Abstract
Malignant gliomas are highly invasive and chemoresistant brain tumors with extremely poor prognosis. Targeting of the soluble factors that trigger invasion and resistance therefore could have a significant impact against the infiltrative glioma cells that are a major source of recurrence. Fibulin-3 is a matrix protein that is absent in normal brain but upregulated in gliomas and promotes tumor invasion by unknown mechanisms. Here, we show that fibulin-3 is a novel soluble activator of Notch signaling that antagonizes DLL3, an autocrine inhibitor or Notch, and promotes tumor cell survival and invasion in a Notch-dependent manner. Using a strategy for inducible knockdown, we found that controlled downregulation of fibulin-3 reduced Notch signaling and led to increased apoptosis, reduced self-renewal of glioblastoma initiating cells, and impaired growth and dispersion of intracranial tumors. In addition, fibulin-3 expression correlated with expression levels of Notch-dependent genes and was a marker of Notch activation in patient-derived glioma samples. These findings underscore a major role for the tumor extracellular matrix in regulating glioma invasion and resistance to apoptosis via activation of the key Notch pathway. More importantly, this work describes a non-canonical, soluble activator of Notch in a cancer model and demonstrates how Notch signaling can be reduced by targeting tumor-specific accessible molecules in the tumor microenvironment.
Keywords: Notch pathway, glioma invasion, chemoresistance extracellular matrix, fibulins, DLL3
Introduction
Malignant gliomas are the most common primary brain tumors and one of the types of cancer with worst prognosis (1, 2). Despite significant advances in neurosurgery and chemo-radiotherapy, gliomas remain highly resistant to conventional treatments and improvements in patient outcome have been modest (3). A major challenge for glioma therapy is the typical scattering of invasive tumor cells in the brain, escaping resection and leading to recurrence (4). Cumulative evidence suggests that these invasive cells are highly resistant to cytotoxic therapies (5, 6) and that their dispersion may be triggered, in part, by these treatments (7, 8). Improved targeting of the mechanisms that promote glioma invasion and facilitate chemoresistance is therefore critical to increase the long-term efficacy of current therapeutic approaches.
The discovery and characterization of glioma-initiating, stem-like cells (GICs) that share properties with neural stem cells (9) has brought attention to a number of signaling pathways in glioma that are also involved in neural development, such as Hedgehog, Wnt and Notch (10, 11). These pathways are necessary for the maintenance of GICs (12, 13) and critical for glioma initiation, self-renewal, and progression through stages of malignancy (11, 14), all of which makes them appealing pharmacological targets. While Hedgehog and Wnt are activated by soluble factors, Notch activation is usually a juxtacrine signaling mechanism that mediates close cell-to-cell signaling (15), which becomes less likely as tumor cells scatter. A few soluble, non-canonical activators of Notch signaling have been described in mammals (e.g.: MAGP-1/2, CCN3, and YB-1 (15)) but their relationship with Notch signaling in cancer progression has not been elucidated.
The fibulins are a family of secreted proteins that associate to the extracellular matrix (ECM) scaffold, forming anchoring structures that can regulate cell proliferation and migration (16, 17). Several fibulins have been associated with the development of solid tumors such as ovarian, breast, and colorectal cancers (16, 17), but their role in cancer initiation and progression has been difficult to define (16–18). Fibulin-3, also known as EFEMP1, is a protein of restricted expression in the body, predominantly localized in the ECM of elastic tissues (19). This protein is absent from normal brain (19, 20) and is downregulated in several types of solid tumors (21, 22). Surprisingly, fibulin-3 is highly upregulated in gliomas, where it promotes tumor growth and invasion (22). Recent results suggest that fibulin-3 is also upregulated in some highly metastatic tumors, where it correlates with the progression of these tumors towards the invasive phenotype (23, 24). The molecular mechanisms of this protein in cancer are still essentially unknown.
Here, we report that fibulin-3 is a novel soluble activator of Notch signaling, acting through an unconventional mechanism that reduces cis-inhibition of Notch. Our results show that fibulin-3 regulates the Notch pathway in glioma and promotes resistance to apoptosis as well as tumor growth and invasion in a Notch-dependent manner. This is the first demonstration of a secreted matrix molecule that regulates the Notch pathway in cancer to promote tumor progression.
Materials and Methods
Cells and tissue specimens
The human glioblastoma cell lines U87 and U251 and the rat glioblastoma cell line CNS1 were cultured as previously described (22) and authenticated using the “Cell Check” service provided by the Research Animal Diagnostic Laboratory (RADIL, Columbia MO). Primary glioblastoma-derived initiating cells (GICs G2, G8, G34 and G146) were kindly provided by Drs. E. Chiocca and I. Nakano of the Department of Neurological Surgery, OSU, and cultured as floating neurospheres in DMEM/F-12 supplemented with 2 μM glutamine, 20 ng/ml EGF, 20 ng/ml bFGF, 2 μg/ml grade-1A heparin and B27 supplement (Invitrogen). These cells were validated for self-renewal, ability to form tumors in low numbers, and multi-lineage differentiation. Tissue microarrays containing multiple replicate cores of formalin-fixed, paraffin-embedded glioma specimens were purchased from US. Biomax, yielding 65 independent samples.
DNA constructs, siRNAs and lentivirus
A clone containing the full-length coding sequence of fibulin-3 was previously described (22). From this clone, the variants fib-3Δn and fib-3Δc were generated by deleting the sequences Glu19-Ser106 and Cys379-Phe493, respectively. Full-length DLL3 cDNA (25) was subcloned into pCDNA4.1-V5/His for expression and epitope-tagging. The following constructs have already been described (26, 27) and were used without modifications: full-length rat Notch-1 (pBOS-rNotch1), full-length rat Jagged-1 (pBOS-SN3T), constitutively active mouse Notch-1 intracellular domain (pSG5-FLAG-NICD), and a Notch-reporter construct carrying firefly luciferase under control of 4xCBF1 binding elements (pGL2Pro-CBF1-Luc). Comparison of N-terminal sequences of fibulin-3 and Notch ligands was performed using ClustalW software (Supplemental Figure S1).
SiRNA oligonucleotides against fibulin-3 and Notch-1 were purchased from Qiagen and validated at the mRNA and protein levels (Supplemental Figure S2).
The lentiviral vector pLVET-tTR-KRAB-eGFP used for doxycycline-dependent shRNA expression has been described (28). shRNAs against fibulin-3 or a non-targeting control sequence (Genscript) were cloned into the shuttle vector pLVTHM and then subcloned into the lentiviral backbone. After stable transduction, tumor cells were treated with 20 μg/ml doxycycline for 48h to induce shRNA and eGFP expression. Inducible fibulin-3 knockdown was confirmed in three independent glioma cell lines (Supplemental Figure S3).
