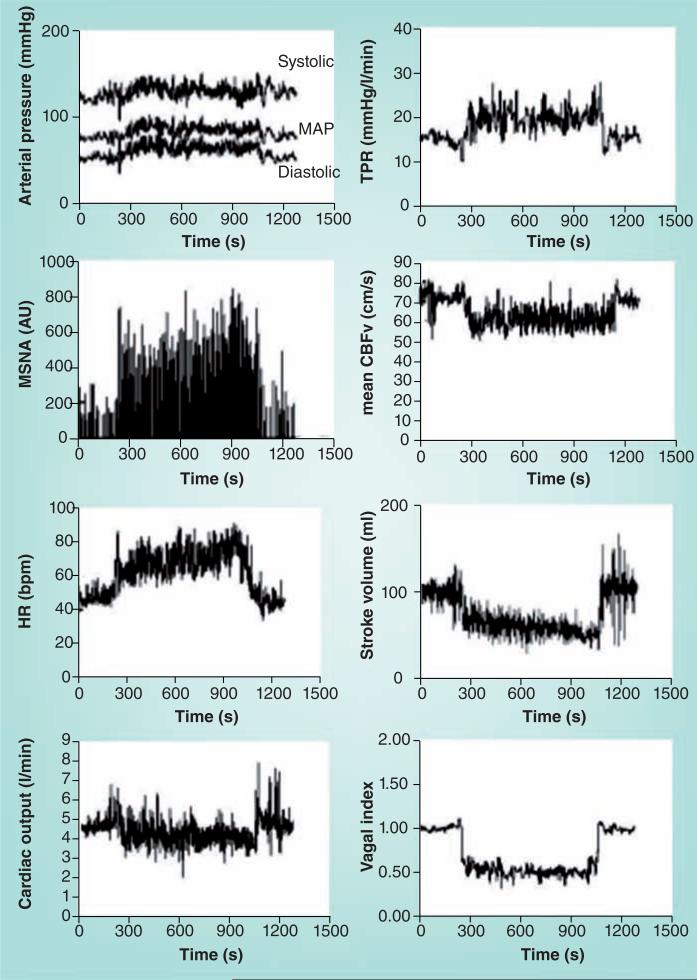
Once hemodynamic stability is reestablished, blood volume is continuously reduced during continued standing still by micro-vascular filtration from plasma to interstitium [36]. While lymphatic activity and reabsorption of interstitial fluid helps to restore blood volume [37], this process is incomplete, and an ongoing reduction in blood volume and venous return occurs. This can be avoided by invoking the skeletal muscle pump by moving around or by external mechanical compression through compression garments [38]. Since venous return equals cardiac output under steady conditions, there is a net reduction in central blood volume, stroke volume, cardiac output and CBF during quiet standing. HR, total peripheral resistance, sympathetic nervous activity, vagal withdrawal and BP increase (Figure 3). Diastolic BP increases more than systolic BP, and pulse pressure decreases coincident with reduced stroke volume.
Figure 3. Hemodynamic and neurovascular changes during upright tilt in a representative healthy volunteer.
The left panel shows from top to bottom: arterial pressure, MSNA from the peroneal nerve, HR and cardiac output. The right panel shows from top to bottom: TPR, CBFv by transcranial Doppler ultrasound, stroke volume and a vagal index calculated from the respiratory sinus arrhythmia component of the frequency spectrum of HR variability. The subject is a representative healthy volunteer. During upright tilt, systolic, diastolic and MAPs increase slightly, while pulse pressure is decreased with a decrease in stroke volume by approximately 40%. HR increases so that cardiac output is only decreased by 20% because of the increase in HR. Cerebral blood flow decreases by 5–10%. Both total peripheral vascular resistance and muscle sympathetic nerve activity increase, while the vagal index decreases, reflecting, respectively, sympathetic activation and parasympathetic withdrawal.
CBFv: Cerebral blood flow velocity; HR: Heart rate; MAP: Mean arterial pressure; MSNA: Muscle sympathetic nerve activity; TPR: Total peripheral vascular resistance.
The restoration of BP and partial restoration of systemic venous return during orthostasis is due in large measure to the reduced stretch and inactivation (unloading) of the inhibitory arterial baroreflexes. This results in vagal withdrawal, sympathetic vasoconstriction variable changes in myocardial sympathoexcitation [39], active venoconstriction within the splanchnic circulation [40] and passive venoconstriction of pooled blood within the splanchnic vasculature and legs by elastic recoil. Venous return is, thus, partially corrected, and central blood volume is partially but incompletely restored [41]. The cardiopulmonary baroreflexes are also unloaded when upright and potentiate the arterial reflexes [42]. Thus, BP is restored and any reduction in BP normally occurs during initial OH. Despite unchanged or even increased BP, increased sympathetic activity continues (Figure 3), which depends on arterial baroreflex resetting by cardiopulmonary reflexes [43] and by the effects of reduced pulse pressure and pulsatility [44]. Thus, diastolic BP, which correlates best with sympathetic nerve activity in humans [45], is increased at the level of the carotid sinus. Diastolic pressure is increased, and reduced stretch, therefore, does not occur while upright. Nevertheless, arterial baroreflexes enhance HR and sympathetic mediated vasoconstriction because the reduction in pulse pressure and the shift of the baroreflex to a higher HR and BP when upright enable HR to remain increased via vagal withdrawal and promotes increased sympathetic nerve activity and vasoconstriction – the normal compensatory response to orthostasis [46].
Orthostatic stress test & tools to study OI
Orthostatic stress tests assess orthostatic capability. The most physiologic test is standing in place without exercising. This allows some muscle pump activity that may mask defects in the ANS [47]. Therefore, clinicians sometimes use devices such as the motorized tilt table [48], which passively places the patient upright while restricting leg movement. Lower body suction or negative pressure, which closely simulates hemorrhage, can duplicate some findings of orthostasis even while remaining supine. Tilt and lower body suction or negative pressure can produce fainting in everyone [2].
Instrumentation that measures BP, HR and cardiac rhythm, cardiac output (e.g., indicator dilution, inert gas rebreathing), regional blood flow (e.g., ultrasound, venous occlusion plethysmography, impedance plethysmography) and blood volume have all been bundled with clinical tilts. Recent conscious human studies of sympathetic control of orthostasis began in earnest with the use of microneurography to measure peripheral sympathetic nerve activity [45]. Other advanced techniques using norepinephrine sympathetic nerve spillover [49] to measure the effect of adrenergic vasoconstriction on local blood flow [50], and, most recently, to directly assess the integrity of norepinephrine synthesis and metabolic products by vascular biopsy [51–53], can be used to find the actual mechanism of OI in sufferers.
