Abstract
Control of Leishmania infantum infection is dependent upon Th1 CD4+ T cells to promote macrophage intracellular clearance of parasites. Deficient CD4+ T cell effector responses during clinical visceral leishmaniasis (VL) are associated with elevated production of IL-10. In the primary domestic reservoir of VL, dogs, we define occurrence of both CD4+ and CD8+ T cell exhaustion as a significant stepwise loss of antigen-specific proliferation and IFNγ production, corresponding to increasing VL symptomatology. Exhaustion was associated with a fourfold increase in the population of T cells with surface expression of Programmed Death 1 (PD-1) between control and symptomatic populations. Importantly, exhausted populations of CD8+ T cells and to a lesser extent CD4+ T cells were present prior to onset of clinical VL. VL exhausted T cells did not undergo significant apoptosis ex vivo after antigen stimulation. Antibody block of PD-1 ligand, B7.H1, promoted return of CD4+ and CD8+ T cell function and dramatically increased reactive oxygen species production in co-cultured monocyte-derived phagocytes. As a result, these phagocytes had decreased parasite load. We demonstrate for the first time that pan-T cell, PD-1-mediated, exhaustion during VL influenced macrophage reactive oxygen intermediate production. Blockade of the PD-1 pathway improved the ability of phagocytes isolated from dogs presenting with clinical VL to clear intracellular parasites. T cell exhaustion during symptomatic canine leishmaniasis has implications for the response to vaccination and therapeutic strategies for control of Leishmania infantum in this important reservoir species.
INTRODUCTION
Zoonotic visceral leishmaniasis (VL) is a fatal systemic illness, resulting in 500,000 annual new human cases and approximately 50,000 deaths per year. Leishmania infantum, a vector-borne, protozoan parasite, is the causative agent of VL in the New World. Natural hosts of L. infantum include dogs and humans [1]. Asymptomatic status is dependent upon a T helper 1 protective response during chronic VL. In patients that progress to disease, the immune response was skewed toward TGF-β, IL-10 or IL-4-producing Th2 and/or T regulatory cells [2, 3]. Parasite-derived protein GP63 is capable of attenuating this immune response through the action of the tyrosine phosphatase SHP-1 [4]. We previously demonstrated that as dogs progress to clinical VL, there was impaired CD4+ T cell proliferation and IFNγ production ex-vivo in response to L. infantum antigen. This was observed to occur in tandem with increased production of IL-10, similar to that observed in VL patients [5].
T cell exhaustion has been defined as antigen-specific effector T cell dysfunction with sustained expression of inhibitory receptors including PD-1 and decreased effector cytokine production [6]. PD-1 exerts this effect on T cells in part through activation of tyrosine phosphatases SHP-1 and SHP-2 via its Immunoreceptor Tyrosine Switch Motif (ITSM) [7]. Exhaustion of CD8+ T cells has been identified in chronic viral infections and parasitic disease including toxoplasmosis and cutaneous leishmaniasis [8–10]. A recent study using murine infection with arginase-deficient L. major demonstrated that impaired priming of T cells can result in PD-1 overexpression, impairment of acquired immunity, and exhaustion [11]. Here we report for the first time concurrent CD4+ and CD8+ T cell exhaustion in symptomatic canine leishmaniasis. Exhaustion was mediated by PD-1 surface expression on CD4+ and CD8+ T cells, associated with progressive disease.
T cell exhaustion progresses incrementally, and during chronic viral infection can result in clonal deletion of CD8+ T cells [12]. Populations of exhausted T cells were maintained by TCR stimulation with cognate antigen, even in the absence of IL-7 and IL-15, with low levels of proliferation [13]. Additional studies during experimental L. major infection indicate that antigen-experienced T cells preferentially respond to presented antigen, to the exclusion of naïve T cell populations [14]. A study using OVA transgenic mice with continual OVA antigen exposure demonstrated that a population of tolerant T cells survived contraction and were quiescent but maintained tolerance without clonal deletion [15]. In this study we evaluated cellular deletion of T cells from L. infantum infected animals after ex vivo antigen stimulation. We demonstrate maintenance of exhausted T cell populations which neither proliferated nor underwent apoptosis. These T cell populations were responsive to blockage of PD-1/B7.H1, increasing proliferative capacity, indicating that this effector memory population was not clonally deleted.
Phagocyte-based reactive oxygen (ROI) and nitrogen intermediates (RNI) are critical for removal of intracellular Leishmania [16, 17]. Production of ROI and RNI required T cell IFN-γ production [18]. In this study, block of B7.H1 resulted in recovery of Th1-effector function, recovery of phagocyte superoxide production and decreased parasite load in peripheral blood-derived monocytes from naturally-infected dogs. The research within this study is a novel characterization of pan-T cell exhaustion in a major domestic reservoir for visceral leishmaniasis. T cell exhaustion during asymptomatic and symptomatic canine leishmaniasis will impact the efficacy of vaccination and therapeutic strategies to reduce the incidence of leishmaniasis in this important reservoir species.
MATERIALS AND METHODS
Sample Population and Study Enrollment
Dogs were enrolled by serology, clinical signs, and quantitative Real Time-PCR (qRT-PCR) positivity as previously described [5]. Dogs were 2 to 7 years old, and had complete veterinary standard of care. Twenty milliliters of blood was collected from each dog at intervals greater than 2 weeks. Procedures were performed with approval from Iowa State University IACUC.
Clinical Staging of Study Animals
Study animals were assessed via physical and laboratory examination and L. infantum diagnostics (L. infantum kinetoplast DNA (kDNA)-specific qPCR, IFAT, and K39/22 (DPP, Chembio, Medford, NY) serologic analysis). Infected animals were classified as: 1) asymptomatic- no clinical signs, qPCR positive, and IFAT <1:256, 2) symptomatic- two or more signs of leishmaniasis (lymphadenomegaly, palpable liver or spleen, alterations in hepatic or renal enzymes), IFAT ≥ 1:256, and qPCR positive. All symptomatic animals had blood harvested for a complete blood count and serum chemistry performed by the Clinical Pathologists at Iowa State University. A single symptomatic animal and no asymptomatic animals evaluated had a leukopenia. This animal had a white blood cell count slightly below the low normal range (5.77×10^3/ml, normal range = 6–17×10^3/ml). Other clinical pathologic findings such as anemia, azotemia, hypocalcemia, hypoalbuminemia, proteinuria, and elevated creatinine were common in the symptomatic group.
Sample Handling and PBMC Isolation
Whole blood samples were separated into PBMC as previously described [5]. Whole blood samples collected in heparin-containing tubes were diluted 1:1 with 1x Hank’s Buffered Salt Solution (Cellgro, Manassas, VA) and 25mM HEPES. Diluted whole blood was centrifuged at 800 rcf (Eppendorf, Hauppauge, NY) for 30 minutes at room temperature through Ficoll/Histopaque 1077 (Sigma Aldrich, St. Louis, MO) as previously described [5]. PBMC were counted with an automated cell counter (Beckman Coulter, Brea, CA). PBMC were washed twice in phosphate-buffered saline (PBS) and suspended in complete medium (CTCM) (RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine, and 25 mM HEPES buffer). PMBC were counted and adjusted to 4 × 106/ml for further analysis. Cells were then split for evaluation of proliferation and intracellular staining for flow cytometry, or for qRT-PCR, nitro-blue tetrazolium assay (NBT) (Sigma Aldrich, St. Louis, MO), or ELIspot Assays.