Immunohistochemistry
Human tissue sections (5 μm) were deparaffinized and processed for immunohistochemistry with antibodies against fibulin-3 and Hes1 (listed in Supplemental Table I). Scoring was performed blindly by a pathologist (GN), using a scale from 0 (absent staining) to 100 (whole visual field stained) (29). Frozen mouse brains were coronally sectioned at 20 μm and every fourth section stained with 0.1 % w/v cresyl violet (22). Imaging software (ImageJ v.1.45) was used to measure maximum coronal area and distance from the geometrical center of the tumor to the most distant border. Sections selected for immunohistochemistry were probed with an antibody against the proliferation marker Ki67 (22) or processed for TUNEL staining (Apoptag Red kit, Millipore).
Biochemical assays
Cells were recovered from culture, lysed and processed for Western blotting using standard protocols (22) (antibodies are listed in Supplemental Table I). To analyze protein phosphorylation, cells were incubated in serum-free media overnight before treatments. In vitro effects of fibulin-3 were analyzed by incubating glioma cells with purified fibulin-3 (100 ng/ml) for 2h. Short-time incubations (15 min) were also performed to compare activity of fibulin-3 against a canonical EGFR ligand (EGF, 5 ng/ml). For semi-quantitative RT-PCR, cells or tissue samples were processed using Trizol reagent (Invitrogen) and total RNA was purified by ethanol precipitation. For Notch-reporter assays, cells were transfected with the Notch-reporter construct and Renilla luciferase as loading control. Reporter cells were exposed to purified fibulin-3 for 8 hours or co-transfected with different constructs and processed after 24 h to quantify luciferase activity.
Migration and invasion assays
Cell migration was quantified with a conventional assay in culture inserts (Transwell™, 8 μm pore size), using bovine fibronectin (5 μg/ml) as chemoattractant. Cells (5,000 cells/well) were allowed to migrate for 16 h and subsequently fixed, stained and counted (30). Invasion of cells out of spheroids implanted in cultured brain slices was performed as described (30) and total dispersion quantified by fluorescence microscopy. The gamma-secretase inhibitor DAPT (25 μM, Tocris) was added to the cells 2h before seeding and maintained in the medium during these experiments. Transfection with cDNAs or siRNAs was performed 48 h before preparing cell spheroids to deposit on brain slices.
Cell viability and self-renewal
Cell viability was monitored using a standard redox assay (Promega CellTiter kit). Cells treated with serum depletion or temozolomide (Tocris) were labeled with propidium iodide/annexin-V following standard protocols and analyzed using a FACSCalibur flow cytometer (Becton-Dickinson). To measure apoptosis/necrosis in multiwell plates, cells were labeled as before and quantified by fluorescence microscopy. To evaluate GIC self-renewal, cells were dissociated, plated in serial dilutions as described (31), and new spheroids quantified after 12–14 days.
Animal studies
All studies involving animals were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Glioma cells were resuspended at 2.5×104 cells/μl in Hanks’ buffered saline solution supplemented with 0.1 % w/v glucose. The cell suspension (2 μl) was injected into the right striatum of 8 week-old nude (nu/nu) mice following standard protocols. Induction of fibulin-3 shRNA was achieved with 1 mg/ml doxycycline in the drinking water, starting 3 days after tumor implantation. Animals were euthanized and tumors harvested for histological analysis 20 days after implantation. For survival studies, animals were kept until they reached physiological criteria for early removal or euthanasia.
Statistics
All experiments in vitro were repeated at least in triplicate with three independent replicates (eight replicates for brain slice invasion assays). Animals studies were performed with N=5 (histology) or N=10 (survival) animals per experimental condition. Results were analyzed by one-factor or multifactorial ANOVA, followed by Bonferroni’s post-hoc test. Immunohistochemical scoring was analyzed using Spearman non-parametric rank correlation. Survival curves were compared by log-rank test.
Results
Fibulin-3 is a paracrine activator of Notch signaling
Fibulin-3 binds to large ECM proteins with lower affinity than other members of this family (19), therefore being a largely soluble factor (22). Other than its binding to ECM proteins, the molecular mechanisms triggered by fibulin-3 are almost completely unknown.
We first investigated if fibulin-3 could activate the EGFR pathway in glioma cells because this mechanism has been described in pancreatic carcinoma cells (32), although opposite results have been shown in nasopharyngeal carcinomas (33). We used a conventional glioma cell line (U87) with moderate expression of endogenous fibulin-3 (22) and well characterized EGFR signaling. Short-time exposure (15 min) of these cells to purified fibulin-3 failed to activate EGFR, MAPK or Akt (Figure 1A); similar results were observed with longer exposure (2 h) or after transfection of the cells with fibulin-3 cDNA. This was not caused by lack of EGFR activity because the pathway was triggered by the canonical ligand, EGF, incubated for the same amounts of time (Figure 1A). The same results were obtained with the glioma cell line U251 (not shown), indicating that this mechanism was not a primary target of fibulin-3 in glioma cells.
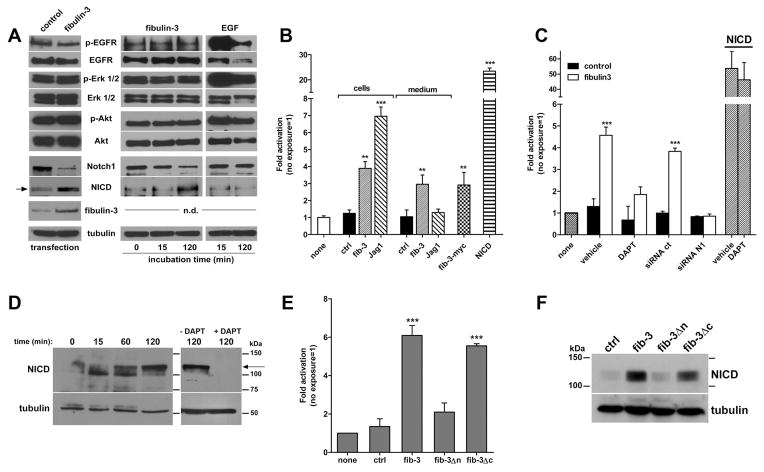
Figure 1. Fibulin-3 activates Notch signaling in glioma cells.
A) U87 cells transfected with fibulin-3 or exposed to purified fibulin-3 (fib-3, 100 ng/ml) did not show significant activation of EGFR signaling compared to controls, but increased the expression of Notch-1 intracellular domain fragment (NICD, arrow). Exposure of the cells to EGF (5 ng/ml) demonstrated that the EGFR pathway was functional. Fibulin-3 levels were determined by Western blotting in serum-free culture medium (n.d.: not determined). B) Notch-reporter HEK293 cells were co-cultured for 24 h with HEK293 cells expressing control (ctrl), fibulin-3, or Jagged-1 (Jag-1) cDNAs, or were exposed for 24 h to the conditioned medium from those cells. The reporter was activated by fibulin-3-secreting cells and their medium (paracrine activation). In contrast, the reporter was activated by Jagged-1-expressing cells but not by their medium (juxtacrine activation). Fib-3-myc: reporter cells were incubated with purified fibulin-3 (100 ng/ml) for 8 h. NICD: reporter cells were transfected with NICD as positive control. C) A Notch-reporter system in CNS1 cells was inhibited by preincubation with DAPT (25 μM) or transfection with Notch-1 siRNA (siRNA N1) before exposure to purified fibulin-3. siRNA ct: control siRNA. Transfection with NICD activated the reporter independently of DAPT. D) CNS1 cells exposed to purified fibulin-3 (100 ng/ml) showed time-dependent increase of NICD (arrow), which was blocked by DAPT. E–F) CNS1 cells carrying the Notch-reporter showed reporter activation (E) and increase of endogenous NICD (F) when transfected with full-length fibulin-3 or fib-3Δc, but not when transfected with fib-3Δn. Results in B, C, E: ** p<0.01, *** p<0.001; one-way ANOVA.