Orthostatic hypotension
OH is defined as a sustained reduction of systolic BP > 20 mmHg or diastolic BP > 10 mmHg within 3 min of standing or head-up tilt to ≥60° [54]. The requirement of a sustained reduction rules out internal OH. This definition is recent (2011) and was assembled by a consensus panel [54]. Before that, there was no consistent definition of OH. Nonneurogenic OH can be caused by drugs, dehydration, blood loss, age and illnesses that secondarily cause acute or chronic hypovolemia. Neurogenic OH is identified with autonomic failure due to inadequate release of norepinephrine from sympathetic vasomotor neurons, leading to vasoconstrictor failure [54]. Neurogenic OH is rare in the young as most causes of autonomic failure are acquired with age either as a primary (e.g., pure autonomic failure) or secondary (diabetes) disease. Autonomic failure can be primary with preganglionic, postganglionic or both forms of sympathetic dysfunction [55]. However, there exist congenital genetic variants such as familial dysautonomia (Riley–Day syndrome) [56] and the exquisitely rare dopamine β-hydroxylase deficiency [57]. Autonomic failure can be autoimmune [58] and may present with the postinfectious Guillain–Barre syndrome, although autonomic dysfunction seems to have little effect on ultimate outcome [59]. Most commonly, autonomic failure is acquired as a secondary aspect of systemic disease, such as diabetes [6]. Sympathetic cardiac denervation is a central aspect of Parkinson's disease [60] and may be found in other forms of autonomic failure. Cardiac parasympathetic innervation is also often defective, resulting in a steady fall in BP with little reflex tachycardia during orthostatic challenge as shown in Figure 4.
Figure 4. Neurogenic orthostatic hypotension.
Arterial blood pressure in the upper panel declines steadily during upright stance, while heart rate is only slightly increased.
Supportive care and treatment of the underlying illness are essential (Table 1). Thus, in the case of dopamine β-hydroxylase deficiency, droxidopa, which bypasses the missing enzyme, can provide definitive remediation [61]. It may also be the drug of choice for most neurogenic OH as it can provide norepinephrine production through alternative pathways [62]. Supportive therapy focuses on decreasing symptomatic OH and syncope. Such therapy would include physical countermaneuvers, including compression garments and dietary changes (increased salt, rapid water drinking). Supportive drug therapy often aims to increase blood volume by promoting salt and water retention (fludrocortisone) or by increasing red blood cell mass (recombinant erythropoietin) [55]. Defects in erythropoietin may occur as part of the denervation in autonomic failure [63]. Short-acting pressor drugs such as midodrine or drugs that enhance autonomic activity (atomoxetine, yohimbine or pyridostigmine) are also used [55,64].
Rapid water ingestion of approximately 16 ounces deserves special mention. Studies in adults have demonstrated that intake of water free of solute can increase BP and improve sympathetic vasoconstriction after a sufficient time has elapsed for the water to reach the small intestine, approximately 20 min [65]. The therapeutic effect of water encompasses all OI, including OH, POTS and VVS [66] and can be successfully used to prevent blood phobic VVS. Effects last for several hours. The mechanism is dependent on osmolarity and may depend on TRPV4 C-fiber receptors within the portal system [67]. This is a very important, simple and effective palliation that is not often considered by clinicians.
Common variant OI: POTS & postural VVS
Postural tachycardia syndrome
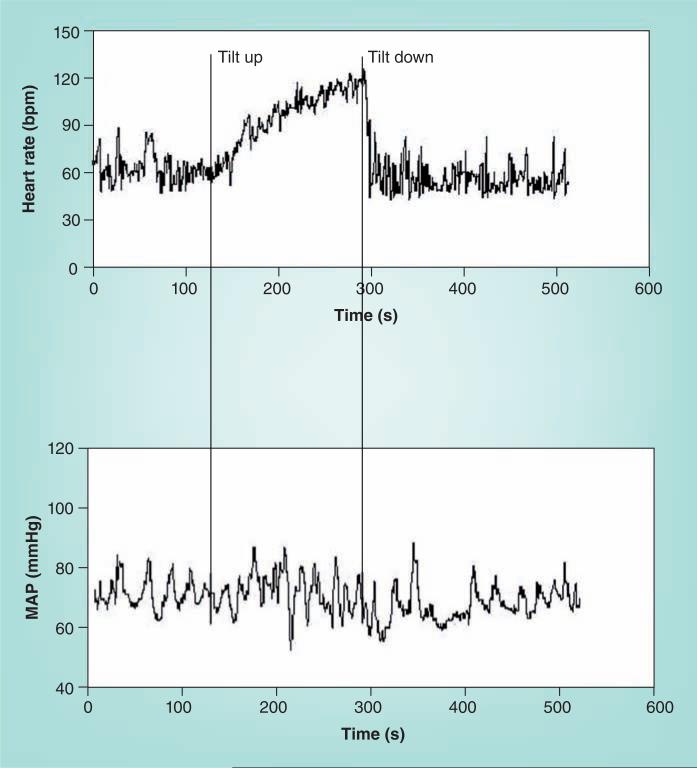
POTS is defined by day-to-day symptoms of OI associated with excessive upright tachycardia, but not with hypotension (Figure 5) [9,68]. Excessive tachycardia in adults is defined by an upright increase in HR exceeding 30 bpm or to a HR exceeding 120 bpm. Recall that the normal HR response to orthostasis is an increase in HR, while the autonomic failure patient often has no significant increase in HR when upright. Larger HR increments on tilt are observed in healthy young subjects without POTS [69], which is important to know in avoiding overdiagnosis. The threshold for an excessive increase in HR in the young exceeds 40 bpm. Symptoms must be concurrent with the excessive tachycardia. No symptoms, no POTS. Tachycardia and concurrent symptoms are very often observed during extremely prolonged orthostatic testing, which are therefore to be avoided if the specific diagnosis of POTS is to be made. POTS has often been divided into subgroups designated ‘neuropathic POTS’, in which it is assumed that partial sympathetic denervation or adrenergic hypoactivity is present, and ‘hyperadrenergic POTS’, in which upright adrenergic overactivity dominates the picture.
Figure 5. Diagram showing representative heart rate in the upper panel and mean arterial pressure in the lower panel during upright tilt in a postural tachycardia syndrome patient.
Heart rate increases, while MAP is stable throughout tilt in postural tachycardia syndrome.
MAP: Mean arterial pressure.
Neuropathic POTS
As originally described, neuropathic POTS is caused by decreased sympathetic adrenergic vasoconstriction in the lower limbs, associated with reduced leg norepinephrine spillover [70] and reduced vasoconstriction of the lower extremities [71]. There is often increased blood flow (‘high flow’) in the lower extremities, even while supine. Another neuropathic variant has normal lower extremity hemodynamics (‘normal flow’) but decreased regional sympathetic adrenergic vasoconstriction in the splanchnic circulation [72]. Neuropathic POTS can represent an autoimmune autonomic neuropathy [58]. Thus, when upright, neuropathic POTS patients have greater than normal redistribution of blood to the dependent vasculature, causing baroreflex-mediated tachycardia and vasoconstriction in the remaining vascular beds that have intact innervation. The cardiac baroreflex response is also blunted in POTS [73]. Central hypovolemia can also result in hyperpnea and hypocapnia in nearly 50% of patients [74] through a baroreflex-mediated mechanism [75].
