Abstract
Extensive research on the rabbit nictitating membrane response (NMR) has shown that the NMR reflex can become exaggerated following classical fear conditioning. This learning-related change is referred to as conditioning-specific reflex modification (CRM) and is observed in the absence of the conditioned stimulus. The aim of the current study was to examine the sensitivity of the CRM paradigm to serotonergic manipulation with fluoxetine, a commonly prescribed selective serotonin reuptake inhibitor for anxiety disorders. To assess the effect of fluoxetine on exaggerated reflexive responding indicative of CRM and on conditioned cued fear, rabbits underwent delay NMR conditioning (pairings of tone and periorbital shock) and were tested for CRM, followed by 5 days of daily fluoxetine (0.03, 0.3, or 3.0 mg/kg) or saline injections. CRM was reassessed 1 day and 1 week later, followed by a retention test of conditioned responses (CRs) to the tone. Fluoxetine (3.0 mg/kg) enhanced CRM and retention of conditioned responses, a week after treatment ceased, and this is in agreement with the reports on increased anxiety-like behaviors in other animal models and humans. The CRM paradigm, therefore, may provide important insight into the mechanisms underlying the paradoxical selective serotonin reuptake inhibitor effects.
Keywords: classical conditioning, eyeblink, fear conditioning, fluoxetine, nictitating membrane response, post-traumatic stress disorder, rabbit, selective serotonin reuptake inhibitor
Introduction
Extensive research on the rabbit nictitating membrane response (NMR) has shown that the NMR reflex, once thought to be invariant and automatic, can become enhanced after classical NMR conditioning (Schreurs, 2003; Burhans et al., 2010). This learning-related change is observed in the absence of the conditioned stimulus (CS) and is referred to as conditioning-specific reflex modification (CRM). Previous work has established that CRM is characterized as an exaggerated reflexive response, particularly to lower intensity stimuli that generated little to no responding before conditioning (Burhans et al., 2008). Other researchers have also reported CRM-like changes during NMR conditioning in rabbits (Gruart and Yeo, 1995; Wikgren et al., 2002) and eyeblink conditioning in rats (Servatius et al., 2001). Because the CRM paradigm can index changes in both cued and reflexive fear responding, we have argued that it may be an important corollary to animal models of fear-based disorders such as post-traumatic stress disorder (PTSD) (Schreurs, 2003; Seager et al., 2003; Schreurs et al., 2006, 2011a, 2011b; Burhans et al., 2008; Burhans and Schreurs, 2008).
The standard CRM paradigm is an ABA design in which rabbits are first exposed to varying intensities and durations of a periorbital shock unconditioned stimulus (US) to obtain a baseline of physiological responsiveness (pretest), followed typically by 6 days of classical delay NMR conditioning involving pairings of a tone CS with the US, and then another US test identical to the pretest (post-test) (Seager et al., 2003; Schreurs et al., 2006, 2011b). The level of CRM is determined by examining the pretest to post-test change in the responsiveness to the US across several NMR parameters including response frequency, amplitude, latency, and area under the response curve. Importantly, CRM does not occur in rabbits receiving an equivalent number of exposures to tones and shocks during unpaired CS/US presentations or equivalent exposure to the training context without stimulus presentations (Schreurs et al., 1995, 2000), demonstrating that CRM is learning-related and not a result of nonassociative factors such as sensitization (Schreurs, 2003).
CRM closely resembles the shape and timing of the NMR response that develops to the tone CS, suggesting that it may in part be a generalized conditioned response (CR). In support of this idea, CRM acquisition is affected by factors that influence the level of NMR conditioning such as the number of paired CS–US presentations (Schreurs et al., 1995), the intensity and aversiveness of the US utilized during conditioning (Buck et al., 2001; Seager et al., 2003), and change in context (Schreurs et al., 2006). However, some dichotomy exists between CRM and NMR conditioning because certain extinction treatments that reduce conditioned NMR responses, such as CS-alone presentations, leave CRM intact (Schreurs et al., 2000). Simultaneous extinction of both is instead best accomplished by less conventional unpaired CS/US presentations using the US presented during conditioning, or using a reduced intensity US as long as treatment continues for 6 days (Schreurs et al., 2000, 2011b). Recent work has also shown that there is a critical time window during which CRM can be exacerbated by incubation (Schreurs et al., 2011a).
To gain understanding of the possible neural substrates behind CRM, we investigated the role of the amygdala, a structure critical for reflex facilitation of the NMR during CS–US pairings (Whalen and Kapp, 1991; Weisz et al., 1992; Canli and Brown, 1996) and also well known for its role in emotional responding (LeDoux, 2000; Davis and Whalen, 2001; Maren, 2001; Kim and Jung, 2006). Inactivation of the central nucleus by infusion of the GABAA agonist muscimol during US testing, but not during NMR conditioning, blocked CRM, demonstrating that CRM expression but not its acquisition is critically dependent on the central nucleus of the amygdala (Burhans and Schreurs, 2008). This study along with previous works showing that CRM is stronger with a more aversive US (Buck et al., 2001; Seager et al., 2003) and is accompanied by an increase in heart rate (Schreurs and Smith-Bell, 2005) supported the idea that there may be an amygdala-mediated fear component to CRM.
A next step in our investigations of the neural mechanisms underlying CRM was to evaluate its sensitivity to serotonergic manipulation. Extensive work by Harvey and colleagues has shown that serotonin plays an important role in NMR conditioning and can affect both conditioned and unconditioned responding (Romano et al., 2000; Harvey, 2003). In addition, selective serotonin reuptake inhibitors (SSRIs) are often a first-line in the treatment of fear-based disorders such as PTSD (Davidson, 2006; Anatai-Otong, 2007; Ipser and Stein, 2011). To elaborate on the potential clinical relevance of the CRM paradigm, we chose to investigate the common SSRI fluoxetine. In the therapeutic setting, SSRIs are typically not introduced until after the onset of symptoms; therefore, the purpose of the following study was to utilize a clinically relevant scenario to examine the effect of fluoxetine treatment on retention/expression of conditioned fear responses and CRM. Rabbits were subjected to the standard CRM experimental design with classical NMR conditioning and CRM testing taking place before the start of the 5-day fluoxetine treatment. CRM was reassessed 1 day after the cessation of treatment and also 1 week later, followed by CS-alone extinction to test the level of remaining cued conditioned fear responses. If shown to be sensitive to fluoxetine, we considered that the CRM paradigm may provide new insight into effects of SSRIs, because of the well-understood pathways and mechanisms involved in NMR and eyeblink conditioning (Christian and Thompson, 2003; Freeman and Steinmetz, 2011).
Methods
Subjects
The subjects were 42 male, New Zealand White rabbits (Oryctolagus cuniculus), 2–3 months of age, weighing ~2.0–2.2 kg upon delivery from the supplier (Harlan, Indianapolis, Indiana, USA). The rabbits were housed in individual cages on a 12-h light–dark cycle and given free access to food and water. They were maintained in accordance with the guide for the care and use of laboratory animals issued by the National Institute of Health, and the research was approved by the West Virginia University Animal Care and Use Committee. One rabbit did not complete the experiment because of an adverse reaction to the drug injection procedure.