To identify other potential mechanisms triggered by fibulin-3 we analyzed its sequence and identified a considerable conservation of a Delta-Serrate-Lag motif, characteristic of the extracellular domain of Notch ligands (Supplemental Figure S1). Accordingly, fibulin-3 promoted Notch-1 cleavage and upregulation of the active Notch-1 intracellular domain (NICD) (Figure 1A), an effect that has never been described for the members of the fibulin family.
To validate this finding, we generated reporter HEK293 cells, which do not express fibulin-3, expressing full-length Notch-1 and a RBPJk-dependent Notch-responsive luciferase reporter (26). These cells were co-cultured in equal proportion with HEK293 cells expressing fibulin-3 or the canonical Notch ligand, Jagged-1. The luciferase reporter was activated in both cases, demonstrating that Notch signaling was activated by fibulin-3 from neighboring cells (Figure 1B). Moreover, when the reporter cells were cultured in the conditioned medium of the ligand-expressing HEK293 cells, only the medium from fibulin-3-secreting cells activated the reporter while the medium from Jagged-expressing cells failed to do so (Figure 1B), confirming the role of fibulin-3 as a paracrine, rather than juxtacrine, Notch activator. Exposure of reporter HEK293 cells to affinity-purified fibulin-3 yielded the same results (Figure 1B).
To further verify these findings, we used rat glioblastoma CNS1 cells, which reproduce the phenotype of highly invasive gliomas and share multiple properties with neural progenitors including high endogenous expression of Notch-1. Transfection of these cells with the Notch-responsive reporter confirmed that purified fibulin-3 was sufficient to activate the reporter (Figure 1C). Moreover, this activation was abolished by pre-incubation of the cells with the gamma-secretase inhibitor DAPT or by transfection with siRNAs against Notch-1, suggesting that this was the predominant Notch receptor mediating the effect of fibulin-3. Exposure of CNS1 cells to fibulin-3 confirmed activation of endogenous Notch-1 in a gamma-secretase dependent-manner (Figure 1D).
Finally, to determine which domain(s) of fibulin-3 were required for Notch activation we tested deletion constructs of fibulin-3 lacking either the N-terminal domain (fib-3Δn) containing the DSL-like motif, or the C-terminal domain (fib-3Δc) that contains a domain common to all fibulins. CNS1 cells expressing fib-3Δn failed to show activation of the Notch-dependent reporter (Figure 1E) or increase of endogenous NICD (Figure 1F), while the effects of fib-3Δc were essentially undistinguishable from those of full-length fibulin-3, suggesting that the DSL-like motif was critical for Notch pathway activation.
Fibulin-3 antagonizes the cis-inhibitor of Notch, DLL3
We next investigated if the effects of fibulin-3 were a result of direct binding to Notch-1 but could not detect co-precipitation of these two proteins. Similarly, we were unable to detect any effect of fibulin-3 on the activity of the proteases ADAM10/ADAM17 involved in Notch activation (not shown).
Therefore, we focused on a possible effect of fibulin-3 on the mechanism of cis-inhibition that regulates Notch activity. Notch ligands of the Delta family are known to increase Notch activity when expressed in a neighboring cell (trans-activation) but inhibit Notch when expressed in the same cell as Notch (cis-inhibition) (15). The Notch ligand DLL3 is unique in that it acts as a cis-inhibitor of Notch receptors expressed in the same cell but lacks trans-activating activity (25).
Using CNS1 glioma cells, we observed that transient upregulation or knockdown of fibulin-3 resulted in opposite changes in the expression of DLL1 and DLL3 (Figure 2A), suggesting that fibulin-3 mechanisms could involve effects on these proteins. In addition, purified fibulin-3 (not shown) as well as endogenous fibulin-3 consistently co-precipitated with DLL3 expressed in glioma cells (Figure 2B).
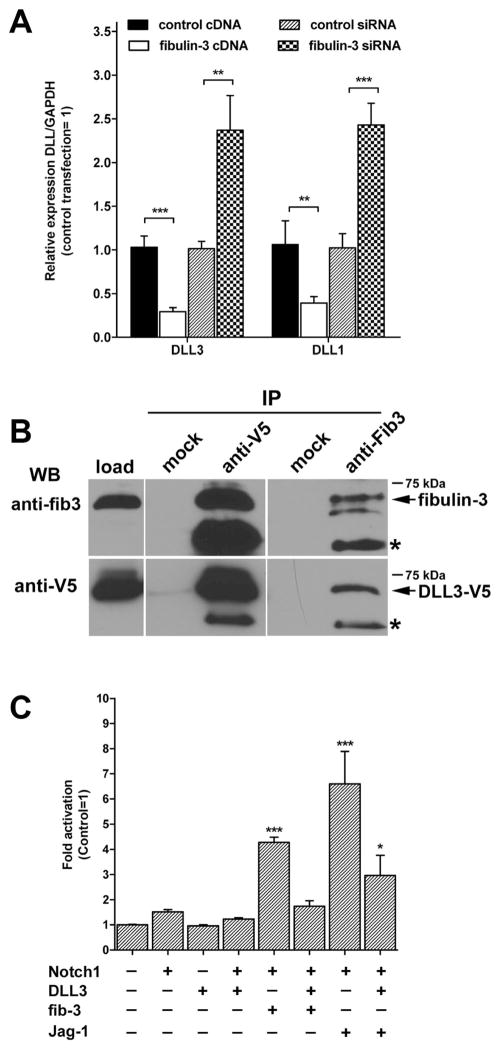
Figure 2. Fibulin-3 antagonizes the effect of DLL3.
A) CNS1 cells were transfected with fibulin-3 cDNA, fibulin-3 siRNA, or their respective controls, and processed for qRT-PCR after 24 h. Overexpression or knockdown of fibulin-3 resulted in the opposite expression of DLL1 and DLL3 (**p<0.01, ***p<0.001, one-way ANOVA). B) CNS1 cells expressing endogenous fibulin-3 and V5-tagged DLL3 (DLL3-V5) were lysed and subjected to co-immunoprecipitation (IP) with anti-fibulin-3 or anti-V5 antibodies. Western blotting (WB) revealed a direct association between fibulin-3 and DLL3 (arrows). Asterisks indicate antibody bands. C) CNS1 cells stably expressing the Notch-responsive reporter were transfected with combinations of plasmids carrying Notch-1, DLL3, fibulin-3 (fib-3), or Jagged-1 (Jag-1) and analyzed after 24 h. DLL3 did not affect the baseline reporter activity, but significantly blocked the enhancing effects of fibulin-3 and Jagged-1 (differences from control at *p<0.05, ***p<0.001, one-way ANOVA).