Therapy for neuropathic POTS (Table 1) includes general supportive measures such as physical countermaneuvers, increased salt and water intake and exercise (see below). Pharmacotherapy has focused on improving sympathetic vasoconstriction, which unfortunately uses medications with widespread systemic effects. Midodrine, an α-1 adrenergic agonist, can be helpful and has few side effects apart from piloerection [76]. Droxidopa is in trials outside the USA and has great expectations. Mestinon® (pyridostigmine) [77] an acetylcholinesterase inhibitor, alone or in combination with midodrine, can be very helpful through its potentiation of cholinergic ganglionic nerve activity and through its muscarinic effects.
Hyperadrenergic POTS
The tachycardia of hyperadrenergic POTS is caused by increased presynaptic or post-synaptic adrenergic potentiation. This might include central sympathetic activity and increased sympathetic nerve activity. Increased supine sympathetic activity has been reported [68], but not universally [78]. To date, the author's laboratory has only observed increased upright muscle sympathetic activity in POTS. One cause of hyperadrenergic POTS is increased synaptic norepinephrine. The norepinephrine transporter deficiency heterozygote [79] is the prime example of this mechanism, but has been found as an autosomal mutation in only one pedigree. Less extensive, possibly epigenetic, NET deficiency has also been demonstrated recently and may have a wider prevalence [52].
Alternative considerations of mechanism include modulation of the adrenergic synapse through enhanced norepinephrine synthesis and release and enhanced post-synaptic affinity, which may be modulated by local and humoral transmitters. Thus, for example, the reciprocal actions of NO and angiotensin-II, respectively, reduce and enhance adrenergic activity. The role of NO as an inhibitory neurotransmitter is now well known [80]. Nitrergic NO released from nerves having parasympathetic activity act at presynaptic and postsynaptic sites to decrease adrenergic transduction [81], the process by which a sympathetic nerve impulse causes vascular smooth muscle contraction. This includes reduction of the release and binding of norepinephrine from the neurovascular synapse [82], interference with postsynaptic neurotransmission [83], chemical denaturing of norepinephrine [84] and downregulation of adrenergic receptors [85]. Conversely, studies of sympatho excitation show that angiotensin-II acts through AT1 receptors to increase production of reactive oxygen and nitrogen species within the brain at presynaptic sympathetic neurons [86], and acts in the periphery, where it produces pre- and post-synaptic augmentation of sympathetic transduction and upregulation of adrenergic receptors [85]. In addition, the release and binding of norepinephrine is facilitated [82], as are the effects of norepinephrine. This depends critically on the formation of reactive oxygen and nitrogen species [87], which also decrease NO [88], often uncoupling NO synthase [89]. This mechanism occurs in a variant of ‘hyperadrenergic POTS’ associated with tachycardia, pallor, vasoconstriction (‘low flow’) and absolute hypovolemia even while supine [90]. NO, plasma renin and serum aldosterone are decreased [91], while plasma angiotensin-II [92] is increased by a defect in angiotensin-converting enzyme-2 [93].
β-blockers have been used in forms of hyperadrenergic POTS with variable success (Table 1) [94,95]. Innovative treatment with angiotensin receptor blockers and droxidopa are under investigation. Exercise has always been a mainstay of rehabilitation in these patients. Recent work indicates that gravitational deconditioning (e.g., bed rest) is a frequent concomitant of the illness and that a graded exercise program can be very effective in improving overall patient well-being [95].
Postural syncope (VVS, acute OI & simple faint)
Syncope (fainting) is defined as ‘complete loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration and spontaneous complete recovery’ [96,97]. Approximately 40% of people will faint during their lives; half of these presenting during adolescence. The peak age for first faint is 15 years [98]. Most syncope is caused by systemic hypotension and reduced CBF. Syncope can be caused by OH as already discussed. OH is easily ruled out by a 3-min standing test (Figure 4). Syncope is divided among cardiovascular syncope, comprising arrhythmic or structural cardiopulmonary disease, and reflex or neurally mediated syncope. Cardiovascular syncope has a poor prognosis unless specifically treated. Reflex syncope has an excellent prognosis [99]. Postural syncope and emotional or phobic syncope comprise VVS [96], the largest subgroup within the reflex syncope category. Regional or system-wide loss of vasoconstriction is an element in all VVS, at least as a terminal event; it may not always be due to loss of sympathetic nerve activity as discussed below. Postural syncope is acute OI wherein loss of consciousness is often preceded by a prodrome of OI symptoms, particularly lightheadedness, nausea, sweating, weakness and blurred vision. Traditionally, postural syncope was believed to be due to reflexes from a hypercontractile underfilled heart analogous to the Bezold–Jarisch reflex [100]. Evidence to the contrary has accrued: the afferent signal would be short-lived because of baroreceptor unloading [101]; very few afferent nerves were excited in the original experiments by Oberg and Thorén in the moribund hemorrhaged cat [102]; VVS can occur in a ventricular denervated transplant recipient [103]; and the heart is neither empty nor hypercontractile prior to syncope [104]. As yet we do not completely understand the pathophysiology of simple faint [105].
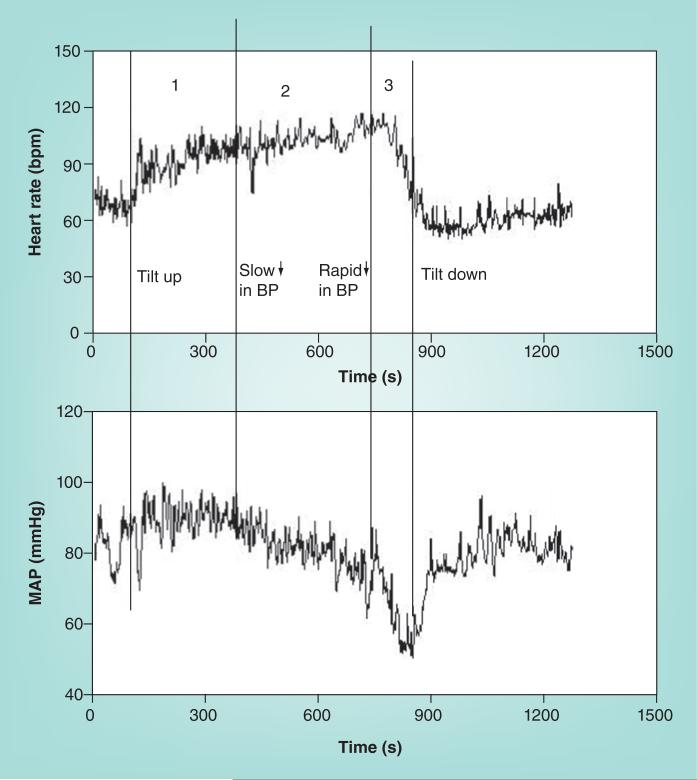
In the most common variant of postural faint occurring in young patients, postural faint comprises three stages (Figure 6), which strongly resemble the circulatory changes found during hemorrhage [106]. After initial OH and restoration of circulatory homeostasis, BP stabilizes while HR increases in Stage 1. BP stability distinguishes postural faint from true OH. BP often exhibits rhythmic fluctuations during this stage referred to as ‘Mayer waves’ [107] with an approximate 10-s period (0.1 Hz). Similar periodicity is shared by fluctuations in HR, sympathetic nerve activity and peripheral resistance. The fluctuations are the closed loop time for sympathetic baroreflex response – that is, the time it takes for detection and compensation for BP changes [108]. Oscillations are accentuated during central blood volume reductions such as occur during orthostasis. During this stage, total peripheral resistance increases to sustain BP in the face of a reduced cardiac output (Figure 3).