Apparatus
The apparatus, recording, and analysis procedures for NMR conditioning have been detailed previously (Schreurs et al., 2005b). Rabbits were restrained in a Plexiglas box placed inside a sound-attenuating, ventilated chamber (Model E10–20; Coulborn Instruments, Allentown, Pennsylvania, USA). Inside the chamber, a stimulus panel containing a speaker and houselight (10 W, 120 V) was mounted at a 45° angle, 15 cm anterior and dorsal to the rabbit’s head. An exhaust fan created a constant ambient noise level of 65 dB inside the chamber. Periorbital electrical stimulation (ES) was delivered by a programmable two-pole stimulator (Model E13–35; Colbourn Instruments) through stainless steel Autoclip wound clips (Stoelting, Wood Dale, Illinois, USA) that were positioned 10 mm ventral and 10 mm posterior to the dorsal canthus of the right eye. Stimulus delivery, data collection, and analysis were all accomplished using the LabVIEW software system (National Instruments, Austin, Texas, USA).
The NMRs were transduced using a potentiometer (Model P2201; Novotechnik US Inc., Southborough, Massachusetts, USA) connected at one end, by a freely moving ball and socket joint, to an L-shaped lever containing a hook that is attached to a 6-0 nylon loop sutured into but not through the nictitating membrane (NM). At the other end, the potentiometer was connected to a 12-bit analog-to-digital converter (5 ms sampling rate, 0.05 mm resolution), and individual A/D outputs were stored on a trial-by-trial basis for subsequent analysis.
Procedure
Classical delay conditioning and unconditioned stimulus testing
One week after arrival, the rabbits received one session per day, starting with adaptation and followed by US pretesting (pretest), six sessions of classical delay conditioning, and US post-testing (Post1). A second US post-test (Post2) occurred after 5 days of drug injections, and two additional sessions were presented 1 week later: a third US post-test (Post3) and a CS-alone test. For adaptation, the subjects were prepared for ES delivery and NMR recording and then adapted to the training chambers for an amount of time equivalent to subsequent training sessions (80 min). For pretest and post-tests, subjects received 80 trials of US presentations with an average intertrial interval of 60 s (range 50–70 s). Each US presentation was one of 20 combinations of ES intensity (0.1, 0.25, 0.5, 1.0, or 2.0 mA) and duration (10, 25, 50, or 100 ms), and these 20 unique USs were presented in four separately randomized blocks, with the restriction that the same intensity or duration could not occur more than three times in succession. For delay conditioning, each session consisted of 80 trials of paired presentations of a 400 ms, 1 kHz, 82 dB tone CS that coterminated with a 100 ms, 2 mA ES US (300 ms interstimulus interval). The CS–US presentations were presented with an average intertrial interval of 60 s (range 50–70 s). The CS-alone test consisted of 80 presentations of the tone CS (parameters same as conditioning).
CRs of the NMR were defined as any extension of the NM exceeding 0.5 mm that was initiated following CS onset but before US onset. For US testing, an unconditioned response (UR) was defined as any extension of the NM exceeding 0.5 mm that was initiated within 300 ms after onset of US. The definition of the UR was based on prior observations that responses to the US after CS–US pairings had onset latencies within the same range as CRs (Schreurs et al., 2000). Amplitude of the response was calculated as the maximum extension (mm) of the NM. Onset latency of the response was the latency (ms) from stimulus onset to when the NM rose to 0.1 mm above baseline, whereas peak latency was the latency (ms) from stimulus onset until maximum NM extension occurred. Area of the response was calculated as the total area of the response curve (arbitrary units) from stimulus onset until the end of the trial (trial length = 2000 ms). For URs during US testing, two additional measures were calculated to overcome the statistical limitations of empty data cells produced by subthreshold responses to ES, particularly at the lower intensities and durations. These measures, magnitude of the response and magnitude of the response area, included the amplitudes and areas of all NMRs above baseline regardless of whether the 0.5 mm criterion was met (Garcia et al., 2003). A significant pretest to post-test increase in any of the UR response measures as a function of classical conditioning is a defining feature of CRM. To increase the sensitivity for detection of CRM and to follow the convention of previous studies on CRM, we collapsed data at the five US intensities across all durations and focused our CRM analyses on the first 20-trial US sequences in which the strongest CRM was observed (Schreurs et al., 2000). To examine the shape and timing of NMRs during the US tests, response topographies were generated at each US intensity by averaging across rabbits and across US durations within each experimental group.
Acute fluoxetine treatment
A solution of fluoxetine hydrochloride (Sigma-Aldrich, St Louis, Missouri, USA) was freshly prepared before each use by dissolving in 0.9% sterile saline. Following Post1, fluoxetine (0.03, 0.3, or 3 mg/kg) or vehicle (0.9% saline) was injected daily for 5 days into the marginal vein of the rabbit’s ear. No behavioral training took place on drug injection days, and rabbits were returned to home cages immediately following injections. Assignment of the drug treatment groups took place after Post1 to match the groups on the basis of levels of NMR conditioning and CRM. The range of doses was centered around a common 20 mg/day dose administered to humans (~0.3 mg/kg) (Mostert et al., 2008) and capped at 3.0 mg/kg, on the basis of findings that a dose of 6 mg/kg elevates intraocular pressure in rabbits (Costagliola et al., 2000).
Statistical analysis
The experiment was conducted in three separate replications. Data were analyzed by analysis of variance (ANOVA; SPSS 18.0, SPSS Inc., Chicago, IL, USA) with violations of the sphericity assumption corrected using the Greenhouse-Geisser correction. Planned and followup comparisons were Bonferroni corrected for the number of comparisons. One rabbit was removed from analyses because of a failure to reach a learning criterion of 80% CRs by the sixth day of delay conditioning. The final subject count for analyses was n = 10 for each group.