Preparation of parasites
L. infantum (LIVT-2) grown for use as positive control for kqRT-PCR or freeze-thaw antigen (f-t L.i.) as previously described [5]. Leishmania infantum (LIVT-2) was grown in complete Grace's medium (incomplete Grace's supplemented with 20% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine). Parasites were harvested, pelleted at 2500 × g for 15 min at 4°C, washed twice with phosphate-buffered salt solution (PBS), and suspended in PBS to a concentration of 107 parasites/mL. Ten 1:5 serial dilutions were made and 50 µL of each dilution was spiked into 150 µL of negative canine blood.
Antigen stimulation
Cells were stimulated with Concanavalin A (ConA; 5 µg/ml) for 4 days and f-t L.i. (10 µg/ml) for 7 days as previously described [5]. Asymptomatic dog PBMC were stimulated with distemper virus vaccine (Pfizer, Kalamazoo, MI), a Non-Leishmania, Specific antigen, positive Control (NSC) for 10 days. 24 hours prior to cell harvest, 5-ethynyl-2´-deoxyuridine (EdU) (Invitrogen, Grand Island, NY) was added at 10 µM, and 10 µg/ml brefeldin A (Sigma, St. Louis, MO) was added 6 hours before harvest. Cells were harvested and washed prior to surface and intracellular labeling.
IL-10 and B7.H1 block
Non-adherent PBMC were removed and saved in separate culture. Adherent cell populations were CD3− CD11b+ by flow cytometry (data not shown). Adherent cells were treated with 10 µg/ml anti-human B7.H1 antibody (Clone MIH-1, eBioscience, San Diego, CA) or isotype for 4 hours, washed, and non-adherent cells were returned to culture for antigen-stimulation. This blocked adherent cell B7.H1 specifically. IL-10 block was performed via 10 µg/ml anti-canine IL-10 antibody (R and D Systems, Minneapolis, MN).
PBMC immunolabeling
PBMC were labeled similar to [5], with the following PBMC labeling panel: Canine PBMC labeling panel: EdU Click-It (Invitrogen, Grand Island, NY), anti-canine CD4/Alexafluor 647 (AbD Serotec, Raleigh, NC), anti-canine CD8/Alexafluor 700 (AbD Serotec, Raleigh, NC), anti-canine IFNγ/Zenon R-PE (R&D systems, Minneapolis, MN)(Invitrogen, Grand Island, NY), anti-canine IL-10/Zenon APC/Alexafluor 750 (R&D systems, Minneapolis, MN)(Invitrogen, Grand Island, NY), and anti-human biotinylated PD-1/PE-Cy7 streptavidin (R&D systems, Minneapolis, MN) (eBioscience, San Diego, CA). Cells blocked with 25 µl of canine serum, with mouse and rat non-specific polyclonal IgG at 20 µg/106 PBMC for 20 minutes at 4°C. Cells were blocked for 20 minutes, 4°C, permeabilized with saponin reagent (Invitrogen, Grand Island, NY) and preserved in BD stabilizing fixative (BD biosciences, San Jose, CA). Samples were analyzed within 48 hours. Data acquired via FACScanto (BD biosciences, San Jose, CA) with FlowJo analysis (TreeStar Inc., Ashland, OR). Cells were progressively sorted by a live lymphocyte gate, then by CD4 or CD8 positivity for subsequent analysis (Supplemental Figure I). All gating was based on pooled fluorescence minus one control samples and applied identically across all samples.
ELISpot
PBMC were stimulated as previous and incubated on anti-canine IFNγ or IL-10 capture antibody coated plates (R&D systems, Minneapolis, MN). 100 µl of 0.5 µg/ml biotinylated detection antibody (R&D systems, Minneapolis, MN) was added. Spots counted as average of duplicate wells over 4 dilutions, standardized to spots per 106 PBMC.
IL-10 ELISA
Supernatants from unlabeled PMBC (2 × 105) were collected at indicated time points and stored at −20°C until analysis. IL-10 production was measured via ELISA (R&D Systems, Minneapolis, MN) per manufacturer's recommendations.
Nitric oxide production
Concentration of nitrite production assessed via Griess reaction. 50 µl cell culture supernatant and Griess reagent (LabChem, Pittsburgh, PA) mixed and incubated at RT, absorbance was measured at 550nm via microplate reader (Molecular Devices, Sunnyvale, CA). Nitrite concentration determined via sodium nitrite standard curve.
Superoxide production
Production of superoxide was assessed using Nitro Blue Tetrazolium (NBT) (Sigma Aldrich, St. Louis, MO) tablets. NBT tablets were dissolved in 1ml water, 30 µl added to cells, and incubated for 60–90 minutes. Coverslips were harvested, fixed, and stained with eosin. Cells were counted as percent cells containing formazan precipitate. All evaluations were based on average of 600 cells per experiment, with blinding.
Polymerase chain reaction assay
DNA was isolated and RT-PCR performed as previously described (12). DNA was isolated using the Qiagen blood DNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. DNA quality and quantity were measured using a NanoDrop ND1000 spectrophotometer (Nanodrop, Wilmington, DE). Leishmania SSU rRNA specific flurorogenic probe (5′-6-carboxyfluorescein [6-FAM]-CGGTTCGGTGTGTGGCGCC-3′) and its flanking primers (F, 5′-AAGTGCTTTCCCATCGCAACT-3’; R, 5′-CGCACTAAACCCCTCCAA-3’ (Applied Biosystems, Foster City, CA) previously designed (Wortman et al. 2001) were used. Blood DNA samples were assayed via qPCR in duplicates of two dilutions (straight, 1:10) using a Stratagene Mx3005P qPCR system (Agilent, Santa Clara, CA) via a 96-well format and perfeCta qPCR SuperMix, Low ROX master mix (Quanta Biosciences, Gaithersburg, MD). Primers were used at 775 nM and probe at 150 nM, with thermocyling at 95°C for 3 min and 50 cycles of 95°C for 15 s, 60°C for 1 min. Results were analyzed via MxPro QPCR software version 4.01, Microsoft Excel, and GraphPad Prism version 5.04 (GraphPad Software, La Jolla, CA).
Statistical analysis
Statistical analysis was conducted with pair-wise student’s t-tests or one way ANOVA with a Tukey’s post-test via Graph Pad Prism version 5.04 (GraphPad Software, La Jolla, CA), significance at α=0.05. qRT-PCR data was log-transformed for one-way ANOVA analysis. Graphical data presented as arithmetic mean ± SEM.