Figure 6. Representative heart rate in the upper panel and mean arterial pressure in the lower panel during upright tilt for a postural syncope patient.
Changes during tilt occur over three stages: during the first stage, following initial hypotension, MAP stabilizes at a slightly higher than resting pressure while heart rate increases. During the second stage, MAP begins to fall gradually, while heart rate continues to increase. Note that the increment in heart rate from supine to upright fulfills tachycardia criteria for postural tachycardia syndrome. During the third stage, MAP and then heart rate fall abruptly and rapidly as loss of consciousness supervenes.
BP: Blood pressure; MAP: Mean arterial pressure.
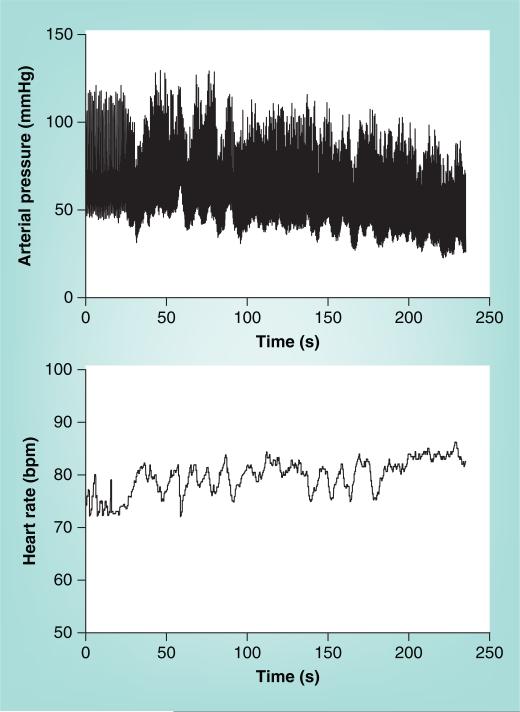
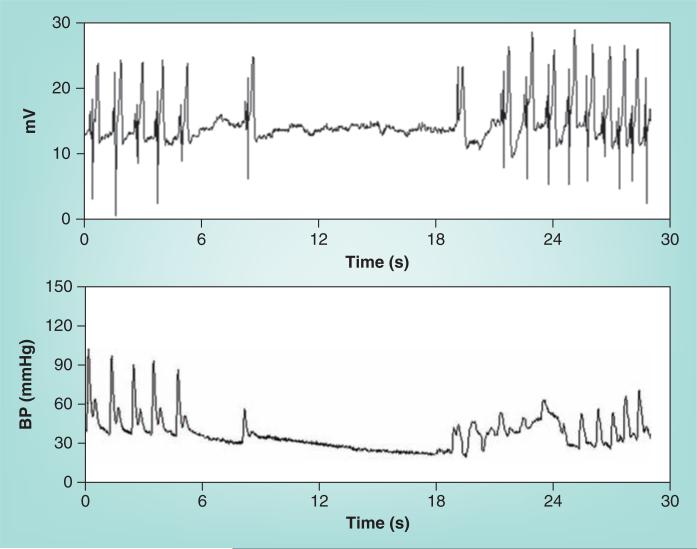
During Stage 2, BP slowly declines as the baroreflex increases HR further. The decrease in BP is most often related to decreased cardiac output [109] even though sympathetic activity [110] and peripheral arterial resistance [111] are sustained. Thereafter, resistance and pressure oscillations decrease despite sustained sympatho excitation. Hyperpnea and hypocapnia occur at this stage in most patients [112]. In some patients, Stage 2 is abbreviated. This is especially true for patients with asystolic syncope (Figure 7) in whom episodes occur abruptly. Asystolic syncope may have some associated tonic posturing, but is distinguished from epilepsy by decreased EEG activity in the former and by nearly immediate resolution of opisthotonic posturing by recumbence. Despite appearances, asystolic faints are not cardiogenic but reflex mediated and are a relatively uncommon form of simple vasovagal fainting that may also be found in phobic fainting.
Figure 7. An asystolic faint.
This is episodic, relatively infrequent and unrelated to intrinsic sinus node disease. Asystolic faints are associated with opisthotonic posturing and have been sometimes referred to as ‘convulsive syncope’.
BP: Blood pressure.
Several mechanisms have been proposed for VVS in some patients. Patients with decreased resting BP can have reduced tyrosine hydroxylase and NE synthesis. A group of normotensive patients can have excess NET [113]. A selective deficit of splanchnic vasoconstriction and venoconstriction have also been demonstrated [72]. Prodromal OI symptoms begin during the second stage and clinicians might, therefore, entertain a diagnosis of POTS in the laboratory setting. Clinical history offers the best way to distinguish patients with acute episodic faints with long periods free of symptoms (postural syncope) from POTS, in which symptoms are chronically present. Indeed, the prodrome of simple faint and the signs and symptoms of POTS are similar because they may have similar initial pathophysiology – excessive reduction in central blood volume resulting in reflex tachycardia [71,72,114]. Postural fainters corresponding to pale and vasoconstricted hyperadrenergic POTS patients are not observed in practice. For the most part, in our experience, POTS patients have day-to-day symptoms but do not faint, while syncope patients have episodic faints, but not daily symptoms. This distinction has become less clear with time: thus, some chronic OI (POTS) patients faint and some episodic fainters also have underlying daily symptoms of OI. However, fainting of POTS patients in the laboratory must be viewed cautiously and cannot, by itself, be regarded as proof of ‘real-world’ fainting. A real-world clinical history compatible with fainting is compulsory.