Results
Classical delay conditioning
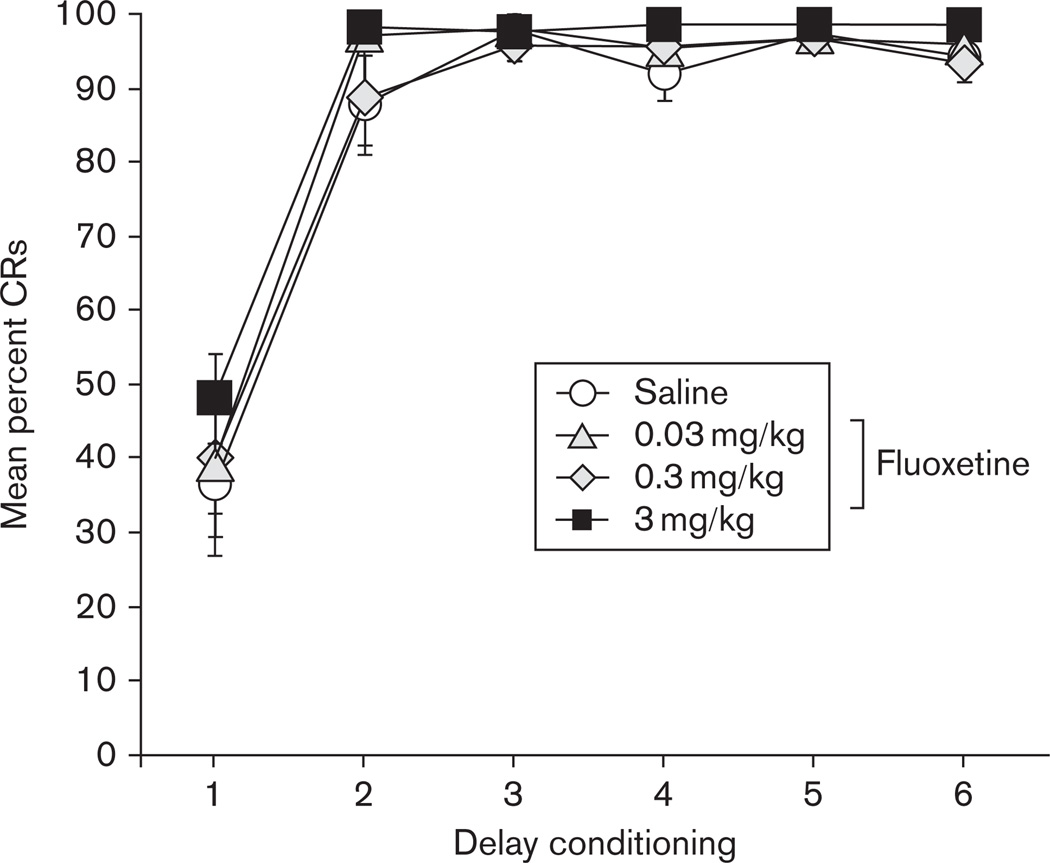
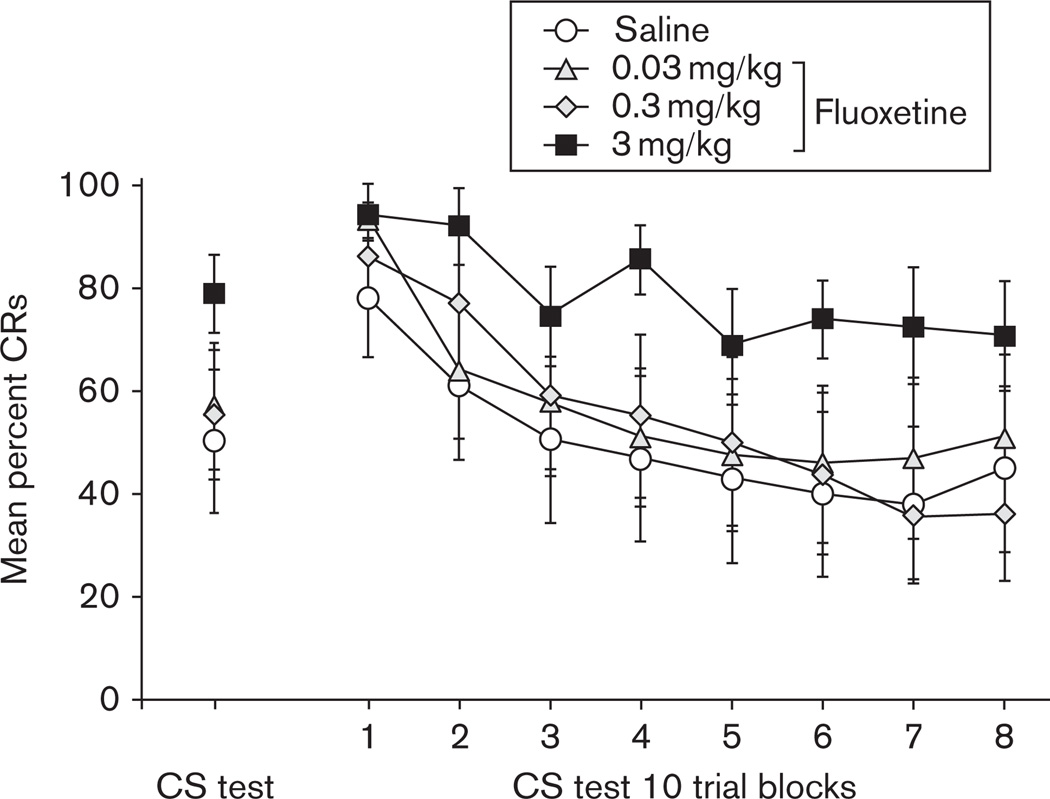
Figure 1 shows the average percentage of CRs to the tone CS across the 6 days of classical delay conditioning, separated into the four experimental groups that received either saline or fluoxetine (0.03, 0.3, and 3.0 mg/kg) injections following conditioning. All groups acquired in excess of 93% CRs to the tone CS by the sixth day of training. Repeated-measures ANOVA revealed a significant main effect of days [F(1.6,58.9) = 163.9, P < 0.001], and no effects or interactions involving Drug Group, indicating that all groups reached an equivalent level of learning before drug treatment.
Fig. 1.
The mean percentage (±SEM) of conditioned responses (CRs) to the tone conditioned stimulus during six daily sessions of delay conditioning before drug injections for groups receiving saline (white circle) or fluoxetine treatment at a dose of 0.03 mg/kg (gray triangle), 0.3 mg/kg (gray diamond), or 3.0 mg/kg (black square).