RESULTS
CD4+ T cells from animals with visceral leishmaniasis were functionally exhausted
Alteration in CD4+ effector function during VL is well established, with demonstrated involvement of IL-10, IL-4, TGFβ, and cells able to produce both IL-10 and IFNγ [2, 3]. We previously demonstrated these alterations in L. infantum-infected dogs as they progress to symptomatic VL [5]. These findings were suggestive of an exhausted phenotype. Since Leishmania protein GP63 was shown to suppress T cell responses via activation of SHP-1 phosphatase [4], and the exhaustion-associated inhibitory receptor PD-1 functions through increased tyrosine phosphatase activity [7], we hypothesized that T cell exhaustion occurred in naturally occurring VL due to overexpression of PD-1. We obtained PBMC from animals with natural, clinical, VL to evaluate whether their CD4+ T cells were functionally exhausted.
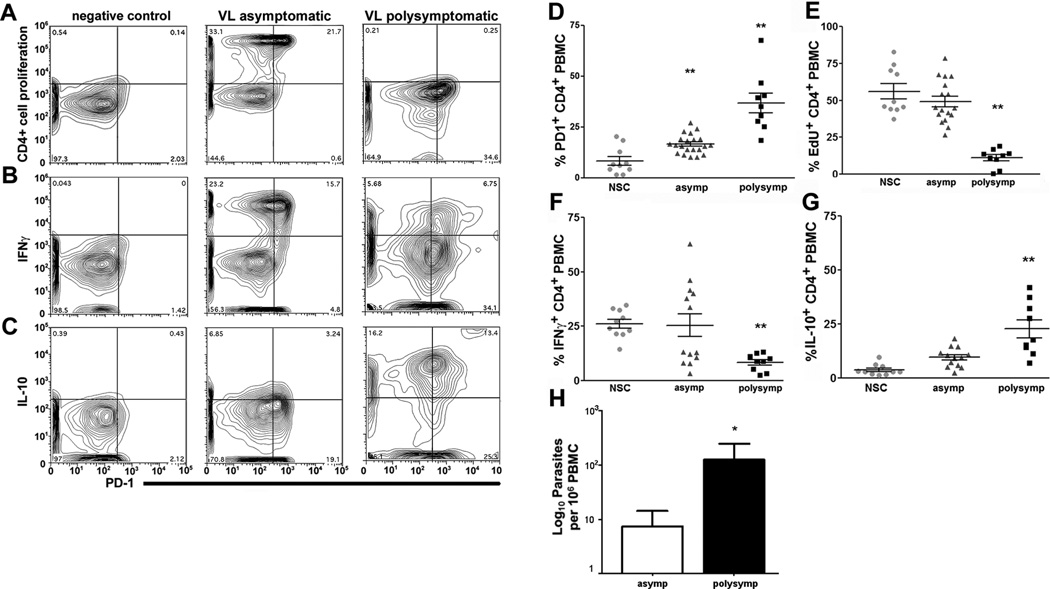
Population surface expression of PD-1 on CD4+ T cells was significantly increased in asymptomatic (two-fold) and symptomatic (four-fold) L. infantum-infected animals (Figure I), as compared to expression on cells stimulated with a positive control antigen (NSC) ex vivo (Figure I D). The percent of CD4+ T cells capable of proliferation in response to f-t L.i. antigen was significantly reduced in animals with symptomatic VL, with five-fold reduction compared to NSC and asymptomatic animals (Figure I A, E). Intracellular staining revealed a 67% reduction in the percentage of CD4+ cells responding to f-t L.i. stimulation with IFNγ production compared to either NSC or asymptomatic animals (Figure I B, F). Consistent with previous studies of leishmaniasis [2] and of T cell exhaustion [19, 20], the percentage of IL-10+ CD4+ T cells in f-t L.i.-stimulated PMBC from symptomatic animals increased almost six-fold and over two-fold compared to NSC and asymptomatic animals respectively (Figure I C, G). CD4+ T cells from symptomatic animals had significantly higher IL-10compared to asymptomatic animals both with and without stimulation, and significant elevation after antigen stimulation compared to unstimulated PBMC (p<0.001, data not shown). Within individual gated populations were multiple dual positive populations including PD-1+ IFNγ Proliferating and IFNγ, and even IFNγ IL-10+ populations. These multiple dual positive populations demonstrate the phenotypic variation possible across a polyclonal, antigen-responsive population of T cells. Alterations in IFNγ and IL-10 production were similar as measured via ELIspot (Supplemental Figure IIA, B). Functional exhaustion and symptomatic VL was also associated with a significant increase in L. infantum burden within PBMC (Figure I, H). The lack of antigen-specific CD4+ effector responses from these cells indicates the presence of CD4+ T cell exhaustion during VL, associated with increased peripheral parasite load.
Figure I. Leishmania infantum infection promotes progressive CD4+ T cell exhaustion.
PBMC from L. infantum- infected dogs were stimulated with f-t L. infantum, or a non-mitogen antigen-specific positive control (NSC) (A–C) - CD4+ T cell plots of negative control (left), asymptomatic (middle), and symptomatic (right) dog PBMC for (A) CD4+ proliferation via EdU incorporation, (B) CD4+ IFNγ+, and (C) CD4+ IL-10+, vs. PD-1+ in response to f-t L.i. (D–G) - Population graphs of CD4+ T cell (D) PD-1 surface expression, (E) proliferation measured via EdU incorporation, (F) IFNγ expression, and (G) IL-10 from NSC-stimulated, asymptomatic and symptomatic animals. (H) Peripheral blood parasite levels in asymptomatic and symptomatic dogs measured by qPCR, expressed as log number of parasites. All experiments included n>21 dogs, 10 asymptomatic dogs for NSC. *p<0.05, **p<0.01 via one-way ANOVA with Tukey’s post-test (A–G), or un-paired Student’s-test (H).
