Abstract
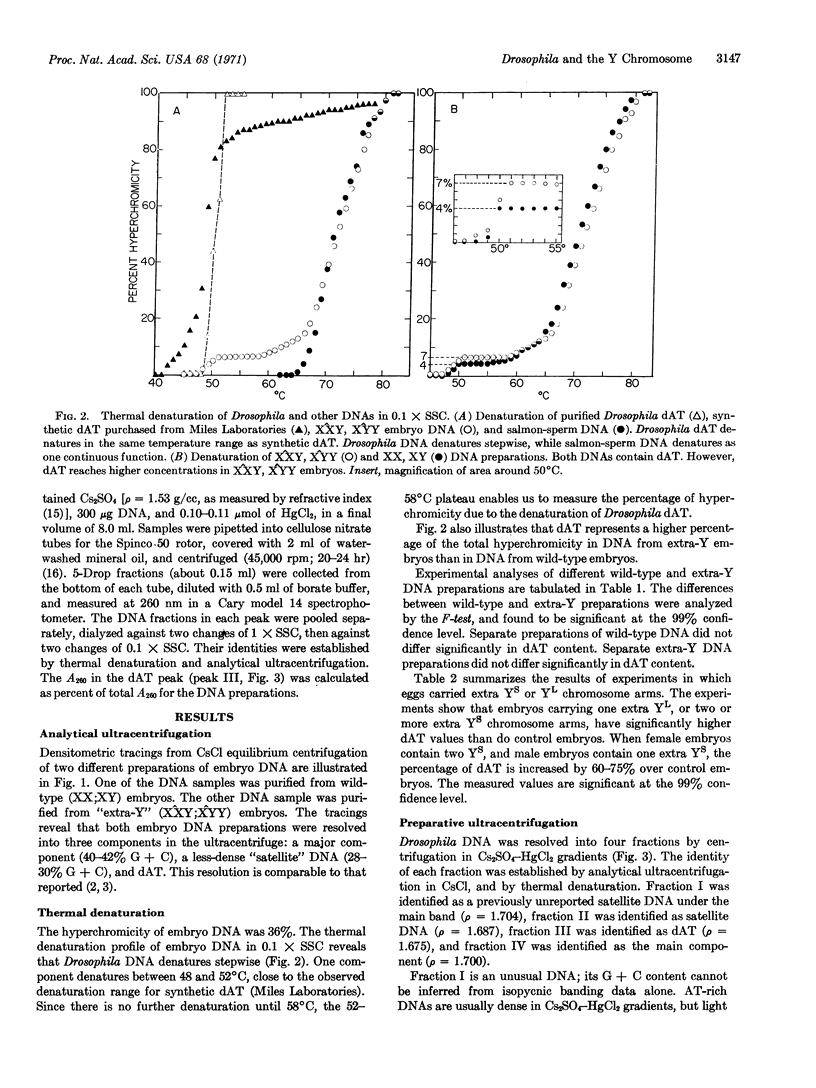
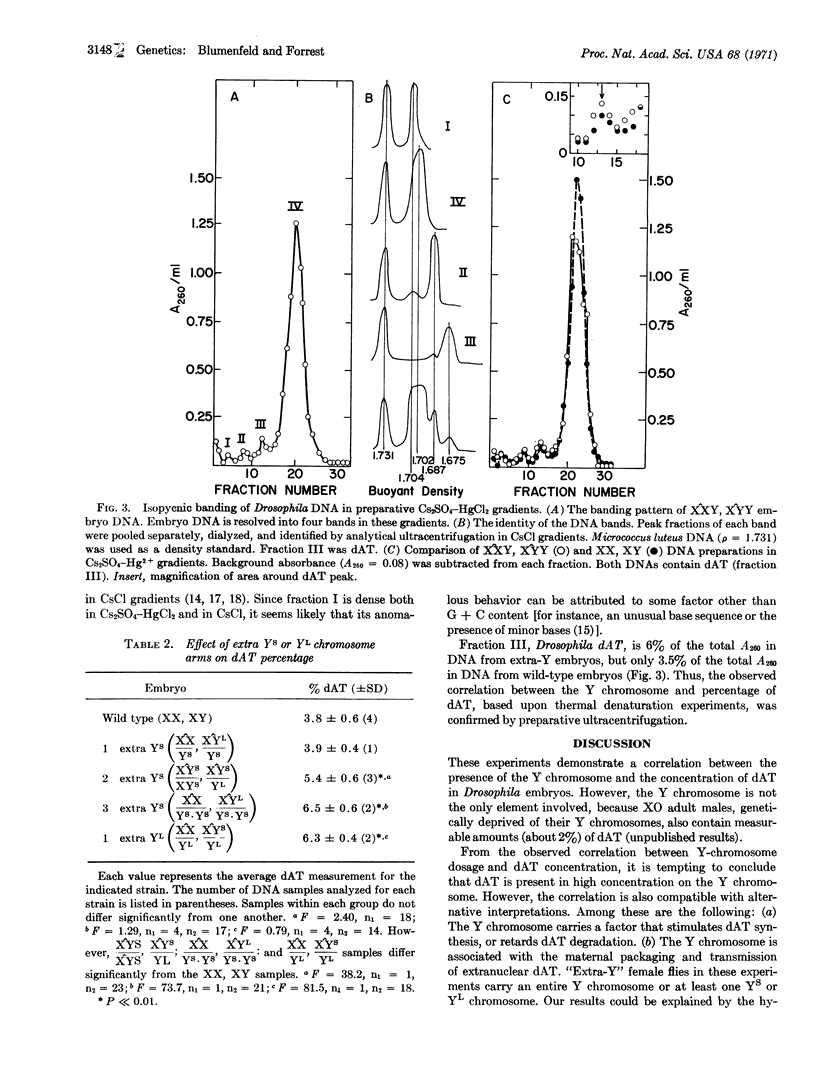
DNA isolated from Drosophila melanogaster embryos contains measurable amounts of dAT, the copolymer of deoxyadenylate and deoxythymidylate. In wild-type (XX,XY) embryos, dAT constitutes 4% of the total DNA. In embryos containing extra Y chromosomes, or even extra portions of Y chromosomes, dAT constitutes as much as 7% of the total. The enrichment for dAT is dependent upon the number of extra short arms of the Y chromosome (YS) or long arms (YL) of the Y chromosome per individual. This enrichment of dAT dependent on the Y chromosome may be interpreted in several ways. The most straightforward interpretation is that dAT is present in high concentrations on the Y chromosome.
Keywords: mutants, ultracentrifugation, embryos
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCOLI F., BOTRE C., LIQUORI A. M. Irreversible changes of ionic activities following thermal denaturation of sodium deoxyribonucleate. J Mol Biol. 1961 Apr;3:202–207. doi: 10.1016/s0022-2836(61)80046-6. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Suzuki D. T., Klett R. P., Smith M., Goldberg I. H. The intracellular location of the adenine- and thymine-rich component of deoxyribonucleate in testicular cells of the crab, Cancer productus. Exp Cell Res. 1969 Jan;54(1):3–10. doi: 10.1016/0014-4827(69)90284-5. [DOI] [PubMed] [Google Scholar]
- DAVIDSON N., WIDHOLM J., NANDI U. S., JENSEN R., OLIVERA B. M., WANG J. C. PREPARATION AND PROPERTIES OF NATIVE CRAB DAT. Proc Natl Acad Sci U S A. 1965 Jan;53:111–118. doi: 10.1073/pnas.53.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fansler B. S., Travaglini E. C., Loeb L. A., Schultz J. Structure of Drosophila melanogaster dAT replicated in an in vitro system. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1266–1272. doi: 10.1016/0006-291x(70)90003-3. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Laird C. D., McCarthy B. J. Magnitude of interspecific nucleotide sequence variability in Drosophila. Genetics. 1968 Oct;60(2):303–322. doi: 10.1093/genetics/60.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Waldvogel F. A., Swartz M. N. Subcellular location of crab poly(dA-dT). Biochim Biophys Acta. 1971 Sep 24;246(3):403–411. doi: 10.1016/0005-2787(71)90776-3. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Yasmineh W. G. Satellite DNA in constitutive heterochromatin of the guinea pig. Science. 1970 Apr 10;168(3928):263–265. doi: 10.1126/science.168.3928.263. [DOI] [PubMed] [Google Scholar]