To confirm if fibulin-3 was competing with DLL3 we expressed both proteins in CNS1 cells carrying the Notch-responsive reporter (Figure 2C). Overexpression of DLL3 did not reduce baseline Notch activity, but significantly inhibited Notch activation mediated by fibulin-3 or the canonical ligand Jagged-1. Together, these results suggested that the Notch-activating effect of fibulin-3 was likely mediated by downregulating DLL3 or blocking the inhibitory effect of DLL3 over Notch.
Fibulin-3 regulates Notch activity and correlates with Notch activation in glioma
We next investigated if the expression of fibulin-3 in gliomas effectively correlated with Notch activity. Overexpression of fibulin-3 in glioma cells resulted in significant upregulation of Notch-dependent genes such as Hes1 (Figure 3A) and Hes5 (Figure 3B), while siRNA-mediated knockdown of fibulin-3 downregulated both genes. More importantly, the effects of fibulin-3 downregulation were reproduced using GICs, suggesting that fibulin-3 levels can modulate Notch activity in this key population of tumor cells.
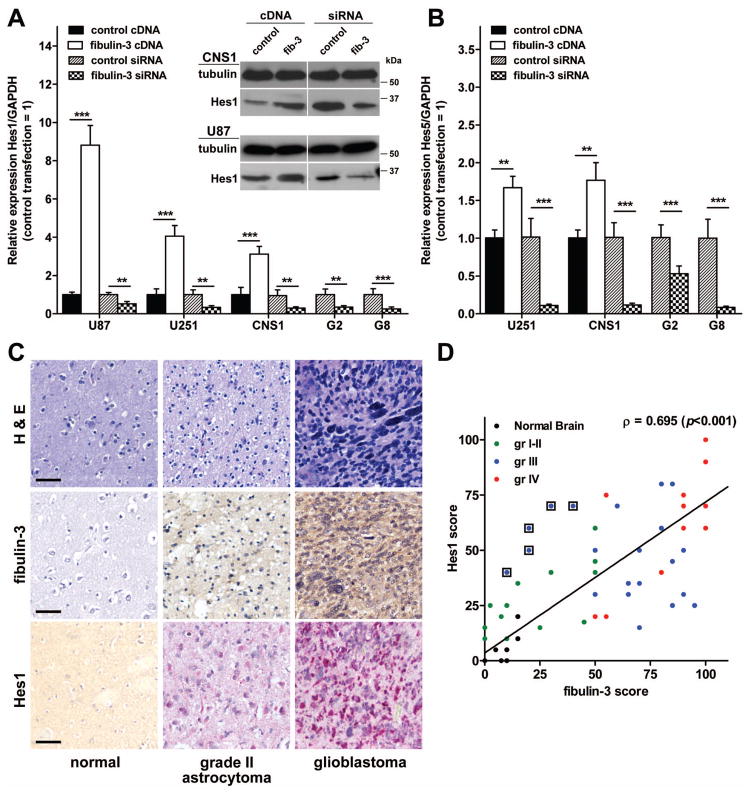
Figure 3. Fibulin-3 is a marker of Notch activation in human gliomas.
A–B) Glioma cells (U87, U251, CNS1) and GICs (G2, G8) were transfected with fibulin-3 cDNA or siRNA and processed for qRT-PCR and Western blotting. Overexpression or knockdown of fibulin-3 resulted in respective up- or down-regulation of the Notch-dependent genes Hes1 (A) and Hes5 (B) (**p<0.01, ***p<0.001; one-way ANOVA for each cell type). Blots show the regulation of Hes1 protein in CNS1 and U87 cells. C) Expression of fibulin-3 and Hes1 in normal human brain, low-grade astrocytoma and glioblastoma; notice the absence of fibulin-3 in normal brain tissue. Bars: 50 μm; H&E: hematoxylin/eosin. D) Scores for expression of fibulin-3 and Hes1 (N=65) were plotted against each other. Expression of both markers increased with tumor grade, with exception of low fibulin-3 levels in anaplastic oligodendrogliomas (circles surrounded by a square). Rank correlation analysis indicated a strong positive correlation of fibulin-3 and Hes1 expression (Spearman’s ρ=0.695). Each dot in the graph is the average of 2–3 scores from separate cores of each specimen.
To determine if the correlation between fibulin-3 levels and Notch activity was maintained in patient-derived specimens, we performed double immunohistochemistry for fibulin-3 and Hes1 in 13 controls and 52 glioma specimens. Results showed that fibulin-3 and Hes1 were very low or absent in normal tissue (Figure 3C) but were co-expressed in tumor cells (not shown) and their expression increased with tumor grade (Figure 3C). There was a significant positive correlation between fibulin-3 and Hes1 expression (Figure 3D) except in a small subset of anaplastic oligodendrogliomas that had low fibulin-3 expression but high levels of Hes1. Interestingly, overexpression of NICD or knockdown of Notch-1 in glioma cells did not affect fibulin-3 expression (Supplemental Figure S4), suggesting that fibulin-3 is not a Notch target. Therefore, the observed correlation in gliomas was most likely due to regulation of Notch signaling by fibulin-3. Taken together, these results underscored fibulin-3 as a marker of Notch activity and potential regulator of this pathway in glioma.
The pro-invasive effect of fibulin-3 in gliomas is Notch-dependent
In a previous study we demonstrated that fibulin-3 promotes glioma invasion (22) but did not identify the underlying molecular mechanism. Therefore, we next asked if the pro-invasive role of fibulin-3 in gliomas would be mediated by Notch signaling.
To test this hypothesis we first analyzed cell migration in a Transwell™ assay using U251 and CNS1 cells. Fibulin-3 overexpression increased total cell migration, as expected, but this effect was abolished by DAPT (Figure 4A). Since DAPT did not affect baseline migration, we concluded that Notch signaling was not necessary for basal cell motility but was required to mediate the pro-migratory effect of fibulin-3.
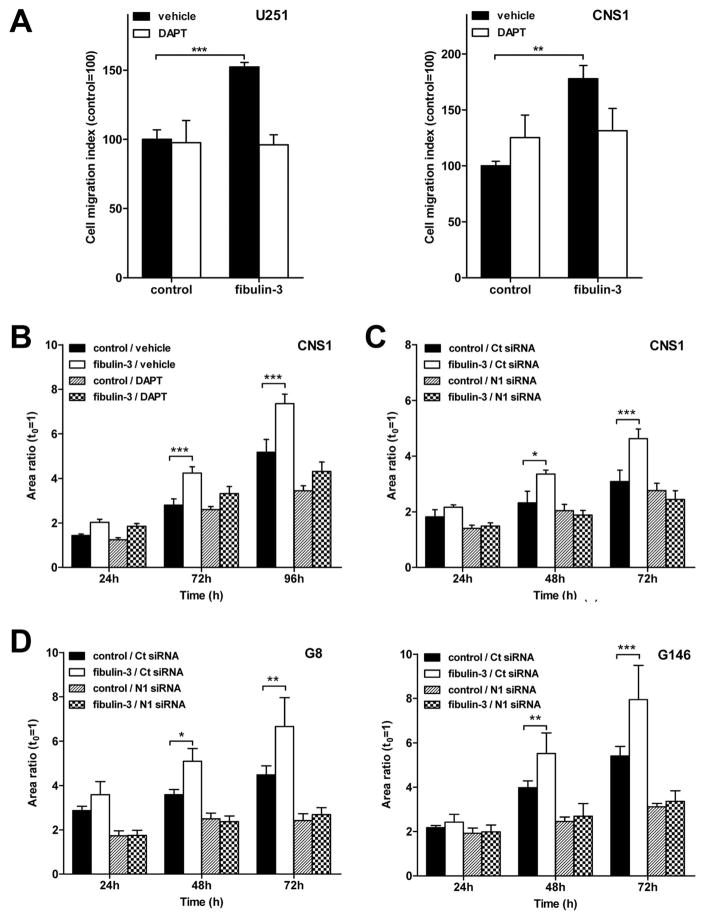
Figure 4. Notch inhibition abolishes the pro-invasive effect of fibulin-3.