CD8+ T cells from asymptomatic and symptomatic VL animals exhibit more severe functional exhaustion than CD4+ T cells
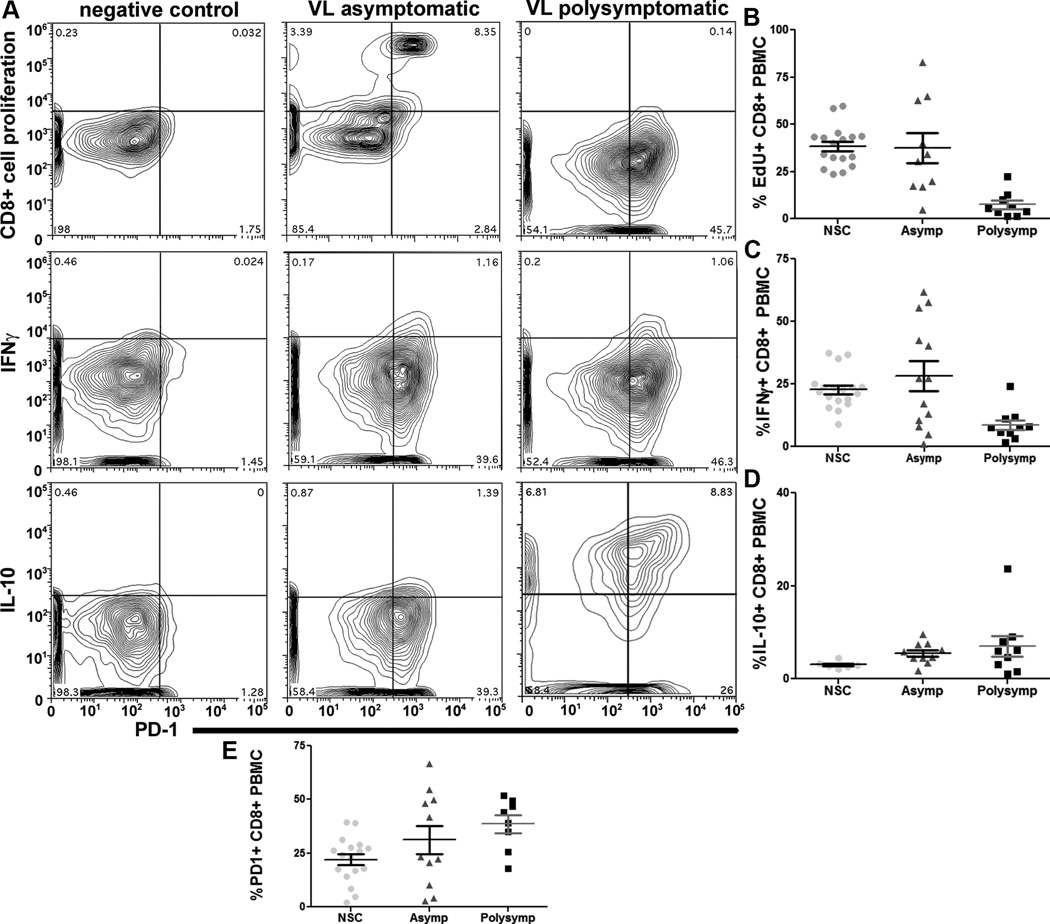
CD8+ T cell exhaustion has been characterized during numerous chronic viral infections, protozoal infections, and cutaneous leishmaniasis [8, 9, 12]. Pro-inflammatory cytokines, including IFNγ, are produced primarily by both CD4+ and CD8+ T cells during chronic VL [21]. CD8+ T cell exhaustion has not been previously reported during naturally-occurring VL, and knowledge of CD4+ vs. CD8+ T cell exhaustion during VL progression was not previously known. Surface expression of PD-1 was elevated in CD8+ T cells from animals with asymptomatic (1.5 fold increase) and symptomatic VL (two-fold increase) as compared to NSC (Figure II). VL symptomatic animal CD8+ T cells had dramatic five-fold reduction in proliferative capacity (Figure II A top row, B), and three-fold reduction in the capacity of CD8+ T cells to produce IFNγ (Figure II A middle row, C) in response to whole f-t L.i. compared either to NSC or asymptomatic animals. There was no significant change in intracellular IL-10 production (Figure II A bottom row, D). During asymptomatic infection, evidence of CD8+ exhaustion was more significant than in CD4+ T cells, with a higher number of dogs with significantly increased populations of PD1+ T cells during asymptomatic infection than of CD4+ T cells (Figure II E, I D), with a bimodal population and an increased CD8+ T cell populations with poor proliferation (Figure II B, I E). Mean population size of PD-1+ CD8+ vs. CD4+ T cells from symptomatic animals was similar (Figure I D, 2E). This data indicates the presence of pan-T cell exhaustion during symptomatic VL, and a large percentage (>40%) of asymptomatic animals with phenotypic CD8+ T cell exhaustion and elevated PD-1 (Figure II). T cell exhaustion during VL was associated with elevated expression of coinhibitory receptor PD-1 which could be identified prior to onset of symptomatic disease. Exhaustion was associated with clinical progression of VL and could be a predominant contributing factor to the onset of symptomatic disease.
Figure II. Leishmania infantum infection promotes progressive CD8+ T cell exhaustion.
PBMC from L. infantum-infected dogs were stimulated with f-t L. infantum, or a non-mitogen antigen-specific positive control (NSC) (A) – CD8+ T cell plots of negative control (left), asymptomatic (middle), and symptomatic (right) dog PBMC for (top) CD8+ proliferation via EdU incorporation, (middle) CD8+ IFNγ+, and (bottom) CD8+ IL-10+, vs. PD-1+ in response to f-t L.i. (B-E) - Population graphs of CD8+ T cell (B) proliferation measured via EdU incorporation, (C) IFNγ expression, (D) IL-10, and (E) PD-1 surface expression from NSC-stimulated, asymptomatic and symptomatic animals. All experiments included n>18 dogs, 6 (D) and 17 (B, C, E) asymptomatic dogs for NSC. *p<0.05, **p<0.01 via one-way ANOVA with Tukey’s post-test.