Fluoxetine effects on conditioning-specific reflex modification
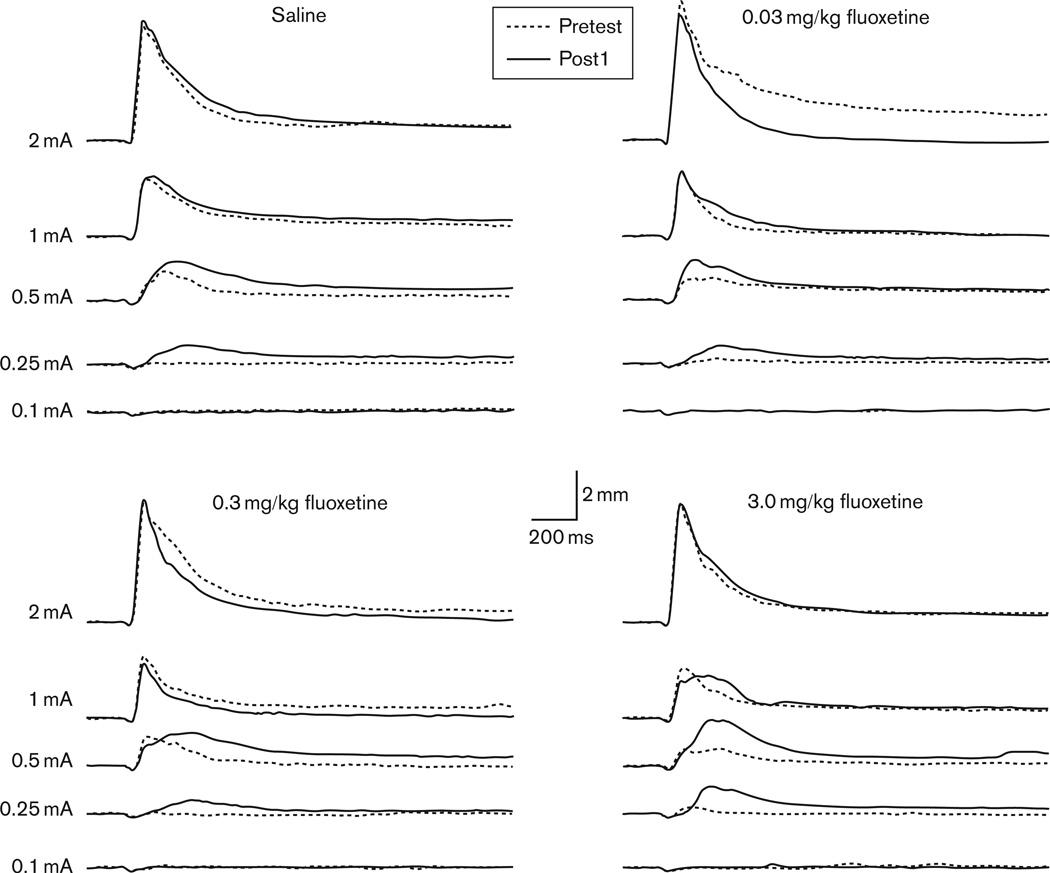
Initial comparisons of the UR changes from pretest to Post1 were conducted to determine the level of CRM before drug treatment. The averaged UR topographies for each drug group during pretest and Post1 are shown in Fig. 2. Repeated-measures ANOVA was focused on the first 20 trial sequence of US presentations where the strongest CRM effects are observed (Schreurs et al., 2000). All groups demonstrated CRM, as shown by enhanced responding at Post1 compared with pretest at the lowest US intensities. This observation was confirmed by a significant interaction of US Intensity (0.1, 0.25, 0.5, 1.0, and 2.0 mA) and Test (pretest, Post1), but no significant interaction with Drug Group for analyses of UR frequency [F(3.1,112.1) = 4.8, P < 0.002], magnitude of the UR [F(2.9,106.0) = 7.1, P < 0.001], and magnitude of the UR area [F(2.9,105.3) = 7.7, P < 0.001]. Planned comparisons showed that CRM occurred at 0.25 mA for all three UR parameters (frequency: P < 0.001; magnitude: P < 0.002; area: P < 0.005) and additionally at 0.1 mA for magnitude of the area (P < 0.05). CRM was also indicated by a shift in peak latency [F(1.5,45.6) = 7.3, P < 0.002] at the 0.5 mA intensity (P < 0.02). Analyses for latency measures did not include the lowest intensities (0.1 and 0.25 mA) because of the limitation of empty data cells (see the Methods section). In summary, CRM was demonstrated before drug treatment and was characterized as pretest to Post1 changes across several UR parameters at the lower US intensities.
Fig. 2.
Average response topographies for the unconditioned response during the first 20 trials of the pretest (Pretest, dotted black line) and first post-test (Post1, solid black line) following delay conditioning and before drug treatment for groups receiving saline or fluoxetine (0.03, 0.3, 3.0 mg/kg). Topographies are shown at the five unconditioned stimulus intensities (2.0, 1.0, 0.5, 0.25, 0.1 mA).