In the last stage, Stage 3, CBF, BP and HR rapidly fall in that order, apparently defying BP–CBF causality [115]. Similar effects are often seen in nonlinear systems of all kinds whenever a sufficiently strong external signal entrains linked signals. Thus, recent work shows that both cardiovagal and sympathetic baro reflex efferent arms are impaired prior to fainting, and Mayer waves disappear. Similarly, cerebral autoregulation becomes impaired with entrainment of CBF, BP and HR by an extrinsic signal, which may be hyperpneic respiration [4,116]. Why baroreflex integrity is lost is not yet known. But this results in abnormal BP–HR and BP–muscle sympathetic nerve activity functional relationships such that HR, BP and sympathetic nerve activity all decrease, resulting in bradycardia, hypotension and sympathetic silence [117]. The faint is associated with marked systemic vasodilation, while CBF falls with declining BP. Recent work challenges the necessity of sympathetic silence as the precipitant of the final hypotension [118]. While vasodilation always occurs, the sympathetic baroreflex can malfunction with or without sympathetic silence because of a loss of the functional relationship between BP and sympathetic activation. Loss of functional connections between BP and sympathetic nerve activity, but not HR, occurs in patients with vasodepressor syncope where vasodilation without bradycardia occurs. While there is a loss of the sympathetic efferent baroreflex causing progressive loss of compensatory vasoconstriction, the cardiovagal baroreflex remains intact.
POTS and postural syncope are both associated with hyperpneic hyperventilation [8,74,112]. Hyperpnea and resultant hypocapnia precede unconsciousness in virtually every VVS patient [112]. Hypotension and bradycardia might be explained by the pulmonary stretch reflex unfettered by compensatory baroreflex effects [116,119]. The cause of hyperpnea is unclear but may relate to the ventilatory efferent arm of the arterial baroreflex [75]. Similar findings of hyperpnea are found in approximately 50% of POTS patients with central hypovolemia who do not faint.
First time postural noncardiogenic fainting with no sequelae requires no pharmacologic treatment. The first-time fainter rarely knows what is happening to him. Once suitably apprised, countermeasures can be employed as shown in Table 1. These include avoidance of precipitants and physical countermaneuvers; the most effective countermaneuvers are lying down with legs up or squatting. Both propel blood from the lower body below the diaphragm back into the central circulation. Other countermaneuvers include those that enhance the skeletal muscle pump (e.g., leg crossing) or activate the exercise pressor reflex (isometric hand grip) [15,17,38]. Generally enhanced salt and water intake is encouraged and has shown some efficacy in small studies employing very large amounts of salt loading [120]. Rapid water ingestion offers an effective therapeutic effect. Thus, once syncope patients have staved off the faint with physical maneuvers, they are counseled to consume 16 ounces of water before attempting to stand up. In older patients, confounding use of antihyper tensives or diuretics need to be considered. Pharmacotherapy (atenelol or fludro cortisone) has not been shown to be more effective than placebo in younger patients in large multicenter studies [121]. Reports of exquisite sensitivity to midodrine are found in Chinese children [122] but are not uniformly born out in other populations [123]. Other pharmacologic strategies tested in small studies include selective serotonin reuptake inhibitors, including paroxetine, which showed efficacy in a 68-patient double-blind randomized study of a select patient subset [124]. Ambulatory asystolic faints have recently been shown to improve with pacemaker insertion in patients >40 years [125]. Work into the fundamental molecular physiology of fainting is ongoing in our laboratory and in others. Our hope is to determine specific therapy based on specific pathophysiology. We have only considered the well-known forms of OI. It is entirely likely that there are other forms that are not well described will emerge.
Expert commentary
OI is very common. Autonomic failure with true neurogenic OH can represent life-threatening disease. Otherwise, OI is not life threatening. Autonomic regulation is usually abnormal or maladaptive in disorders of orthostatic tolerance and often relate to suboptimal sympathetic adrenergic function. Mild OI, especially initial OI, is universal and rarely harmful. Other forms of OI can seriously impact on quality of life. True neurogenic OH, which can signal the presence of potential life-threatening autonomic failure, has only recently been unequivocally defined by consensus and simple testing devised. Postural vasovagal faint and POTS are two well-described common forms of OI. The ability of simple nonpharmaceutical measures of physical countermaneuvers and rapid water ingestion to aid in improving OI must be emphasized.
Five-year view
The mechanisms of chronic and acute OI are incompletely understood. This even includes simple postural faint that had been treated for years by practitioners with drugs with placebo effects only. Placebo therapy might be helpful if unorthodox. Treatments are therefore often nonspecific. However, several investigations hold promise for specific therapy targeting specific mechanisms of OI.
Key issues.
Orthostasis means standing up.
Orthostatic intolerance (OI) is defined by signs and symptoms that make remaining upright impossible and improve when lying down.
Initial OI is a normal, common, short-lived form of OI in the young. It is the most common form of OI in the young.
Physical countermeasures and rapid water ingestion can improve most forms of OI.
With the exception of neurogenic orthostatic hypotension, OI can even be ‘normal’ (in the sense that anyone can be made to faint with sufficient provocation, and fainting occurs in 40% of people). Normalcy is not the issue. Rather OI becomes a problem when it interferes with the quality of life.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute (grants RO1-HL074873 and RO1-HL087803).