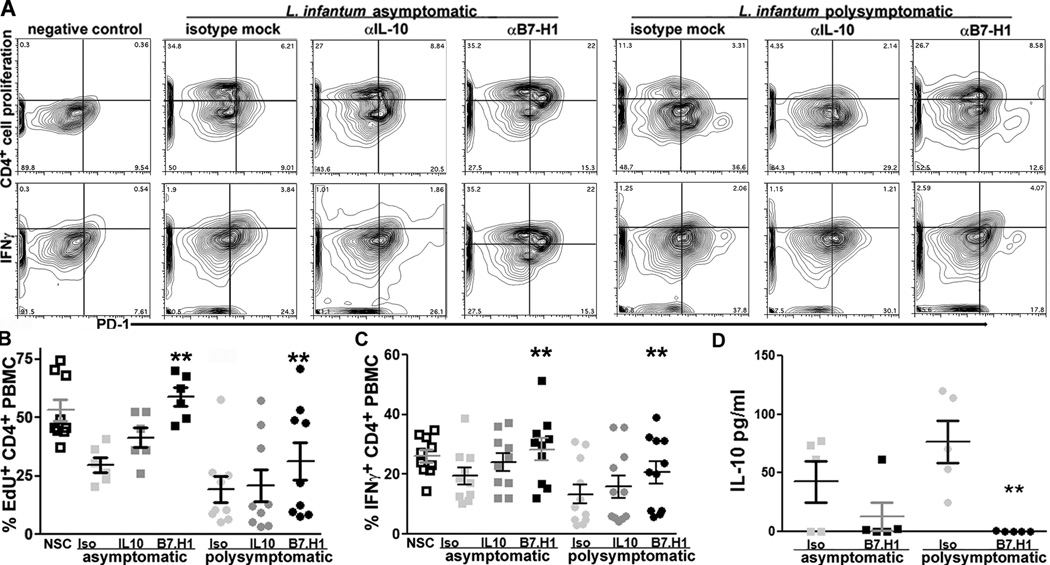
T cell exhaustion in CD4+ T cells from symptomatic VL animals reversed by B7.H1 block
An increased percentage of PD-1 expressing CD4+ T cells was progressively associated with CD4+ T exhaustion during symptomatic VL. Previous studies utilizing B7.H1 antibody block demonstrated recovery of CD8+ T cell effector function [19, 22]. Our previous studies demonstrated an increased percentage of IL-10-producing CD4+ T cells and increased IL-10 production during symptomatic VL. Others have postulated that IL-10 may induce CD4+ T cell suppression during chronic VL [2, 23]. Based on this, our hypothesis was that IL-10/IL-10R or PD-1/PDL-1 (B7.H1) signaling was necessary for CD4+ T cell exhaustion in PBMC from symptomatic VL animals. Block of B7.H1 resulted in significant recovery in CD4+ T cells able to proliferate to f-t L.i. antigen both in asymptomatic and symptomatic infection-derived PBMC (Figure III A, B). IL-10 block trended toward increased proliferation-capable CD4+ T cells in PBMC from both asymptomatic and symptomatic animals but was not statistically significant (Figure III). B7.H1 block resulted in significant recovery of a population of CD4+ IFNγ+ cells compared to isotype or IL-10 antibody-treated PBMC from symptomatic animals and compared to isotype-treated PBMC from asymptomatic animals (Figure III A, C). The number of IFNγ-producing PBMC was also significantly increased after B7.H1 blockage compared to isotype or to IL-10 treatment as measured via ELISpot (Supplemental Figure III A). Recovery of a population of IFNγ producing cells after anti-IL-10 antibody treatment trended towards significance but was not significantly altered compared to isotype treated-PBMC (Figure III A, C). IL-10 production as measured by ELISA was significantly reduced after B7.H1 block as compared to IL-10 production from PBMC treated with isotype antibody (Figure III D). The population of IL-10+ PBMC quantified via ELISpot increased after blockage of B7.H1 and stimulation with f-t. L.i., perhaps suggesting transformation of IL-10 expressing cells into IFN-γ co-expression, similar to previous findings [2] (Supplemental Figure III B).
Figure III. PD-1/B7.H1 interaction necessary for CD4+ T cell exhaustion during symptomatic VL.
Adherent PBMC treated with B7.H1 blocking antibody or IL-10 blocking antibody. PBMC stimulated as previous. (A) CD4+ cellular proliferation (top row) and CD4+ T cell IFNγ (bottom row), compared to PD-1 (x-axis) in PBMC from negative control (left), asymptomatic VL (middle), and symptomatic VL (right) dogs, with isotype control (left), anti-IL-10 antibody (center), or anti-B7.H1 antibody treatment (right). (B) CD4+ cellular proliferation, n=15. (C) CD4+ PBMC IFNγ intracellular production, n=21. (D) Production of IL-10 via ELISA, n=11. **p<0.01, via one way ANOVA with Tukey’s post-test.
Blockage of B7.H1 was consistently effective in increasing the antigen-responsive population of CD4+ T cells from both asymptomatic and symptomatic VL animals, significantly increasing the percentage of cells able to proliferate and produce IFN-γ after B7.H1 treatment. PD-1 is therefore necessary for CD4+ T cell exhaustion during VL, and blocking the PD-1/B7.H1 interaction recovered a population of functional Th1 CD4+ T cells.
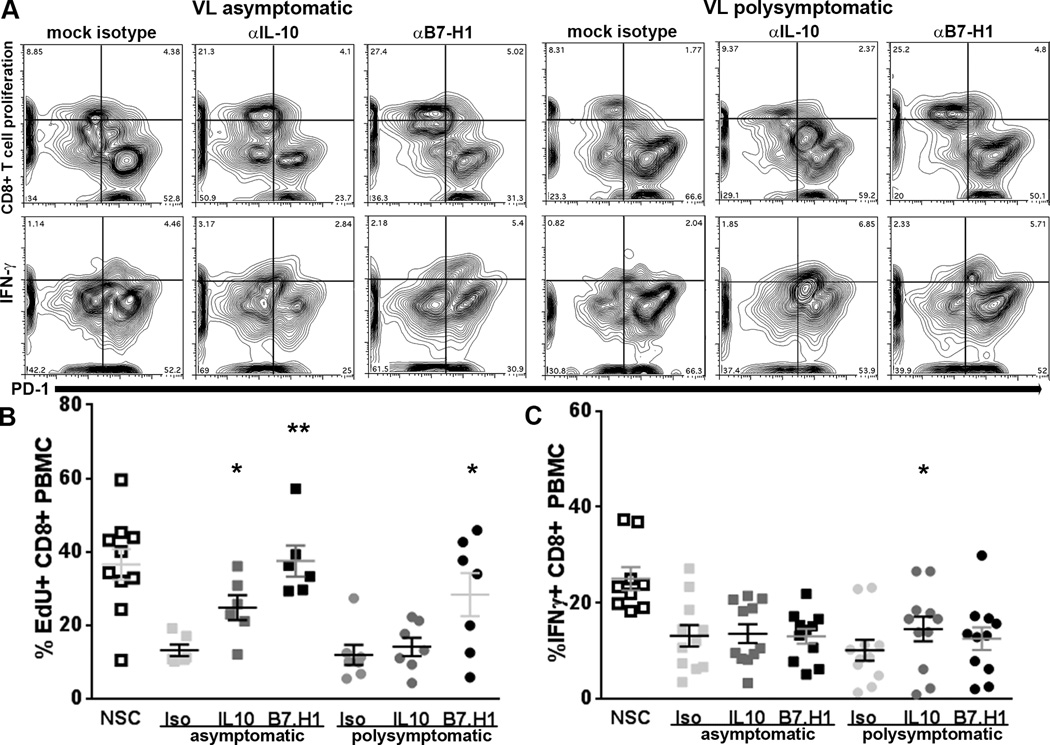
Blockage of B7.H1/PD-1 recovers proliferation in exhausted CD8+ T cells but not IFNγ production
Exhaustion of CD8+ T cells from peripheral blood in both asymptomatic and symptomatic animals was associated with a significant elevation of PD-1 surface expression (Figure II). Blockage of the B7.H1/PD-1 interaction but not IL-10 significantly recovered both proliferation and IFNγ production within the CD4+ T cell population in response to stimulation with f-t L.i. (Figure III). To evaluate the role of PD-1 and IL-10 in suppressing CD8+ T cell function, we blocked B7.H1 and IL-10 prior to stimulation with f-t L.i. and evaluated CD8+ T cell function (Figure IV). Consistent with B7.H1 blockage in the CD4+ T cell population, B7.H1/PD-1 blockage on adherent PBMC significantly increased CD8+ T cell proliferative capacity in response to f-t L.i. in both asymptomatic and symptomatic animals (Figure IV A, B). As opposed to the CD4+ T cell population, blockage of IL-10 also significantly recovered CD8+ T cell proliferation after f-t L.i. stimulation in asymptomatic animals (Figure IV A, B). However, within the CD8+ T cell population, blockage of B7.H1 did not significantly recover IFNγ production in either asymptomatic or symptomatic dogs (Figure IV B). In symptomatic dogs, blockage of IL-10 significantly recovered IFNγ production, although recovery was minimal compared to positive control (Figure IV B). This indicates in the CD8+ T cell population of asymptomatic and symptomatic dogs with VL, IFNγ production is largely non-responsive to blockage of B7.H1/PD-1.