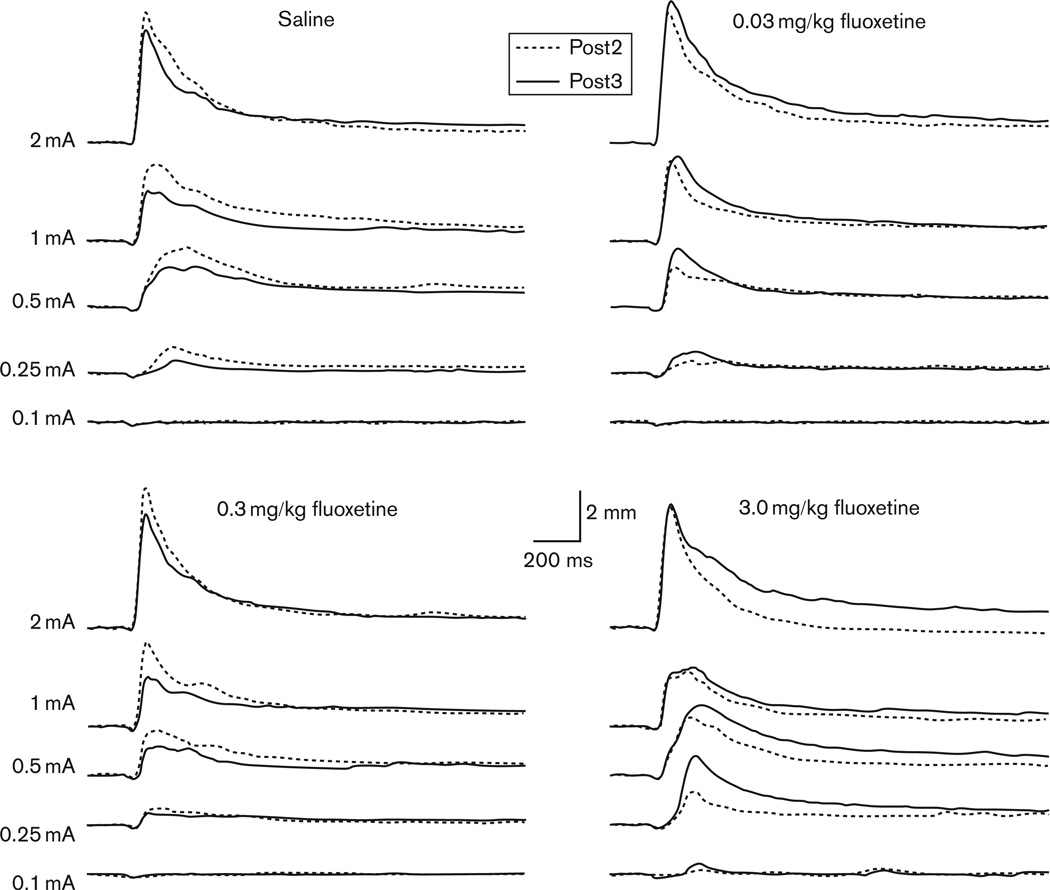
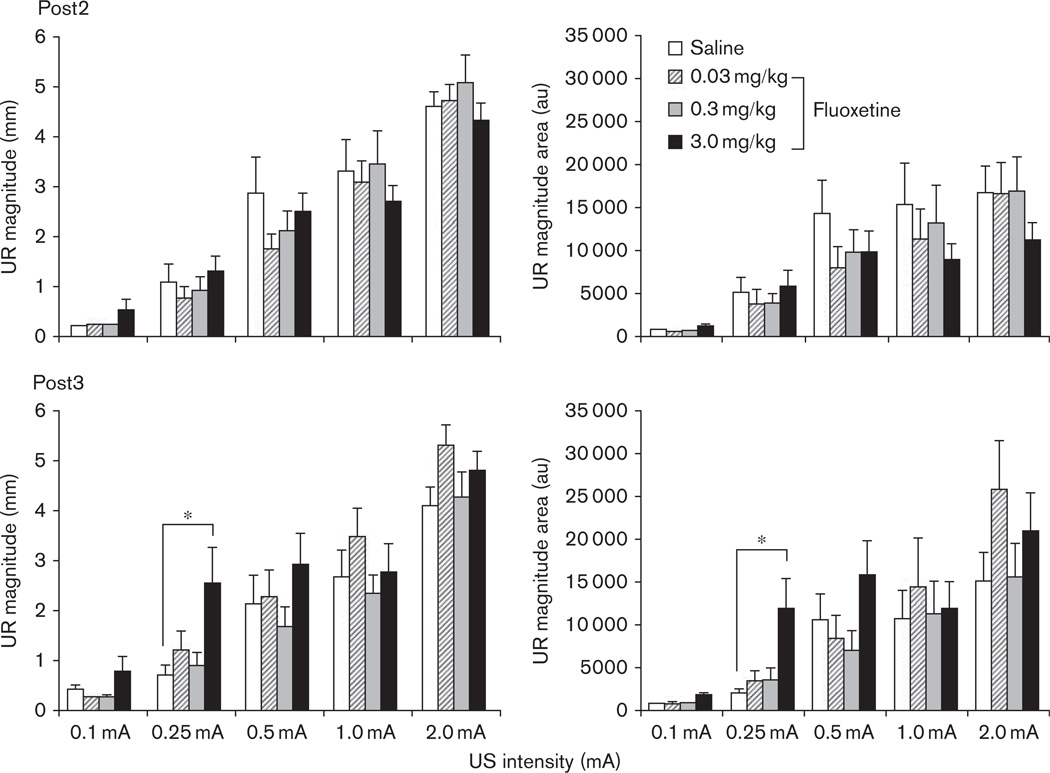
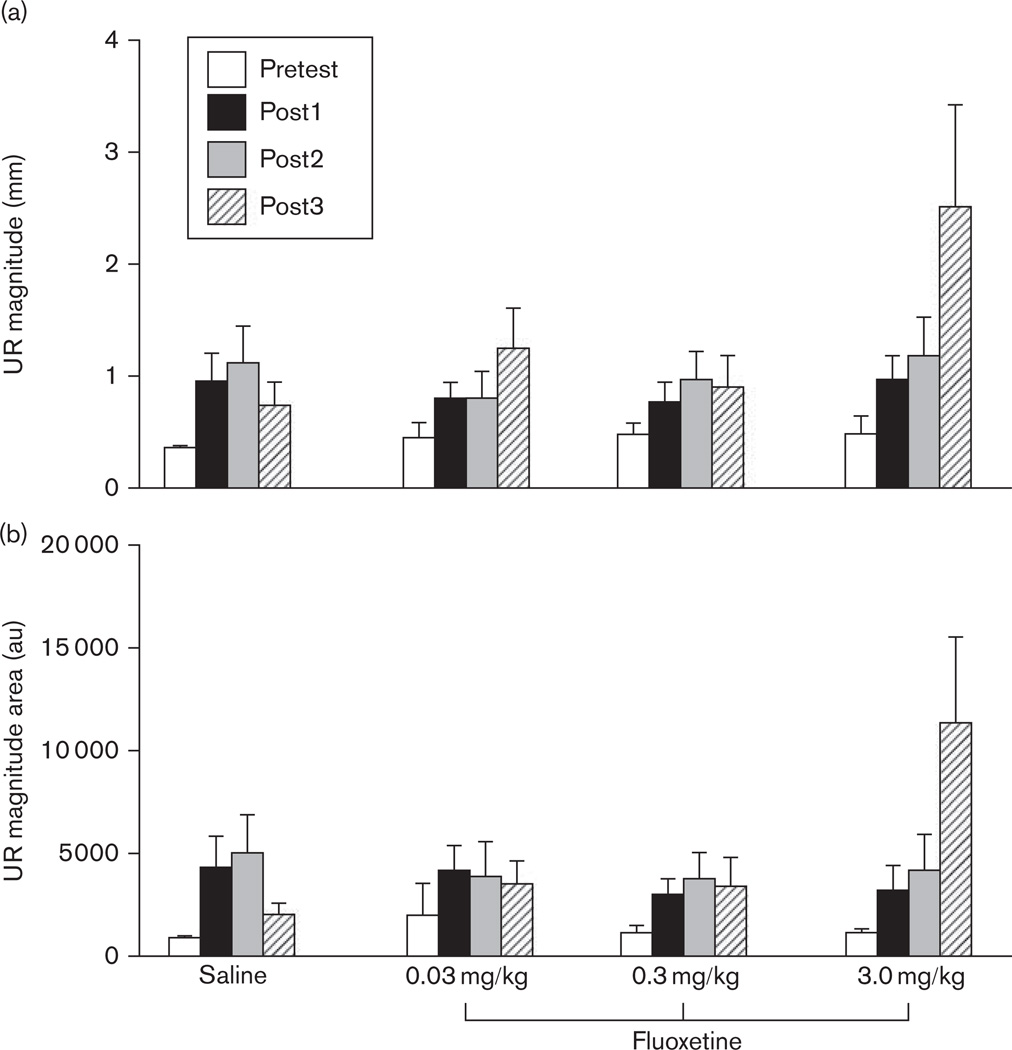
The immediate and delayed effects of fluoxetine treatment on CRM are indicated in Fig. 3 as Post2 and Post3, respectively. There were no obvious effects of fluoxetine at the first post-test following drug treatment (Post2), with all groups showing a similar level of CRM. However, the post-test conducted 1 week later (Post3) revealed an enhanced level of CRM in the highest dose group (3.0 mg/kg) suggesting a delayed drug effect. In contrast, rabbits in the saline and lower dose drug groups showed either a similar level or decreased CRM at Post3 relative to Post2. These observations are quantified in Fig. 4, which compares responding across groups at each of the US intensities for response magnitude and magnitude of the area. Repeated-measures ANOVAs with factors of Test (Posts 1–3) and Drug Group were focused on the intensities for which CRM was significant before conditioning (0.1 and 0.25 mA). For the analysis at the 0.1 mA intensity, there were no significant drug effects. For 0.25 mA, there was a significant Test × Drug Group interaction for magnitude of the area [F(4.6,55.1) = 2.4, P < 0.05] and a trend for response magnitude [F(4.3,51.1) = 2.0, P = 0.075]. Planned comparisons indicated that responding in the 3.0 mg/kg dose group had a greater area (P < 0.01) and magnitude (P < 0.05) than the saline group at Post3. There were no differences between the lower dose groups and saline group for Post3, and no differences between any groups at Post1, Post2, or at the higher US intensities. Figure 5 provides an overview of responding at the 0.25 mA US intensity across all US tests within each drug group. In summary, these findings demonstrated that the highest dose of fluoxetine enhanced CRM a week after cessation of treatment.