A) U251 and CNS1 cells were transfected with fibulin-3 or control cDNAs and assayed in a Transwell migration assay. The pro-migratory effect of fibulin-3 was prevented by DAPT (**p<0.01, ***p<0.001; two-way ANOVA). B–C) Invasion of CNS1 cells was measured in a brain-slice invasion assay. The pro-invasive effect of fibulin-3 was abolished by DAPT (B) or transfection of Notch-1 (N1) siRNA (C). D) Notch-1 knockdown was assessed in two GIC cultures (G8 and G146), showing the same inhibitory results as in (C). Results in B, C, D: significant differences versus control at *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA for repeated measures.
To further determine if Notch was necessary for the pro-invasive role of fibulin-3 we analyzed cell invasion through neural ECM, using glioma spheroids implanted in cultured brain slices. This is an extensively validated model (22, 30, 34) to study glioma cell invasion under conditions that accurately reproduce the natural barriers to cell motility in the brain. Using highly invasive CNS1 cells we confirmed that the pro-invasive effect of fibulin-3 could be suppressed by DAPT (Figure 4B) or by transfection of the cells with Notch-1 siRNA (Figure 4C).
We finally investigated the pro-invasive effects of fibulin-3 on GICs, which are thought to be a key infiltrative population in gliomas (6). Knockdown of Notch-1 reduced GIC invasion through neural ECM and completely abolished the pro-invasive effect of fibulin-3 (Figure 4D). Together, these results support a novel role for Notch signaling as a pro-invasive pathway in gliomas and the major underlying mechanism for the invasive effects of fibulin-3.
Fibulin-3 promotes glioma cell survival in a Notch-dependent manner
The Notch pathway is critically required for survival of glioma cells (35). Given our results demonstrating the regulation of Notch signaling by fibulin-3, we next asked if this ECM protein could regulate glioma cell viability, making it a potential chemosensitizing target.
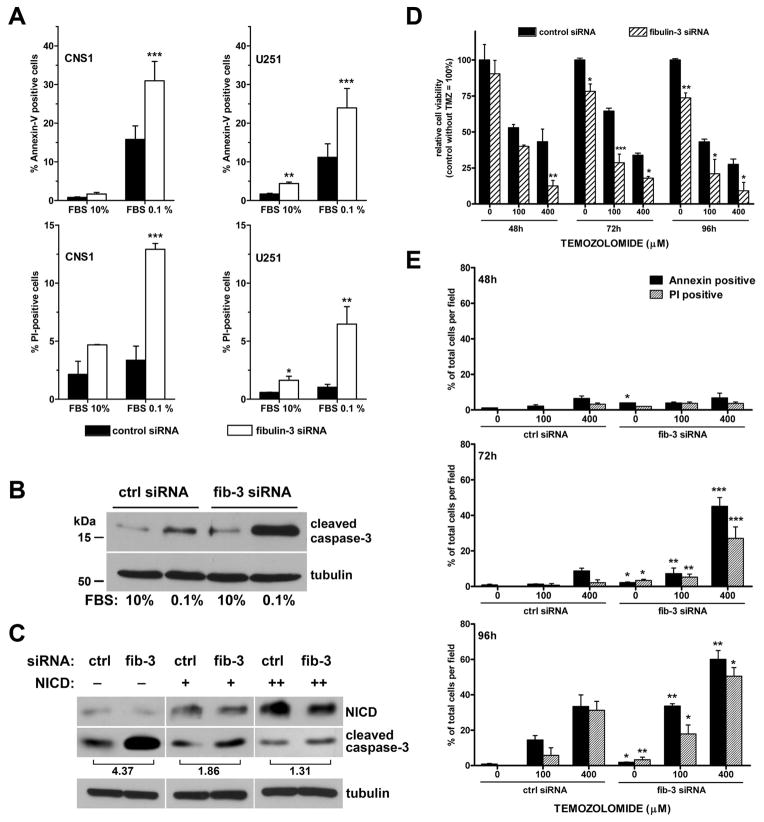
To test this hypothesis, U251 and CNS1 cells were transfected with control or fibulin-3-specific siRNAs and subjected to serum starvation as a simple pro-apoptotic condition. Transient knockdown of fibulin-3 was sufficient to increase the stress sensitivity of glioma cells, resulting in higher percentages of apoptotic (annexin V-positive) and necrotic (propidium iodide-positive) cells (Figure 5A) as well as overall reduced viability in the cultures (Supplemental Figure S5). Biochemical analysis of starved CNS1 cells demonstrated that fibulin-3 knockdown increased the expression of cleaved caspase-3, a typical marker of apoptosis (Figure 5B). However, when fibulin-3 siRNA was co-transfected with increasing amounts of the NICD fragment, the upregulation of cleaved caspase-3 was significantly reduced upon starvation (Figure 5C). This result suggested that the anti-apoptotic effect of fibulin-3 was Notch-dependent and could be rescued by independent activation of Notch signaling.
Figure 5. Downregulation of fibulin-3 promotes apoptosis and is rescued by Notch-1.
A) CNS1 and U251 cells transfected with fibulin-3 or control siRNAs were cultured for 24 h in normal (FBS 10%) or serum-depleted (FBS 0.1%) conditions and analyzed by flow cytometry. There was a significant increase in the percentages of early-apoptotic (annexin-V positive) and necrotic (PI-positive) cells following fibulin-3 knockdown and serum starvation (differences from control at *p<0.05, ***p<0.001; two-way ANOVA for each cell type). B) Western blotting of CNS1 cells showed increase of cleaved caspase-3 following fibulin-3 knockdown. C) CNS1 cells transfected with fibulin-3 siRNA and increasing amounts of NICD were cultured in serum deprivation. NICD reduced the increase of cleaved caspase caused by fibulin-3 downregulation. Numbers show the attenuation in increase of cleaved caspase (optical density fib-3 siRNA/control siRNA) as NICD increases. D–E) U251 cells were transfected with fibulin-3 or control siRNAs and treated with temozolomide. Viability of control cells without temozolomide was calculated as 100% for each day (D). In parallel wells, cells were labeled with annexin-V/PI and counted each day (E). Results show increased chemosensitivity following fibulin-3 knockdown. Results in D and E: significant differences between fibulin-3 and control siRNA for each condition at *p<0.05, **p<0.01, ***p<0.001; multifactorial ANOVA.