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am. J. Med. Sci. 1999;317(2):75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 2.LeLorier P, Klein GJ, Krahn A, Yee R, Skanes A, Shoemaker JK. Combined head-up tilt and lower body negative pressure as an experimental model of orthostatic syncope. J. Cardiovasc. Electrophysiol. 2003;14(9):920–924. doi: 10.1046/j.1540-8167.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- 3.Low PA, Opfer-Gehrking TL, McPhee BR, et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin. Proc. 1995;70(7):617–622. doi: 10.4065/70.7.617. [DOI] [PubMed] [Google Scholar]
- 4.Ocon AJ, Kulesa J, Clarke D, Taneja I, Medow MS, Stewart JM. Increased phase synchronization and decreased cerebral autoregulation during fainting in the young. Am. J. Physiol. Heart Circ. Physiol. 2009;297(6):H2084–H2095. doi: 10.1152/ajpheart.00705.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2009;297(2):H664–H673. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukai S, Lipsitz LA. Orthostatic hypotension. Clin. Geriatr. Med. 2002;18(2):253–268. doi: 10.1016/s0749-0690(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 7.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol. Rev. 2008;16(1):4–20. doi: 10.1097/CRD.0b013e31815c8032. [DOI] [PubMed] [Google Scholar]
- 8.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29(9):1876–1881. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 9.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43(1):132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 10.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20(1):45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 1959;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki KI, Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279(6):R2189–R2199. doi: 10.1152/ajpregu.2000.279.6.R2189. [DOI] [PubMed] [Google Scholar]
- 13.The European Society of Cardiology. The European Society of Cardiology guidelines for the diagnosis and management of syncope reviewed by Angel Moya, MD, FESC, Chair of the Guideline Taskforce with J. Taylor, MPhil. Eur.Heart J. 2009;30(21):2539–2540. doi: 10.1093/eurheartj/ehp393. [DOI] [PubMed] [Google Scholar]
- 14.Rowell LB. Human Cardiovascular Control. Oxford University Press; NY, USA: 1993. [Google Scholar]
- 15.Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J. Physiol. (Lond.) 2005;563(Pt 3):925–943. doi: 10.1113/jphysiol.2004.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Marsgall RJ, Shepherd JT. The effect of changes in posture and graded exercise on stroke volume in man. J. Clin. Invest. 1960;39:1051–1061. doi: 10.1172/JCI104120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thijs RD, Wieling W, van den Aardweg JG, van Dijk JG. Respiratory countermaneuvers in autonomic failure. Neurology. 2007;69(6):582–585. doi: 10.1212/01.wnl.0000266671.61599.a0. [DOI] [PubMed] [Google Scholar]
- 18.van Dijk N, de Bruin IG, Gisolf J, et al. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J. Appl. Physiol. 2005;98(2):584–590. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]
- 19.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J. Appl. Physiol. 1998;84(3):914–921. doi: 10.1152/jappl.1998.84.3.914. [DOI] [PubMed] [Google Scholar]
- 20.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ. Res. 2002;90(12):1316–1324. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 21.Shipley RD, Kim SJ, Muller-Delp JM. Time course of flow-induced vasodilation in skeletal muscle: contributions of dilator and constrictor mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2005;288(4):H1499–H1507. doi: 10.1152/ajpheart.00489.2004. [DOI] [PubMed] [Google Scholar]
- 22.Raczak G, La Rovere MT, Mortara A, et al. Arterial baroreflex modulation of heart rate in patients early after heart transplantation: lack of parasympathetic reinnervation. J. Heart Lung Transplant. 1999;18(5):399–406. doi: 10.1016/s1053-2498(98)00071-0. [DOI] [PubMed] [Google Scholar]
- 23.Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2+ transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone – epicardial Ca2+ fluorescence studies using fura-2 AM in the isolated innervated beating rabbit heart. Exp. Physiol. 2010;95(1):80–92. doi: 10.1113/expphysiol.2009.048215. [DOI] [PubMed] [Google Scholar]
- 24.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol. Rev. 2009;61(1):62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- 25.Liu JL, Murakami H, Zucker IH. Angiotensin II-nitric oxide interaction on sympathetic outflow in conscious rabbits. Circ. Res. 1998;82(4):496–502. doi: 10.1161/01.res.82.4.496. [DOI] [PubMed] [Google Scholar]
- 26.Macarthur H, Wilken GH, Westfall TC, Kolo LL. Neuronal and non-neuronal modulation of sympathetic neurovascular transmission. Acta Physiol. (Oxf) 2011;203(1):37–45. doi: 10.1111/j.1748-1716.2010.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Euler US. Identification of the sympathomimetic ergone in adrenergic nerves of cattle (sympathin N) with levo-noradrenaline. Acta Physiol. Scandinav. 1948:1663–1674. [Google Scholar]
- 28.Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am. J. Physiol. Heart Circ. Physiol. 2007;292(3):H1420–H1426. doi: 10.1152/ajpheart.01096.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheriff DD, Nådland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J. Appl. Physiol. 2010;108(3):523–532. doi: 10.1152/japplphysiol.01185.2009. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JM. Transient orthostatic hypotension is common in adolescents. J. Pediatr. 2002;140(4):418–424. doi: 10.1067/mpd.2002.122643. [DOI] [PubMed] [Google Scholar]
- 31.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin. Sci. 2007;112(3):157–165. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 32.Clarke DA, Medow MS, Taneja I, Ocon AJ, Stewart JM. Initial orthostatic hypotension in the young is attenuated by static handgrip. J. Pediatr. 2010;156(6):1019–1022. 1022.e1. doi: 10.1016/j.jpeds.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart JM, Clarke D. He's dizzy when he stands up”: an introduction to initial orthostatic hypotension. J. Pediatr. 2011;158(3):499–504. doi: 10.1016/j.jpeds.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard C. Sur les effets de la section de la portion cephalique du grand sympathique. C.R.Soc. Biol. Paris. 1852:4168–1170. [Google Scholar]
- 35.Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin. Proc. 2009;84(9):822–830. doi: 10.4065/84.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010;87(2):198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 37.Huxley VH, Scallan J. Lymphatic fluid: exchange mechanisms and regulation. J. Physiol. (Lond.) 2011;589(Pt 12):2935–2943. doi: 10.1113/jphysiol.2011.208298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platts SH, Tuxhorn JA, Ribeiro LC, Stenger MB, Lee SM, Meck JV. Compression garments as countermeasures to orthostatic intolerance. Aviat. Space. Environ. Med. 2009;80(5):437–442. doi: 10.3357/asem.2473.2009. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein DS, Holmes C, Frank SM, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002;106(18):2358–2365. doi: 10.1161/01.cir.0000036015.54619.b6. [DOI] [PubMed] [Google Scholar]
- 40.Hill L. The influences of the force of gravity on the circulation of the blood. J. Physiol. (Lond.) 1951:1815–1853. [Google Scholar]