Fig. 3.
Average response topographies for the unconditioned response to the first 20 trials of the post-test (Post2, dotted black line) following drug treatment with 5 days of saline or fluoxetine (0.03, 0.3, 3.0 mg/kg) and then the post-test 1 week later (Post3, solid black line). Topographies are shown at the five unconditioned stimulus intensities (2.0, 1.0, 0.5, 0.25, 0.1 mA).
Fig. 4.
The mean (±SEM) unconditioned response (UR), magnitude of the response (left graphs), and magnitude of the area (right graphs) for the first 20 trials of Post2 (top graphs) and Post3 (bottom graphs) presented 1 day and 1 week, respectively, following the cessation of 5 days of drug treatment with saline (white bar) or fluoxetine (0.03 mg/kg, striped bar; 0.3 mg/kg, gray bar; 3.0 mg/kg, black bar). *P < 0.05.
Fig. 5.
The mean (±SEM) unconditioned response (UR), magnitude of the response (a), and magnitude of the area (b) to the 0.25 mA unconditioned stimulus (US) intensity for the first 20 trials of each of four US tests for groups receiving saline or fluoxetine (0.03, 0.3, 3.0 mg/kg). Pretest (white bar) and Post1 (black bar) took place before and following conditioning, respectively, whereas Post2 (gray bar) and Post3 (striped bar) occurred 1 day and 1 week following the cessation of 5 days of drug treatment.
Fluoxetine effects on retention of conditioned responding
The effect of five daily injections of either saline or fluoxetine on retention of CRs, as measured by the tone alone presentations during the CS test, is shown in Fig. 6. Examination of responding averaged across the whole session (left side of Fig. 6) suggested that the highest dose group had an enhanced retention of CRs. An ANOVA on the CS Test, however, did not reveal a significant effect of Drug Group. When separate analyses were carried out to examine responding within each group across sessions (6 days conditioning plus the CS test), post-hoc comparisons for a significant main effect of session found for each group revealed that during the CS test, only the 3.0 mg/kg dose failed to reduce responding back to levels exhibited during day 1 of conditioning (P < 0.01) [F(1.7,15.1) = 36.2, P < 0.001].
Fig. 6.
The mean percentage (±SEM) of conditioned responses (CRs) to the tone conditioned stimulus (CS) during a session of 80 CS-alone presentations (CS test, left side of graph), following cessation of saline (white circle) or fluoxetine treatment at a dose of 0.03 mg/kg (gray triangle), 0.3 mg/kg (bold gray diamond), or 3.0 mg/kg (black square). The right side of the graph (CS test 10 trial blocks) shows the breakdown of the 80 trial session into eight blocks of 10 trials each.
Data from the CS test session were then graphed into blocks of 10 trials to analyze whether there were within-session changes in responding across groups (right side of Fig. 6). The 3.0 mg/kg fluoxetine drug group appeared to maintain a slightly higher level of responding across the session but similar to the other groups, did show within-session decreases in responding. Repeated-measures ANOVA with factors of Trial Block and Drug Group did not indicate any significant group effects, but the latter observation was confirmed by a significant main effect of Trial Block [F(3.1,111.9) = 20.1, P < 0.001]. These findings suggested that the slightly elevated level of CRs in the 3.0 mg/kg fluoxetine group was possibly because of enhanced retention.
Discussion
The aim of the current experiment was to examine the sensitivity of the CRM paradigm to serotonergic manipulation with the commonly prescribed SSRI fluoxetine. The main finding was that fluoxetine treatment at the highest dose (3.0 mg/kg) enhanced CRM and retention of CRs. In addition, the enhancement in CRM was not immediately apparent, only being detected after a week had elapsed following the cessation of treatment. In summary, the results show that short-term fluoxetine treatment can increase exaggerated reflexive responding and cued fear behaviors that may be representative of anxiogenic-like responding. Examples of types of behavioral tests widely used as measures of anxiety-like responding in animals include social interaction, open field testing, elevated plus maze, and fear conditioning to cues and contexts associated with shock (Salchner and Singewald, 2002; Burghardt et al., 2007; Liu et al., 2010; Homberg et al., 2011; Ravinder et al., 2011; Robert et al., 2011) We argue that the CRM paradigm may also represent a test of anxiety-like behavior in rabbits, as we are examining conditioned cued and reflexive NMR responses to shock.
It is widely established that fluoxetine and other SSRIs used to treat anxiety often require several weeks of treatment to establish efficacy, producing initial side-effects that can be interpreted as a worsening of symptoms (Ravindran and Stein, 2010). Anxiogenic effects of acute SSRIs have been explicitly documented in healthy humans (Grillon et al., 2007), and anxiogenic-like effects of acute fluoxetine have been documented in animal models of anxiety and PTSD (Salchner and Singewald, 2002; Burghardt et al., 2007; Liu et al., 2010; Homberg et al., 2011; Ravinder et al., 2011; Robert et al., 2011). In these studies, ‘acute’ treatment is characterized as a single dose administered shortly before testing. The current study utilized a treatment of 5 days, which could be considered subacute; therefore, the results presented here may be at least partially interpreted as being in line with the documented acute treatment effects. There also have been reports of anxiogenic-like effects of chronic administration of fluoxetine for 15 days or more in animal models (Schulz et al., 2007b; Oh et al., 2009; Homberg et al., 2011; Robert et al., 2011). Of particular interest are the studies that established specific conditions under which chronic fluoxetine can increase anxiety-like behaviors. Oh et al. (2009) and Homberg et al. (2011) showed that juvenile rats had more adverse behavioral reactions to chronic fluoxetine than adults, mirroring the clinical literature showing that adolescents are especially vulnerable to the negative effects of SSRIs resulting in an increased risk of suicide (Jick et al., 2004). Robert et al. (2011) demonstrated in rats that the stressful handling associated with daily injections had a stand-alone anxiolytic-like effect that mitigated anxiogenic-like behaviors to fluoxetine. Our experiment could have been influenced by both of these factors. We used juvenile rabbits that may be more prone to adverse reactions to fluoxetine, and those rabbits then underwent a stressful daily handling experience of intravenous injections that possibly dampened the effects of fluoxetine.