To confirm if fibulin-3 downregulation could be used as a chemosensitizing strategy, U251 cells were subjected to fibulin-3 knockdown followed by briefly treatment with temozolomide, which is the standard of care cytotoxic agent for malignant gliomas. Downregulation of fibulin-3 significantly reduced cell viability in presence of temozolomide (Figure 5D) and increased the percentages of apoptotic and necrotic cells in a time- and dose-dependent manner (Figure 5E). This underscored the potential relevance of fibulin-3 downregulation for chemosensitizing strategies in gliomas.
Downregulation of fibulin-3 reduces glioma stem cell viability and self-renewal
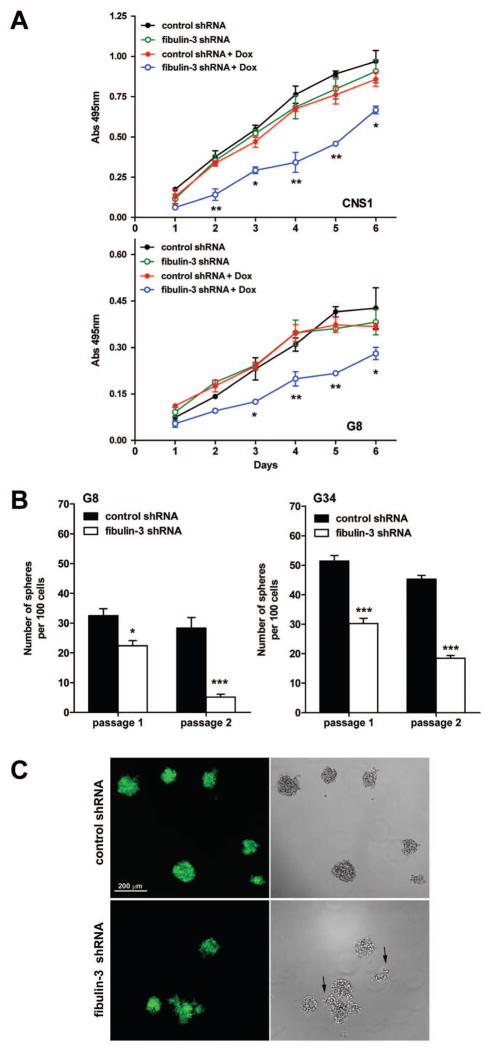
We next attempted to generate glioma cells with stable knockdown of fibulin-3 but these cells showed deficient growth and cell clumping (Supplemental Figure S5), possibly due to sustained negative effects on Notch signaling. Therefore, we used a lentiviral system for doxycycline-inducible expression of shRNAs (described in methods and Supplemental Figure S3). Lentiviruses carrying inducible shRNAs were used for stable transduction of glioma cells (U251 and CNS1) and GICs (G8 and G34).
Cultures transduced with shRNAs and maintained in absence of doxycycline showed similar growth curves (Figure 6A). In contrast, induction of fibulin-3 shRNA caused a significant loss of viability in conventional glioma cells and GICs (Figure 6A). We investigated the effect in GICs in further detail, asking whether fibulin-3 knockdown would affect the self-renewal ability of these cells. G8 and G34 GICs cultured in limiting dilutions after induction of fibulin-3 shRNA showed significantly reduced formation of tumorspheres, an effect that was more noticeable when the cells were processed for a second round of sphere formation (Figure 6B). Visual inspection of the tumorspheres showed in many cases the formation of partially dissociated cell aggregates (Figure 6C). These results strongly supported the pro-survival role of fibulin-3 in glioma cells.
Figure 6. Controlled downregulation of fibulin-3 reduces tumor cell viability and self-renewal.
A) CNS1 and G8 cells transduced with inducible fibulin-3 or control shRNAs were treated with doxycycline (Dox, 20 μg/ml) for 48 h and analyzed for viability. Continuous fibulin-3 knockdown reduced viability in both cell types (*p<0.05, **p<0.01; two-way ANOVA). B) GICs (G8 and G34) transduced with inducible shRNAs were dissociated, cultured at limiting dilutions (1–10 cells/well) in presence of doxycycline, and quantified for formation of neurospheres. Spheres from individual wells were dissociated and plated for a second passage. Fibulin-3 knockdown caused a significant loss of self-renewal (*p<0.05, ***p<0.001; two-way ANOVA for repeated measures). C) Representative images of G8 cells after two weeks in presence of doxycycline. Fibulin-3 knockdown resulted in aberrant formation of neurospheres (arrows).
Donwregulation of fibulin-3 reduces tumor growth and increases overall survival
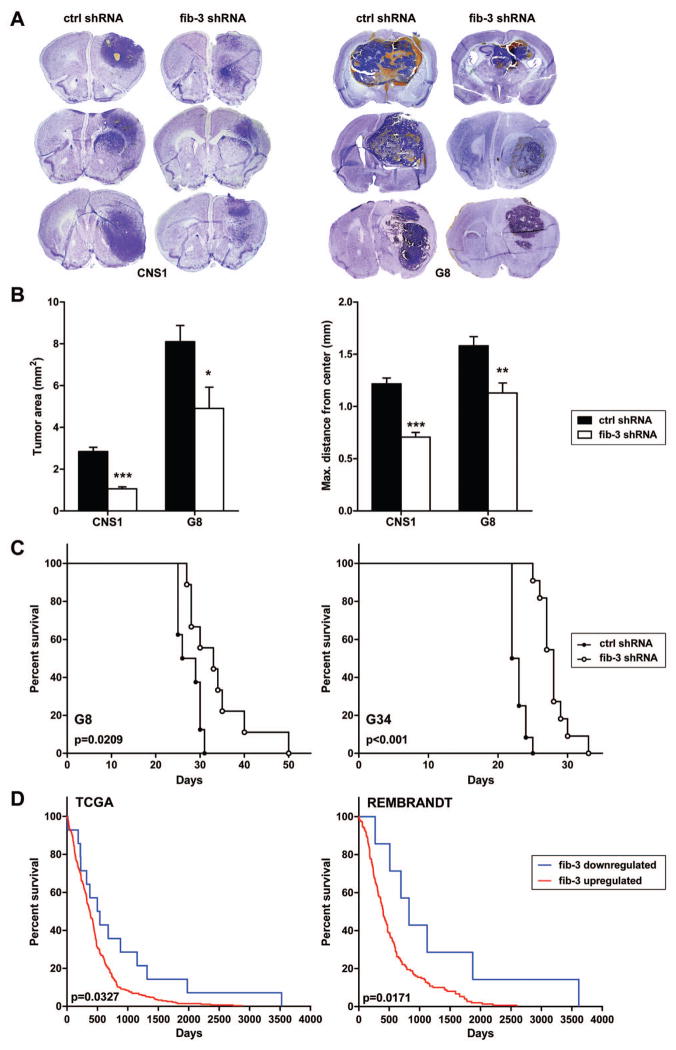
Finally, we determined whether controlled downregulation of fibulin-3 would affect tumor development in vivo. We implanted CNS1, G8 and G34 cells intracranially and induced shRNA expression after tumor implantation. The resulting gliomas showed efficient long-term downregulation of fibulin-3 and Notch-regulated genes in the tumor tissue (Supplemental Figure S6). Histological (Figure 7A) and morphometric (Figure 7B) analysis demonstrated a significant reduction in size and dispersion of tumors with fibulin-3 knockdown compared to controls. This was not due to defects in cell proliferation because the proportion of Ki67-positive cells in fibulin-3-defficient tumors was not significantly different from controls (Supplemental Figure S6). However, fibulin-3 knockdown resulted in a significant increase in the percentage of apoptotic cells (Supplemental Figure S6), explaining the reduced growth. In agreement with these results, animals bearing tumors with controlled downregulation of fibulin-3 showed significantly extended survival compared to controls (Figure 7C).