- 41.Donegan JF. The physiology of the veins. J. Physiol. (Lond.) 1921;55(3–4):226–245. doi: 10.1113/jphysiol.1921.sp001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victor RG, Mark AL. Interaction of cardiopulmonary and carotid baroreflex control of vascular resistance in humans. J. Clin. Invest. 1985;76(4):1592–1598. doi: 10.1172/JCI112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp. Physiol. 2003;88(6):671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- 44.Chapleau MW, Abboud FM. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs. Circ. Res. 1987;61(5):648–658. doi: 10.1161/01.res.61.5.648. [DOI] [PubMed] [Google Scholar]
- 45.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J. Physiol. (Lond.) 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper VL, Hainsworth R. Carotid baroreceptor reflexes in humans during orthostatic stress. Exp. Physiol. 2001;86(5):677–681. doi: 10.1113/eph8602213. [DOI] [PubMed] [Google Scholar]
- 47.Claydon VE, Hainsworth R. Increased postural sway in control subjects with poor orthostatic tolerance. J. Am. Coll. Cardiol. 2005;46(7):1309–1313. doi: 10.1016/j.jacc.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: a useful test for investigating unexplained syncope. Lancet. 1986;1(8494):1352–1355. doi: 10.1016/s0140-6736(86)91665-x. [DOI] [PubMed] [Google Scholar]
- 49.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol. Rev. 1990;70(4):963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 50.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2004;287(6):H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- 51.Bayles R, Kn H, Lambert E, et al. Epigenetic modification of the norepinephrine transporter gene in postural tachycardia syndrome. Arterioscler. Thromb. Vasc. Biol. 2012;32(8):1910–1916. doi: 10.1161/ATVBAHA.111.244343. [DOI] [PubMed] [Google Scholar]
- 52.Lambert E, Eikelis N, Esler M, et al. Altered sympathetic nervous reactivity and norepinephrine transporter expression in patients with postural tachycardia syndrome. Circ. Arrhythm. Electrophysiol. 2008;1(2):103–109. doi: 10.1161/CIRCEP.107.750471. [DOI] [PubMed] [Google Scholar]
- 53.Esler M. The 2009 Carl Ludwig Lecture: pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J. Appl. Physiol. 2010;108(2):227–237. doi: 10.1152/japplphysiol.00832.2009. [DOI] [PubMed] [Google Scholar]
- 54.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 55.Shibao C, Okamoto L, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol. Ther. 2012;134(3):279–286. doi: 10.1016/j.pharmthera.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hilz MJ, Ehmann EC, Pauli E, et al. Combined counter-maneuvers accelerate recovery from orthostatic hypotension in familial dysautonomia. Acta Neurol. Scand. 2012;126(3):162–170. doi: 10.1111/j.1600-0404.2012.01670.x. [DOI] [PubMed] [Google Scholar]
- 57.Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA, 3rd, Biaggioni I. Dopamine β-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension. 1991;18(1):1–8. doi: 10.1161/01.hyp.18.1.1. [DOI] [PubMed] [Google Scholar]
- 58.Klein CM, Vernino S, Lennon VA, et al. The spectrum of autoimmune autonomic neuropathies. Ann. Neurol. 2003;53(6):752–758. doi: 10.1002/ana.10556. [DOI] [PubMed] [Google Scholar]
- 59.Singh NK, Jaiswal AK, Misra S, Srivastava PK. Assessment of autonomic dysfunction in Guillain–Barré syndrome and its prognostic implications. Acta Neurol. Scand. 1987;75(2):101–105. doi: 10.1111/j.1600-0404.1987.tb07902.x. [DOI] [PubMed] [Google Scholar]
- 60.Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol. Dis. 2012;46(3):572–580. doi: 10.1016/j.nbd.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Man in ‘t Veld AJ, Boomsma F, van den Meiracker AH, Schalekamp MA. Effect of unnatural noradrenaline precursor on sympathetic control and orthostatic hypotension in dopamine-β-hydroxylase deficiency. Lancet. 1987;2(8569):1172–1175. doi: 10.1016/s0140-6736(87)91318-3. [DOI] [PubMed] [Google Scholar]
- 62.Mathias CJ. l-dihydroxyphenylserine (Droxidopa) in the treatment of orthostatic hypotension: the European experience. Clin. Auton. Res. 2008;18(Suppl.):125–129. doi: 10.1007/s10286-007-1005-z. [DOI] [PubMed] [Google Scholar]
- 63.Biaggioni I, Robertson D, Krantz S, Jones M, Haile V. The anemia of primary autonomic failure and its reversal with recombinant erythropoietin. Ann. Intern. Med. 1994;121(3):181–186. doi: 10.7326/0003-4819-121-3-199408010-00004. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto LE, Shibao C, Gamboa A, et al. Synergistic effect of norepinephrine transporter blockade and α-2 antagonism on blood pressure in autonomic failure. Hypertension. 2012;59(3):650–656. doi: 10.1161/HYPERTENSIONAHA.111.184812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water. Lancet. 1999;353(9154):723. doi: 10.1016/S0140-6736(99)99015-3. [DOI] [PubMed] [Google Scholar]
- 66.Mathias CJ, Young TM. Water drinking in the management of orthostatic intolerance due to orthostatic hypotension, vasovagal syncope and the postural tachycardia syndrome. Eur. J. Neurol. 2004;11(9):613–619. doi: 10.1111/j.1468-1331.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 67.McHugh J, Keller NR, Appalsamy M, et al. Portal osmopressor mechanism linked to transient receptor potential vanilloid 4 and blood pressure control. Hypertension. 2010;55(6):1438–1443. doi: 10.1161/HYPERTENSIONAHA.110.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furlan R, Jacob G, Snell M, et al. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98(20):2154–2159. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 69.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J. Pediatr. 2012;160(2):222–226. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N. Engl. J. Med. 2000;343(14):1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 71.Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105(19):2274–2281. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 72.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2006;290(2):H665–H673. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute α-1 adrenergic agonist and a β-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. 2002;106(23):2946–2954. doi: 10.1161/01.cir.0000040999.00692.f3. [DOI] [PubMed] [Google Scholar]
- 74.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am. J. Physiol. Heart Circ. Physiol. 2006;291(2):H904–H913. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart JM, Rivera E, Clarke DA, et al. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am. J. Physiol. Heart Circ. Physiol. 2011;300(4):H1492–H1500. doi: 10.1152/ajpheart.01217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, Wang L, Sun J, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ. J. 2011;75(4):927–931. doi: 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- 77.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111(21):2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 78.Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation. 2004;110(20):3193–3198. doi: 10.1161/01.CIR.0000147280.90339.E9. [DOI] [PubMed] [Google Scholar]
- 79.Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N. Engl. J. Med. 2000;342(8):541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 80.Zanzinger J, Czachurski J, Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ. Res. 1994;75(6):1073–1077. doi: 10.1161/01.res.75.6.1073. [DOI] [PubMed] [Google Scholar]
- 81.Storgaard T, Nedergaard OA. Prejunctional modulation by angiotensins of noradrenaline release from sympathetic neurons in isolated rabbit aorta. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356(6):706–711. doi: 10.1007/pl00005109. [DOI] [PubMed] [Google Scholar]
- 82.Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am. J. Physiol. Heart Circ. Physiol. 2004;286(1):H296–H303. doi: 10.1152/ajpheart.00668.2003. [DOI] [PubMed] [Google Scholar]