To our knowledge, this is the first documentation of the effects of fluoxetine in the rabbit NMR preparation. In this study, the dose range selected was capped at 3.0 mg/kg because of a report that a higher dose of 6 mg/kg can increase intraocular pressure (Costagliola et al., 2000). The range utilized in rodent studies is typically higher, with many studies administering doses of 10 mg/kg (Burghardt et al., 2007; Schulz et al., 2007a; Liu et al., 2010; Ravinder et al., 2011; Robert et al., 2011). It is, therefore, possible that the behavioral effects of fluoxetine treatment would have been more pronounced with a higher dose; however, those effects may be confounded by nonassociative effects on intraocular pressure. Although the current study examined the actions of fluoxetine on retention of CRs and CRM following delay conditioning, there has been at least one study on fluoxetine effects on acquisition of trace eyeblink conditioning in the rat (Leuner et al., 2004). This study demonstrated that a chronically administered dose of 5 mg/kg – closer to that used in current study – did not have any effects on acquisition of trace eyeblink conditioning but did prevent a stress-induced learning deficit in female rats. This study suggests that the dose utilized here should not affect NMR conditioning per se, but possibly is a large enough dose to be sensitive to modulation by stress.
Despite a lack of earlier studies specifically investigating fluoxetine or other SSRIs in the rabbit NMR preparation, there is extensive work by Harvey and colleagues on the role of the serotonergic system on the acquisition, maintenance, and retention of trace NMR conditioning in the rabbit (Welsh et al., 1998; Harvey et al., 1999, 2004; Romano et al., 2000, 2006; Harvey, 2003). A large portion of their work has focused on the serotonin receptor 2A subtype (5-HT2A) and has demonstrated that agonists of the 5-HT2A receptor can enhance the rate of conditioning, whereas certain inverse agonists acting as antagonists can impair it (Harvey, 2003). They also have shown that manipulation of the 5-HT2 receptor can affect the NMR reflex itself, with agonists and antagonists increasing and decreasing the UR magnitude, respectively (Harvey et al., 1988; Harvey et al., 1999). These findings therefore show that serotonin plays an important role in the modulation of both CRs and URs during NMR conditioning, and our work extends this also to CRM and retention of CRs.
Research from animal models of anxiety may provide additional clues for the possible mechanism by which fluoxetine may lead to enhanced CRs and CRM. These studies have focused on determining the neuronal substrates behind the acute anxiety-like behavioral effects of fluoxetine and other SSRIs (Burghardt et al., 2007; Homberg et al., 2011; Ravinder et al., 2011). Work by Burghardt and colleagues have shown that acute SSRI treatment with fluoxetine and citalopram leads to anxiety-like responses through facilitation of 5-HT2C receptor-mediated neurotransmission. Other work has shown that the anxiety-like effects of systemic fluoxetine can be blocked by injection of a 5-HT2C receptor agonist directly into the basolateral nucleus of the amygdala (Vicente and Zangrossi, 2011). In addition, fluoxetine treatment can lead to increased neuronal excitability in the basolateral nucleus (Ravinder et al., 2011) and to an increased number of amygdalar neurons immunopositive for a marker of synaptic remodeling (Homberg et al., 2011). The amygdala, therefore, is a strong candidate site for these serotonergic actions as it is a well-known locus for the acquisition, expression, and extinction of fear conditioning (Maren, 2001; Myers and Davis, 2002; Pare et al., 2004; Bouton et al., 2006), and amygdala dysfunction is also strongly implicated in fear-based disorders such as PTSD (Gilboa et al., 2004; Protopopescu et al., 2005; Hughes and Shin, 2011). Taken together, these findings, along with work by our laboratory showing that the central nucleus is critical for CRM expression (Burhans and Schreurs, 2008), give support to the amygdala as a possible substrate for the fluoxetine-induced enhancement of both CRs and CRM. Earlier work characterizing CRM has consistently shown that it is associative in nature, as it does not occur in rabbits receiving unpaired CS/US presentations (Schreurs et al., 1995) and is affected by factors that influence the level of NMR conditioning (Burhans et al., 2008). Without an unpaired control group comparison in the current study, the question is raised whether the enhanced CRM observed with the highest dose of fluoxetine might represent nonassociative sensitization. Against this idea is the finding that the enhanced UR responsiveness was not uniform and instead was specific to the US intensity (0.25 mA), with the greatest amount of CRM before drug treatment. In addition, the parallel increase in CRM and CRs, two behaviors that are associatively linked, suggest fluoxetine may enhance retention of the CS–US association, thus enhancing CRM.
Conclusion
An enhancement of CRM and CRs following subacute fluoxetine treatment is in agreement with the effects of acute and chronic SSRIs reported in animal models of anxiety in other species with different behavioral assays. Findings such as these, however, do raise the question of whether animal models of anxiety are valid if they produce paradoxical responses to anxiolytic drugs (Borsini et al., 2002). However, it is important to note that anxiogenic responses to acute SSRI treatment are reported in humans, with juveniles being a particularly vulnerable population, and there are also questions about the efficacy of SSRI treatment in anxiety disorders like PTSD (Bajor et al., 2011). Also, it is possible that anxiolytic-like effects would be found in the CRM model if long-term treatment and adult rabbits were utilized or if pharmacological and behavioral treatments were combined (Karpova et al., 2011). The fact that the CRM model is sensitive to the SSRI fluoxetine demonstrates that it has potential as a screening tool for pharmacological treatments and may provide insight into the mechanisms behind paradoxical SSRI effects.