Figure 7. Fibulin-3 downregulation reduces tumor growth and increases survival.
CNS1 and G8 cells carrying inducible shRNAs were implanted intracranially (N=5 per condition) and tumors analyzed as indicated in the methods section. A) Representative Nissl-stained sections from three control and fibulin-3-deficient tumors. B) Fibulin-3 knockdown resulted in a significant decrease of maximum tumor area and distance from the geometrical center to the visible border of the tumor (*p<0.05; **p<0.01, ***p<0.001; two-tailed t-test for each cell type). C) Survival curves for animals bearing GIC-derived tumors (G8 and G34) show significantly increased survival after controlled fibulin-3 downregulation. D) Survival data from glioblastoma patients with upregulated (≥ 2-fold) or downregulated (≥ 1.5 fold) fibulin-3, queried from the databases TCGA and REMBRANDT. Results show significantly increased overall survival of the patient populations with downregulated fibulin-3.
Our results from animal models agreed with data from human glioblastomas queried from the databases The Cancer Genome Atlas (TCGA) and Repository for Molecular Brain Neoplasia Data (REMBRANDT). Fibulin-3 was highly upregulated in the vast majority of samples from those databases (Supplemental Figure S7). Nevertheless, survival analysis demonstrated that the small subpopulation of patients with downregulated fibulin-3 had significantly longer overall survival (Figure 7D). Furthermore, meta-analysis of high-grade gliomas grouped by transcriptional profile (Gene Expression Omnibus dataset GSE4271 (36)) showed that fibulin-3 levels were always higher in gliomas identified as “mesenchymal” (Supplemental Figure S7), which have poor overall survival compared to other molecular subtypes. Together, these results underscored the potential relevance of fibulin-3 for high-grade glioma classification and prognosis.
Discussion
Malignant gliomas are highly resistant to conventional radio- and chemotherapy, which, coupled to their invasive ability, facilitates tumor recurrence. Novel pharmacological and molecular strategies are focused on targeting signaling pathways to increase apoptotic sensitivity (37, 38) and improve the efficacy of adjuvant therapies. Importantly, accumulated evidence suggests that an invasive cellular phenotype is associated with increased chemoresistance (4, 6). Therefore, targeting pro-invasive factors in glioma could contribute not only to reducing tumor dispersal but also to increasing the sensitivity of already dispersed malignant cells (37, 39).
Anti-invasive treatments for glioma have targeted several potential candidates, including cytoskeletal proteins, cell-surface adhesion receptors, secreted proteases, and soluble paracrine/autocrine factors (40). However, there has been little direct evidence of how targeting these pro-invasive factors could have a direct impact on tumor cell survival. Our results provide evidence of a molecule that is specifically expressed in gliomas compared to normal neural tissue (22), increases tumor cell invasion and, in addition, promotes tumor cell survival by regulating a critical pathway for glioma growth and resistance to apoptosis (35).
Fibulin-3 is a member of the “small fibulins” group (fibulins -3, -4 and -5), which have been shown to act either as pro-tumoral or tumor suppressor genes in different types of solid cancer and different stages of tumor progression (16, 17). Their molecular mechanisms have been difficult to identify due to the multiple functions of the fibulins in cancer and the fact that their molecular partners are largely unknown. Fibulin-3 is downregulated in several types of solid tumors (21, 22) but, surprisingly, is the most upregulated member of the fibulin family in malignant gliomas (22). We have shown clear tumor-promoting effects of fibulin-3 in gliomas, including enhanced cell invasion, increased tumor volume, and reduced animal survival (22). A similar role of fibulin-3 has been proposed in other highly invasive tumors, such as high-grade pancreatic and cervical carcinomas (23, 41). Surprisingly, a tumor-suppressor effect of fibulin-3 was recently reported in a non-invasive glioma model and indirectly attributed to an anti-angiogenic effect (42). Although this result is difficult to explain, it is worth noting that a previously described anti-angiogenic effect of fibulin-3 (21) was caused by a recombinant form that is absent in gliomas and lacks its critical N-terminal domain (22). On the other hand, data from large populations of high-grade glioma patients, available from multiple databases, strongly supports the pro-tumoral role of fibulin-3 in glioma. Our previous meta-analysis of REMBRANDT and ONCOMINE data (22) and current analysis of REMBRANDT and TCGA data (Figures 7 and S7) demonstrate that fibulin-3 is highly and specifically upregulated in glioma patients, increases with grade, and correlates with reduced patient survival. Taken together, the specific upregulation of this protein in gliomas and its enhancing effect on tumor growth and invasion emphasize the potential of fibulin-3 as a relevant target in the tumor microenvironment.
The Notch signaling pathway is one of the major pathways dysregulated in cancers (43), including gliomas (10). Notch signaling contributes to maintaining the glioma initiating cell population (12) and disruption of this pathway critically reduces cell proliferation and survival (35). Recent results also suggest the involvement of Notch signaling in glioma cell motility (44, 45), increasing the interest in targeting this pathway as an anti-invasive and chemosensitizing strategy. Interestingly, Notch receptors and their ligands are upregulated in gliomas (10, 35) but the expression of DLL and Jagged family members does not seem to follow a common pattern with tumor grade (35). On the other hand, upregulation of downstream genes regulated by Notch (Hes1, Hes2, Hey1) correlates better with tumor grade (10, 46), suggesting that Notch signaling may be activated by additional mechanisms, such as the one we have described here for fibulin-3.
Canonical Notch signaling is initiated by cell-cell interaction between cells expressing Notch receptors and those expressing DLL and Jagged ligands. Notch activation by secreted molecules is uncommon and the evidence in cancer models is virtually absent. Soluble factors that activate Notch in mammalian cells include the proteins MAGP-1/2, CCN3 and YB-1 (15). Of these, CCN3 and YB-1 have been shown to promote tumor growth (47, 48) but it has not been elucidated if they achieve this effect through Notch activation. In contrast, our results are the first to show a soluble factor in a cancer model that activates the Notch pathway and likely achieves its pro-tumoral effects in gliomas by reducing cis-inhibition of Notch and increasing Notch signaling in tumor cells.