Figure IV. PD-1/B7.H1 interaction necessary for suppression of proliferation but not IFNγ production during symptomatic VL– associated CD8+ T cell exhaustion.
Adherent PBMC treated with B7.H1 blocking antibody or IL-10 blocking antibody. PBMC stimulated as previous. (A) CD8+ cellular proliferation (top row) and CD8+ T cell IFNγ (bottom row), compared to PD-1 (x-axis) in PBMC from negative control (left), asymptomatic VL (middle), and symptomatic VL (right) dogs, with isotype control (left), anti-IL-10 antibody (center), or anti-B7.H1 antibody treatment (right). (B) CD8+ cellular proliferation, n=13. (C) CD8+ PBMC IFNγ intracellular production, n=22. *p<0.05, **p<0.01, via one way ANOVA with Tukey’s post-test.
Antigen stimulation does not induce further apoptosis in exhausted T cells
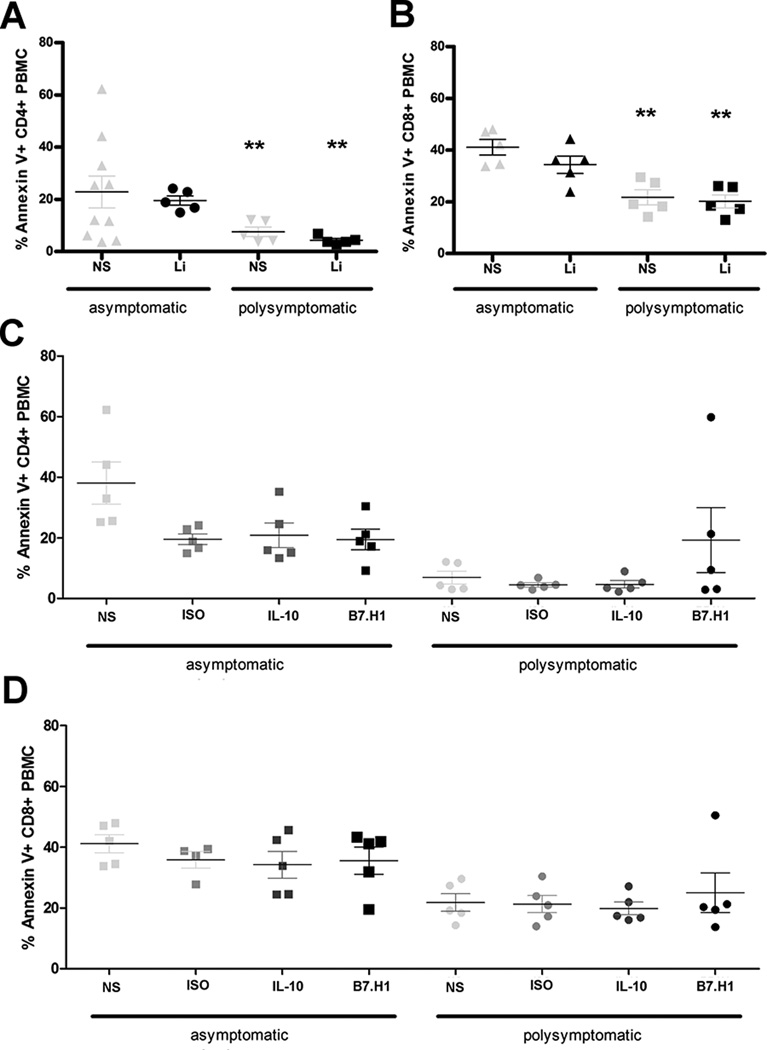
Previous research has demonstrated a step-wise manner in which CD4+ and CD8+ T cell populations progressively exhaust, ending with clonal deletion of exhausted cells [10, 12]. Conversely, Shin et al. demonstrated persistence of exhausted T cells in vivo, maintained through basal levels of proliferation and antigen stimulation [13]. To evaluate whether there was maintenance of exhausted T cells during symptomatic VL, we evaluated PBMC for Annexin V and propidium iodide positivity (Figure V). Annexin V positivity was higher within the CD8+ T cell population than within the CD4+ T cell population from both symptomatic and asymptomatic animals across all experimental treatments (Figure V, A–D). However, Annexin V positivity was higher in CD4+ and CD8+ T cells from asymptomatic animals than in T cells from symptomatic animals (Figure V A–D). Propidium iodide positivity indicative of cell death was minimal in all groups (data not shown). After IL-10 or B7.H1 block, CD4+ and CD8+ T cell apoptosis was still significantly lower within the more phenotypically exhausted symptomatic T cell populations as compared to cells from asymptomatic animals (Figure V C, D). There were no significant differences in Annexin V positivity between experimental treatments (Figure V C, D). Blocking of B7.H1 in CD4+ T cells from symptomatic VL animals resulted in a trend toward increased Annexin V positivity, corresponding with more pronounced proliferation recovery. Three VL symptomatic dogs had increased PBMC proliferation and increased Annexin V positivity, while two dogs, animals with lowest proliferative and IFNγ responses to B7.H1 block (Figure III B, C), had no alteration in PBMC Annexin V positivity.
Figure V. L. infantum exhausted T cells do not undergo apoptosis or cell death in response to antigen stimulation.
(A, B) Annexin V and PI (not shown) positivity was measured in asymptomatic or poly-symptomatic VL dogs after no stimulation and stimulation with 10 µg/ml f-t L.i. for 7 days. PBMC were gated on CD4+ (A) or CD8+ (B) live lymphocytes based on isotype control. (C, D) Annexin V positivity was measured similarly to 3A, after blocking with isotype, IL-10, or B7.H1 blocking antibodies in CD4+ (C) and CD8+ (D). **p<0.01 via one way ANOVA with Tukey’s post-test in GraphPad Prism 5. Experiments included n=10.