Acknowledgements
This work was supported by the National Institute of Mental Health (NIMH) (Grant MH081159) and the Blanchette Rockefeller Neurosciences Institute. The contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Anatai-Otong D. The art of prescribing. Pharmacological management of posttraumatic stress disorder. Perspect Psychiatr Care. 2007;43:55–59. doi: 10.1111/j.1744-6163.2007.00096.x. [DOI] [PubMed] [Google Scholar]
- Bajor LA, Ticlea AN, Osser DN. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an update on posttraumatic stress disorder. Harv Rev Psychiatry. 2011;19:240–258. doi: 10.3109/10673229.2011.614483. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Buck DL, Seager MA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: generality and nature of the phenomenon. Behav Neurosci. 2001;115:1039–1047. [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans LB, Schreurs BG. Inactivation of the central nucleus of the amygdala abolishes conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response and delays classical conditioning. Behav Neurosci. 2008;122:75–88. doi: 10.1037/0735-7044.122.1.75. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell C, Schreurs BG. Conditioning-specific reflex modification of the rabbit’s nictitating membrane response and heart rate: behavioral rules, neural substrates, and potential applications to posttraumatic stress disorder. Behav Neurosci. 2008;122:1191–1206. doi: 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell C, Schreurs BG. Effects of extinction on classical conditioning and conditioning-specific reflex modification of rabbit heart rate. Behav Brain Res. 2010;206:127–134. doi: 10.1016/j.bbr.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Brown TH. Amygdala stimulation enhances the rat eyeblink reflex through a short-latency mechanism. Behav Neurosci. 1996;110:51–59. doi: 10.1037//0735-7044.110.1.51. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Costagliola C, Mastropasqua L, Capone D, Verolino M, Ciancaglini M, Pisanti N. Effect of fluoxetine on intraocular pressure in the rabbit. Exp Eye Res. 2000;70:551–555. doi: 10.1006/exer.1999.0773. [DOI] [PubMed] [Google Scholar]
- Davidson JRT. Pharmacological treatment of acute and chronic stress following trauma: 2006. J Clin Psychiatry. 2006;67(Suppl 2):34–39. [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behav Neurosci. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Exp Brain Res. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem. 2003;10:355–362. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA, Gormezano I, Cool-Hauser VA, Schindler CW. Effects of LSD on classical conditioning as a function of CS-UCS interval: relationship to reflex facilitation. Pharmacol Biochem Behav. 1988;30:433–441. doi: 10.1016/0091-3057(88)90477-7. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Welsh SE, Hood H, Romano AG. Effect of 5-HT2 receptor antagonists on a cranial nerve reflex in the rabbit: evidence for inverse agonism. Psychopharmacology (Berl) 1999;141:162–168. doi: 10.1007/s002130050820. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Quinn JL, Liu R, Aloyo VJ, Romano AG. Selective remodeling of rabbit frontal cortex: relationship between 5-HT2A receptor density and associative learning. Psychopharmacology (Berl) 2004;172:435–442. doi: 10.1007/s00213-003-1687-4. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Blom T, Arentsen T, van BC, Schipper P, et al. Fluoxetine exerts age-dependent effects on behavior and amygdale neuroplasticity in the rat. PLoS One. 2011;6:e16646. doi: 10.1371/journal.pone.0016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CH, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) Int J Neuropsychopharmacol. 2011;29:1–16. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Mostert JP, Koch MW, Heerings M, Heersema DJ, De KJ. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci Ther. 2008;14:153–164. doi: 10.1111/j.1527-3458.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–2207. doi: 10.1038/npp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Ravinder S, Pillai AG, Chattarji S. Cellular correlates of enhanced anxiety caused by acute treatment with the selective serotonin reuptake inhibitor fluoxetine in rats. Front Behav Neurosci. 2011;5:1–10. doi: 10.3389/fnbeh.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;71:839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- Robert G, Drapier D, tue-Ferrer D, Renault A, Reymann JM. Acute and chronic anxiogenic-like response to fluoxetine in rats in the elevated plus-maze: modulation by stressful handling. Behav Brain Res. 2011;220:344–348. doi: 10.1016/j.bbr.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Romano AG, Hood H, Harvey JA. Dissociable effects of the 5-HT(2) antagonist mianserin on associative learning and performance in the rabbit. Pharmacol Biochem Behav. 2000;67:103–110. doi: 10.1016/s0091-3057(00)00298-7. [DOI] [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Liu R, Dave KD, Schwab D, Alexander G, et al. Effect of serotonin depletion on 5-HT2A-mediated learning in the rabbit: evidence for constitutive activity of the 5-HT2A receptor in vivo. Psychopharmacology (Berl) 2006;184:173–181. doi: 10.1007/s00213-005-0245-7. [DOI] [PubMed] [Google Scholar]
- Salchner P, Singewald N. Neuroanatomical substrates involved in the anxiogenic-like effect of acute fluoxetine treatment. Neuropharmacology. 2002;43:1238–1248. doi: 10.1016/s0028-3908(02)00329-5. [DOI] [PubMed] [Google Scholar]
- Schreurs BG. Classical conditioning and modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Behav Cogn Neurosci Rev. 2003;2:83–96. doi: 10.1177/1534582303255014. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA. Heart rate changes during conditioning-specific reflex modification of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response. Neurobiol Learn Mem. 2005;84:148–158. doi: 10.1016/j.nlm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Oh MM, Hirashima C, Alkon DL. Conditioning-specific modification of the rabbit’s unconditioned nictitating membrane response. Behav Neurosci. 1995;109:24–33. doi: 10.1037//0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Shi T, Pineda S, III, Buck DL. Conditioning the unconditioned response: modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. J Exp Psychol Anim Behav Process. 2000;26:144–156. doi: 10.1037//0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behav Neurosci. 2005a;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behav Neurosci. 2005b;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gonzalez-Joekes J, Smith-Bell CA. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response is sensitive to context. Learn Behav. 2006;34:315–324. doi: 10.3758/bf03192886. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Incubation of conditioning-specific reflex modification: implications for post traumatic stress disorder. J Psychiatr Res. 2011a;45:1535–1541. doi: 10.1016/j.jpsychires.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Unpaired extinction: implications for treating post-traumatic stress disorder. J Psychiatr Res. 2011b;45:638–649. doi: 10.1016/j.jpsychires.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D, Buddenberg T, Huston JP. Extinction-induced ‘despair’ in the water maze, exploratory behavior and fear: effects of chronic antidepressant treatment. Neurobiol Learn Mem. 2007a;87:624–634. doi: 10.1016/j.nlm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Schulz D, Huston JP, Buddenberg T, Topic B. ‘Despair’ induced by extinction trials in the water maze: relationship with measures of anxiety in aged and adult rats. Neurobiol Learn Mem. 2007b;87:309–323. doi: 10.1016/j.nlm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Seager MA, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learn Behav. 2003;31:292–298. doi: 10.3758/bf03195990. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K. Stress facilitates acquisition of the classical conditioned eyeblink response at both long and short interstimulus intervals. Learn Motiv. 2001;32:178–192. [Google Scholar]
- Vicente MA, Zangrossi H. Serotonin-2C receptors in the basolateral nucleus of the amygdala mediate the anxiogenic effect of acute imipramine and fluoxetine administration. Int J Neuropsychopharmacol. 2011 doi: 10.1017/S1461145711000873. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Weisz DJ, Harden DG, Xiang Z. Effects of amygdala lesions on reflex facilitation and conditioned response acquisition during nictitating membrane response conditioning in rabbit. Behav Neurosci. 1992;106:262–273. doi: 10.1037//0735-7044.106.2.262. [DOI] [PubMed] [Google Scholar]
- Welsh SE, Romano AG, Harvey JA. Effects of serotonin 5-HT(2A/2C) antagonists on associative learning in the rabbit. Psychopharmacology (Berl) 1998;137:157–163. doi: 10.1007/s002130050605. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS. Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behav Neurosci. 1991;105:141–153. doi: 10.1037//0735-7044.105.1.141. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Ruusuvirta T, Korhonen T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus) Behav Neurosci. 2002;116:1052–1058. doi: 10.1037//0735-7044.116.6.1052. [DOI] [PubMed] [Google Scholar]