- 83.Hatanaka Y, Hobara N, Honghua J, et al. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J. Pharmacol. Exp. Ther. 2006;316(2):490–497. doi: 10.1124/jpet.105.094656. [DOI] [PubMed] [Google Scholar]
- 84.Macarthur H, Mattammal MB, Westfall TC. A new perspective on the inhibitory role of nitric oxide in sympathetic neurotransmission. Biochem. Biophys. Res. Commun. 1995;216(2):686–692. doi: 10.1006/bbrc.1995.2676. [DOI] [PubMed] [Google Scholar]
- 85.Hu ZW, Shi XY, Okazaki M, Hoffman BB. Angiotensin II induces transcription and expression of α 1-adrenergic receptors in vascular smooth muscle cells. Am. J. Physiol. 1995;268(3 Pt 2):H1006–H1014. doi: 10.1152/ajpheart.1995.268.3.H1006. [DOI] [PubMed] [Google Scholar]
- 86.Head GA. Role of AT1 receptors in the central control of sympathetic vasomotor function. Clin. Exp. Pharmacol. Physiol. 1996;23(Suppl. 3):S93–S98. doi: 10.1111/j.1440-1681.1996.tb02820.x. [DOI] [PubMed] [Google Scholar]
- 87.Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am. J. Physiol. Heart Circ. Physiol. 2004;287(2):H695–H703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- 88.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler. Thromb. Vasc. Biol. 2000;20(6):1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 89.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raj SR, Biaggioni I, Yamhure PC, et al. Renin–aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 91.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J. Appl. Physiol. 2011;111(1):20–26. doi: 10.1152/japplphysiol.01448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin. Sci. 2006;110(2):255–263. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1-7) production in postural tachycardia syndrome. Hypertension. 2009;53(5):767–774. doi: 10.1161/HYPERTENSIONAHA.108.127357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120(9):725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58(2):167–175. doi: 10.1161/HYPERTENSIONAHA.111.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gowers WR. A lecture on vagal and vasovagal attacks. Lancet. 1907;173:716–724. [Google Scholar]
- 97.Lewis T. A lecture on vasovagal syncope and the carotid sinus mechanism. Br. Med. J. 1932;1(3723):873–876. doi: 10.1136/bmj.1.3723.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am. J. Cardiol. 2003;91(8):1006–1008. A8. doi: 10.1016/s0002-9149(03)00127-9. [DOI] [PubMed] [Google Scholar]
- 99.Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N. Engl. J. Med. 2002;347(12):878–885. doi: 10.1056/NEJMoa012407. [DOI] [PubMed] [Google Scholar]
- 100.Aviado DM, Guevara Aviado D. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann. NY Acad. Sci. 2001;940:48–58. [PubMed] [Google Scholar]
- 101.Hainsworth R. Syncope: what is the trigger? Heart. 2003;89(2):123–124. doi: 10.1136/heart.89.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oberg B, Thorén P. Increased activity in left ventricular receptors during hemorrhage or occlusion of caval veins in the cat. A possible cause of the vaso-vagal reaction. Acta Physiol. Scand. 1972;85(2):164–173. doi: 10.1111/j.1748-1716.1972.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 103.Scherrer U, Vissing S, Morgan BJ, Hanson P, Victor RG. Vasovagal syncope after infusion of a vasodilator in a heart-transplant recipient. N. Engl. J. Med. 1990;322(9):602–604. doi: 10.1056/NEJM199003013220906. [DOI] [PubMed] [Google Scholar]
- 104.Liu JE, Hahn RT, Stein KM, et al. Left ventricular geometry and function preceding neurally mediated syncope. Circulation. 2000;101(7):777–783. doi: 10.1161/01.cir.101.7.777. [DOI] [PubMed] [Google Scholar]
- 105.Mosqueda-Garcia R, Furlan R, Tank J, Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation. 2000;102(23):2898–2906. doi: 10.1161/01.cir.102.23.2898. [DOI] [PubMed] [Google Scholar]
- 106.Barcroft H, Mcmichael JE, Sharpey-Schafer EP. Posthaemorrhagic fainting. Study by cardiac outputand forearm flow. Lancet. 1944;1:489–491. [Google Scholar]
- 107.Julien C. The enigma of Mayer waves: facts and models. Cardiovasc. Res. 2006;70(1):12–21. doi: 10.1016/j.cardiores.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288(6):R1637–R1648. doi: 10.1152/ajpregu.00050.2004. [DOI] [PubMed] [Google Scholar]
- 109.Verheyden B, Liu J, van Dijk N, et al. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart Rhythm. 2008;5(12):1695–1701. doi: 10.1016/j.hrthm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J. Physiol. (Lond.) 2009;587(Pt 20):4987–4999. doi: 10.1113/jphysiol.2009.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am. J. Physiol. Heart Circ. Physiol. 2008;295(1):H372–H381. doi: 10.1152/ajpheart.00101.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lagi A, Cencetti S, Corsoni V, Georgiadis D, Bacalli S. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation. 2001;104(22):2694–2698. doi: 10.1161/hc6172.099397. [DOI] [PubMed] [Google Scholar]
- 113.Vaddadi G, Guo L, Esler M, et al. Recurrent postural vasovagal syncope: sympathetic nervous system phenotypes. Circ. Arrhythm. Electrophysiol. 2011;4(5):711–718. doi: 10.1161/CIRCEP.111.962332. [DOI] [PubMed] [Google Scholar]
- 114.Stewart JM, McLeod KJ, Sanyal S, Herzberg G, Montgomery LD. Relation of postural vasovagal syncope to splanchnic hypervolemia in adolescents. Circulation. 2004;110(17):2575–2581. doi: 10.1161/01.CIR.0000145543.88293.21. [DOI] [PubMed] [Google Scholar]
- 115.Dan D, Hoag JB, Ellenbogen KA, Wood MA, Eckberg DL, Gilligan DM. Cerebral blood flow velocity declines before arterial pressure in patients with orthostatic vasovagal presyncope. J. Am. Coll. Cardiol. 2002;39(6):1039–1045. doi: 10.1016/s0735-1097(02)01719-9. [DOI] [PubMed] [Google Scholar]
- 116.Ocon AJ, Medow MS, Taneja I, Stewart JM. Respiration drives phase synchronization between blood pressure and RR interval following loss of cardiovagal baroreflex during vasovagal syncope. Am. J. Physiol. Heart Circ. Physiol. 2011;300(2):H527–H540. doi: 10.1152/ajpheart.00257.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am. J. Physiol. Heart Circ. Physiol. 2002;282(5):H1804–H1809. doi: 10.1152/ajpheart.00640.2001. [DOI] [PubMed] [Google Scholar]
- 118.Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur. Heart J. 2010;31(16):2027–2033. doi: 10.1093/eurheartj/ehq071. [DOI] [PubMed] [Google Scholar]
- 119.Looga R. Reflex cardiovascular responses to lung inflation: a review. Respir. Physiol. 1997;109(2):95–106. doi: 10.1016/s0034-5687(97)00049-2. [DOI] [PubMed] [Google Scholar]
- 120.Claydon VE, Hainsworth R. Salt supplementation improves orthostatic cerebral and peripheral vascular control in patients with syncope. Hypertension. 2004;43(4):809–813. doi: 10.1161/01.HYP.0000122269.05049.e7. [DOI] [PubMed] [Google Scholar]
- 121.Sheldon RS, Amuah JE, Connolly SJ, et al. Prevention of Syncope Trial. Effect of metoprolol on quality of life in the Prevention of Syncope Trial. J. Cardiovasc. Electrophysiol. 2009;20(10):1083–1088. doi: 10.1111/j.1540-8167.2009.01518.x. [DOI] [PubMed] [Google Scholar]
- 122.Qingyou Z, Junbao D, Chaoshu T. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J. Pediatr. 2006;149(6):777–780. doi: 10.1016/j.jpeds.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 123.Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace. 2011;13(11):1639–1647. doi: 10.1093/europace/eur200. [DOI] [PubMed] [Google Scholar]
- 124.Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 1999;33(5):1227–1230. doi: 10.1016/s0735-1097(98)00694-9. [DOI] [PubMed] [Google Scholar]
- 125.Brignole M, Menozzi C, Moya A, et al. International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation. 2012;125(21):2566–2571. doi: 10.1161/CIRCULATIONAHA.111.082313. [DOI] [PubMed] [Google Scholar]