B7.H1 blockage improves the production of superoxide and parasite clearance in phagocytic monocytes
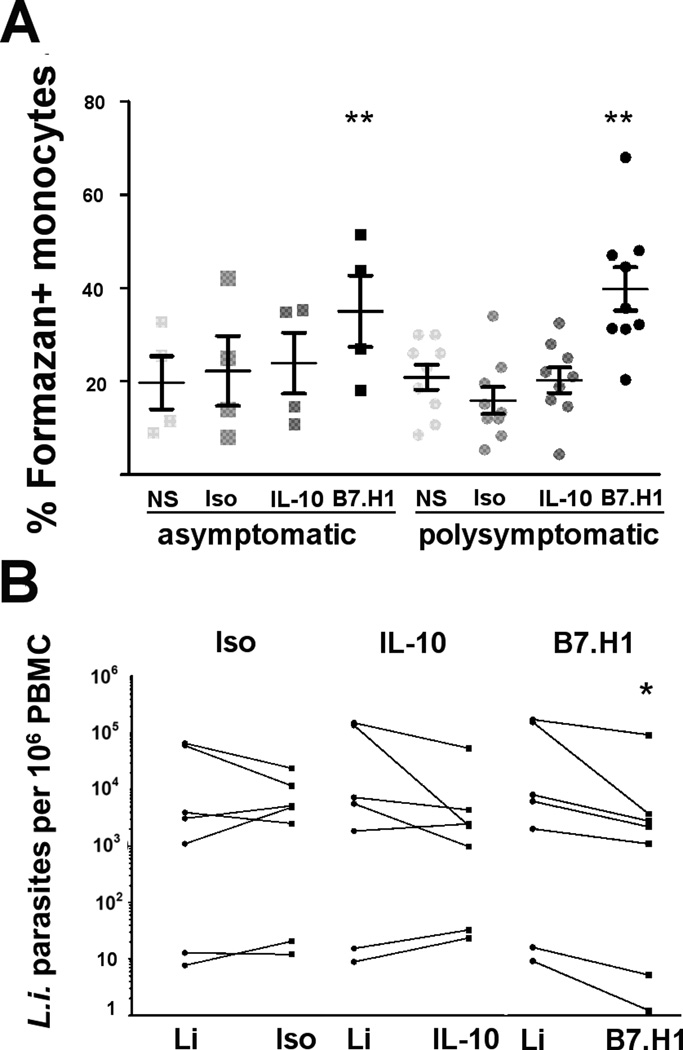
Previous reports demonstrated the importance of ROI and RNI in reducing intracellular Leishmania burden [1, 17]. Phagocyte production of ROI and RNI required IFNγ production by T cells [18]. We demonstrated that blocking PD-1/B7.H1 signaling recovered a population of CD4+ T cells with IFN-γ production. We sought to determine how functional Th1 recovery altered phagocyte function, as phagocytes are both the target of L. infantum infection and necessary for L. infantum removal. After PD-1/B7.H1 block, L.i. antigen-stimulated monocyte-derived phagocytes from asymptomatic and symptomatic dogs produced significantly more superoxide compared to all other treatments (Figure VI A, Supplemental Figure III D). Nitric oxide production, as measured by Griess reaction performed on cell supernatants from identical PBMC cultures, had no significant differences between any experimental groups (Supplemental Figure III C). Dogs, like humans, have limited iNOS activity. There is continuing debate as to the importance of RNI in the killing of intracellular pathogens in species other than mice, including humans and dogs [18, 24].
Figure VI. B7.H1 block increased phagocyte superoxide production and decreased parasite load.
(A) NBT assay performed on adherent PBMC from asymptomatic and symptomatic VL dogs after isotype, B7.H1, or IL-10 antibody treatment and stimulation with f-t L.i.. (B) L. infantum-specific quantitative RT-PCR performed on PBMC DNA. Data presented as number of genomic copies of L. infantum/106 PBMC from symptomatic dogs, after f-t L.i. stimulation (Li) compared to stimulation and addition of isotype, (left, Iso), anti-IL-10 (middle, IL-10), or anti-B7.H1antibody (right, B7.H1). (A) n=13 and (B) n=7. *p<0.05, **p<0.01, via one way ANOVA with Tukey’s post-test on averaged (A) or log-transformed (B) data.
Blocking PD-1/B7.H1 increased superoxide production in adherent monocytes from symptomatic dogs chronically infected with L. infantum (Figure VI A). Regaining an ability in these cells to produce parasite-lethal reactive oxygen species provides a link between restoration of CD4+ T helper function and improved monocyte-derived phagocyte function. We next wanted to identify whether this increase in ROI-producing phagocytes led to reduced intra-PBMC parasites ex vivo from VL asymptomatic and symptomatic dogs. We quantified PBMC parasite load after PD-1/B7.H1 treatment via RT-PCR. After B7.H1 block, parasite-stimulated PBMC from symptomatic animals had significantly fewer L. infantum parasites than f-t L.i.-stimulated PBMC or isotype-antibody treated PBMC using paired PBMC from the same dogs (Figure VI B). Average reduction in L. infantum after B7.H1 blockage was 0.58 log or 67.36%. While not significant, IL-10 block reduced parasite load in the four dogs with highest parasitemia (p=0.16) (Figure VI B, IL-10). Reduction of L. infantum in PBMC after blockage of B7.H1/PD-1 strongly suggests that PD1-PD1L signaling within the phagocyte has a significant suppressive role on the ability of phagocytes to induce an oxidative burst, permissive to L. infantum survival.
DISCUSSION
Effective vaccination and immunotherapeutic strategies for VL have remained elusive [25]. Host and parasite factors regulating the balance of an asymptomatic state versus symptomatic clinical disease are incompletely understood for VL. A better understanding is integral for addressing VL immunity [1]. Dogs with leishmaniasis have a similar pathogenesis to human VL. In addition, canine leishmaniasis is a primary target for public health interventions in multiple endemic areas, including culling, vaccination and/or therapy [5, 26].
Using naturally-occurring canine VL, we identified decreased PBMC production of IFNγ, decreased CD4+ T cell proliferation to antigen stimulation, and increased IL-10 production during clinical disease [5]; an exhausted phenotype. CD8+ T cell exhaustion has been identified in numerous infections, including disseminated cutaneous leishmaniasis [9, 10, 12, 27]. CD8+ exhaustion is characterized by absence of antigen-specific responses including proliferation, CD8+ cytotoxicity, and IFNγ production [28]. Knowledge of CD4+ T cell exhaustion is less extensive [10]. Here we demonstrate for the first time concurrent CD4+ and CD8+ T cell exhaustion during naturally-occurring canine VL, mediated by PD-1/B7.H1. We demonstrate CD8+ T cell exhaustion, partially mediated by PD-1, which occurs prior to the onset of symptomatic disease, and prior to the onset of functional CD4+ T cell exhaustion. It is logical, from an evolutionary standpoint that CD8+ T cells are more sensitive than CD4+ to tolerogenic stimuli, including prolonged antigen stimulation during chronic infection. This would protect infected cells from being the target of cytotoxic responses, and significantly dampen pro-inflammatory stimuli that induce host pathology [29].
Blockade of B7.H1/PD-1 significantly recovered CD4+ and CD8+ T cell proliferation and CD4+ IFNγ production in response to L. infantum antigen. In addition, this blockade also significantly reduced the presence of IL-10 in cell culture supernatants. This is consistent with the findings and hypothesis suggested by Mou et al. that PD-1 exerts its effects both directly through ITSM-associated SHP phosphatases and indirectly through regulation of IL-10 production from monocytes during Leishmania infection [11]. Based on clinical data in this study, it is likely that concurrent CD4+ and CD8+ T cell exhaustion preceding the onset of clinical disease contributes to precipitation of symptomatic VL.
CD4+ T cells from symptomatic animals had a significant reduction in Annexin V positivity, with minimal apoptosis. CD8+ populations also had reduced Annexin V positivity compared to control, although the CD8+ T cell population overall had a greater degree of apoptosis in culture than CD4+ T cells. Reductions in apoptosis could be due to previous clonal deletion of responsive antigen specific T cells. Within both CD4+ and CD8+ T cell populations, blockage of B7.H1 significantly increased proliferative capacity and in the CD4+ population recovered IFNγ. This makes clonal deletion of all responsive cells unlikely, as an antigen-specific memory T cell population could be recovered. A more likely explanation is the antigen-addiction theory put forth by Wherry and others [10, 13]. This would suggest that both CD8+ and CD4+ exhausted T cell populations contain antigen-experienced cells which do not respond to antigen by undergoing proliferation, but also do not undergo apoptosis after antigen stimulation. Possible mechanisms for this would include antigen-driven survival, perhaps through cytokines such as IL-2 and T cell receptor engagement, or less likely through replicative senescence due to epigenetic modification and telomere shortening during aging and prolonged cellular replication [30–32].
We identified increased APC superoxide production and reduced intracellular parasites ex vivo after PD-1/B7.H1 block, indicating a role for this signaling pathway in hindering phagocyte function as VL progresses. The importance of ROI and RNI production is well documented for effective immunity against Leishmania species [1]. This is the first report linking T cell exhaustion and B7.H1 signaling directly to macrophage responses. Due to dramatic alterations in phagocyte, CD4+ T cell, and CD8+ T cell effector function after ex vivo PD-1/B7.H1 block, this treatment may be a means of inducing increased pro-inflammatory phagocyte function, and therefore a strong target for VL immunotherapy.
A recent report demonstrates the importance of CD4+ T cell exhaustion in the development of CD8+ T cell exhaustion [33]. In the presence of chronic infection it is likely that these two processes are extensively intertwined. Previous studies aimed at VL immunotherapy have targeted IFNγ, blocking IL-10, or using TLR agonists [23, 34]. Limited success of these immunotherapeutic approaches suggests there are other, perhaps more significant, mechanisms of immune dysregulation during VL. This study demonstrates concurrent CD4+ and CD8+ T cell exhaustion, often present prior to the onset of symptomatic VL was mediated by significantly elevated surface expression of coinhibitory molecule PD-1. This may have significant ramifications for prevention and treatment interventions for dogs as a reservoir of Leishmania infantum, as T cell exhaustion may reduce the efficacy of vaccination or therapeutic strategies.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Mary E. Wilson and Selma M.B. Jeronimo, for their critical review regarding this manuscript. We would like to thank our collaborating Foxhound hunts for assistance with their priceless dogs.
Funding: work supported by National Institutes of Health grant AI50803 and Pfizer Animal Health/Morris Animal Foundation Fellowship for Advanced Study.
References
- 1.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 2.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson ME, Recker TJ, Rodriguez NE, Young BM, Burnell KK, Streit JA, Kline JN. The TGF-beta response to Leishmania chagasi in the absence of IL-12. Eur J Immunol. 2002;32:3556–3565. doi: 10.1002/1521-4141(200212)32:12<3556::AID-IMMU3556>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Gomez MA, Contreras I, Halle M, Tremblay ML, McMaster RW, Olivier M. Leishmania GP63 alters host signaling through cleavage-activated protein tyrosine phosphatases. Sci Signal. 2009;2:ra58. doi: 10.1126/scisignal.2000213. [DOI] [PubMed] [Google Scholar]
- 5.Boggiatto PM, Ramer-Tait AE, Metz K, Kramer EE, Gibson-Corley K, Mullin K, Hostetter JM, Gallup JM, Jones DE, Petersen CA. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin Vaccine Immunol. 2010;17:267–273. doi: 10.1128/CVI.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death-1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 8.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Ruiz J, Salaiza-Suazo N, Carrada G, Escoto S, Ruiz-Remigio A, Rosenstein Y, Zentella A, Becker I. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 11.Mou Z, Muleme HM, Liu D, Jia P, Okwor IB, Kuriakose SM, Beverley SM, Uzonna JE. Parasite-derived arginase influences secondary anti-Leishmania immunity by regulating programmed cell death-1-mediated CD4+ T cell exhaustion. J Immunol. 2013;190:3380–3389. doi: 10.4049/jimmunol.1202537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray PMM, Reiner SL, Smith DF, Kaye PM, Scott P. Antigenexperienced T cells limit the priming of naive T cells during infection with Leishmania major . J Immunol. 2006;177:925–933. doi: 10.4049/jimmunol.177.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barron L, Knoechel B, Lohr J, Abbas AK. Cutting edge: contributions of apoptosis and anergy to systemic T cell tolerance. J Immunol. 2008;180:2762–2766. doi: 10.4049/jimmunol.180.5.2762. [DOI] [PubMed] [Google Scholar]
- 16.Gantt KRR, Goldman TL, McCormick ML, Miller MA, Jeronimo SM, Nascimento ET, Britigan BE, Wilson ME. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi . J Immunol. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- 17.Murray HWW, Nathan CF. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani . J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan C. Role of iNOS in human host defense. Science. 2006;312:1874–1875. doi: 10.1126/science.312.5782.1874b. author reply 1874–1875. [DOI] [PubMed] [Google Scholar]
- 19.Barber DLL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17:1462–1470. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 22.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautam S, Kumar R, Maurya R, Nylen S, Ansari N, Rai M, Sundar S, Sacks D. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis. 2011;204:1134–1137. doi: 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panaro MA, Brandonisio O, de Caprariis D, Cavallo P, Cianciulli A, Mitolo V, Otranto D. Canine leishmaniasis in Southern Italy: a role for nitric oxide released from activated macrophages in asymptomatic infection? Parasit Vectors. 2008;1:10. doi: 10.1186/1756-3305-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 26.Esch KJ, Pontes NN, Arruda P, O'Connor A, Morais L, Jeronimo SM, Petersen CA. Preventing zoonotic canine leishmaniasis in northeastern Brazil: pet attachment and adoption of community leishmania prevention. Am J Trop Med Hyg. 2012;87:822–831. doi: 10.4269/ajtmh.2012.12-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare-Pelletier A, Sharpe AH, Freeman GJ, Turley SJ. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182:2102–2112. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 31.van Baarle D, Nanlohy NM, Otto S, Plunkett FJ, Fletcher JM, Akbar AN. Progressive telomere shortening of Epstein-Barr virus-specific memory T cells during HIV infection: contributor to exhaustion? J Infect Dis. 2008;198:1353–1357. doi: 10.1086/592170. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 33.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman VS, Bhatia A, Picone A, Whittle J, Bailor HR, O'Donnell J, Pattabhi S, Guderian JA, Mohamath R, Duthie MS, Reed SG. Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J Immunol. 2010;185:1701–1710. doi: 10.4049/jimmunol.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.