A possible mechanism of fibulin-3 as antagonist of DLL3 is particularly interesting because the role of this Notch ligand in cancer is poorly, if at all, defined. Current evidence indicates that DLL3 does not trans-activate Notch but inhibits this pathway when expressed in the same cell as the Notch receptor (25). It has been proposed that this could be partly due to the formation of a Notch-DLL3 complex retained in the endoplasmic reticulum (49), although the localization of DLL3 is still subject of debate (25) and has not been analyzed in cancer cells. Fibulin-3 could bind to and inhibit DLL3 intracellularly, or trigger DLL3 downregulation by additional mechanisms in order to activate Notch signaling.
Interestingly, DLL3 is a signature gene of high-grade gliomas defined as “proneural” by their transcriptional profile (36). These tumors are characterized by expression of typical markers of neural differentiation and have better prognosis than other molecularly-defined subtypes. In contrast, both fibulin-3 and its close homologue fibulin-4 are expressed at much higher levels in “mesenchymal” gliomas compared to proneural tumors (Supplemental Figure S7). The mesenchymal subtype can be clearly differentiated from proneural gliomas by its molecular signature and is characterized by increased angiogenesis and poorer prognosis. It is tempting to speculate whether increased expression of fibulins may upregulate Notch signaling in the tumor or its microenvironment (e.g., blood vessels) and contribute to tumor progression towards the more aggressive mesenchymal phenotype, which is also the predominant phenotype upon recurrence (36).
In conclusion, our results highlight fibulin-3 as an upstream marker of Notch signaling in gliomas and a novel Notch activator increasing tumor cell self-renewal, chemoresistance, invasion and tumor growth. This evidence of Notch regulation by the tumor microenvironment opens a way to potential strategies for targeting this pathway more efficiently. Targeting fibulin-3 as part of anti-Notch strategies could restrict anti-invasive and chemosensitizing effects to the tumor cells without the pleiotropic effects of current pharmacological inhibitors.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R01CA152065-01) and the National Brain Tumor Society to MSV, and the Joel Gingras Jr. Research Fellowship from the American Brain Tumor Association to BH. The authors thank the valuable advice from Dr. E. A. Chiocca and the technical support from Jessica De Jesus (Department of Neurological Surgery) and Susie Jones (Pathology Core Facility), The Ohio State University Wexner Medical Center.
Footnotes
Conflicts of interest: None
References
- 1.Berens ME, Giese A. …those left behind. Biology and oncology of invasive glioma cells. Neoplasia. 1999;1:208–19. doi: 10.1038/sj.neo.7900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesari S, Ramakrishna N, Sauvageot C, Stiles CD, Wen PY. Targeted molecular therapy of malignant gliomas. CurrNeurolNeurosciRep. 2005;5:186–97. doi: 10.1007/s11910-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. JClinOncol. 2005;23:2411–22. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 5.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Demuth T, Ross KR, et al. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J Neurooncol. 2001;53:161–76. doi: 10.1023/a:1012253317934. [DOI] [PubMed] [Google Scholar]
- 6.Johannessen TC, Wang J, Skaftnesmo KO, Sakariassen PO, Enger PO, Petersen K, et al. Highly infiltrative brain tumours show reduced chemosensitivity associated with a stem cell-like phenotype. Neuropathol Appl Neurobiol. 2009;35:380–93. doi: 10.1111/j.1365-2990.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhai GG, Malhotra R, Delaney M, Latham D, Nestler U, Zhang M, et al. Radiation enhances the invasive potential of primary glioblastoma cells via activation of the Rho signaling pathway. JNeurooncol. 2006;76:227–37. doi: 10.1007/s11060-005-6499-4. [DOI] [PubMed] [Google Scholar]
- 8.Miletic H, Niclou SP, Johansson M, Bjerkvig R. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets. 2009;13:455–68. doi: 10.1517/14728220902806444. [DOI] [PubMed] [Google Scholar]
- 9.Germano I, Swiss V, Casaccia P. Primary brain tumors, neural stem cell, and brain tumor cancer cells: where is the link? Neuropharmacology. 2010;58:903–10. doi: 10.1016/j.neuropharm.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. JNeurosurg. 2007;106:417–27. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- 11.Natsume A, Kinjo S, Yuki K, Kato T, Ohno M, Motomura K, et al. Glioma-initiating cells and molecular pathology: implications for therapy. Brain Tumor Pathol. 2011;28:1–12. doi: 10.1007/s10014-010-0011-3. [DOI] [PubMed] [Google Scholar]
- 12.Hu YY, Zheng MH, Cheng G, Li L, Liang L, Gao F, et al. Notch signaling contributes to the maintenance of both normal neural stem cells and patient-derived glioma stem cells. BMC Cancer. 2011;11:82. doi: 10.1186/1471-2407-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu J, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 2011;102:1306–12. doi: 10.1111/j.1349-7006.2011.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida H, Arita K, Yunoue S, Yonezawa H, Shinsato Y, Kawano H, et al. Role of sonic hedgehog signaling in migration of cell lines established from CD133-positive malignant glioma cells. J Neurooncol. 2011 doi: 10.1007/s11060-011-0552-2. [DOI] [PubMed] [Google Scholar]
- 15.D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher WM, Currid CA, Whelan LC. Fibulins and cancer: friend or foe? Trends MolMed. 2005;11:336–40. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4:1127–31. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wlazlinski A, Engers R, Hoffmann MJ, Hader C, Jung V, Muller M, et al. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67:1770–80. doi: 10.1002/pros.20667. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. JBiolChem. 2007;282:11805–16. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 20.Giltay R, Timpl R, Kostka G. Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol. 1999;18:469–80. doi: 10.1016/s0945-053x(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 21.Albig AR, Neil JR, Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006;66:2621–9. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS. Fibulin-3 Is Uniquely Upregulated in Malignant Gliomas and Promotes Tumor Cell Motility and Invasion. Mol Cancer Res. 2009;7:1756–70. doi: 10.1158/1541-7786.MCR-09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeliger H, Camaj P, Ischenko I, Kleespies A, De Toni EN, Thieme SE, et al. EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol Cancer Res. 2009;7:189–98. doi: 10.1158/1541-7786.MCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 24.En-lin S, Sheng-guo C, Hua-qiao W. The expression of EFEMP1 in cervical carcinoma and its relationship with prognosis. Gynecol Oncol. 2010;117:417–22. doi: 10.1016/j.ygyno.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Ladi E, Nichols JT, Ge W, Miyamoto A, Yao C, Yang LT, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol. 2005;170:983–92. doi: 10.1083/jcb.200503113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. MolCell Biol. 1996;16:952–9. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsell CE, Shawber CJ, Boulter J, Weinmaster G. Jagged: a mammalian ligand that activates Notch1. Cell. 1995;80:909–17. doi: 10.1016/0092-8674(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 28.Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3:109–16. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 29.Nuovo GJ, Hagood JS, Magro CM, Chin N, Kapil R, Davis L, et al. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod Pathol. 2011 doi: 10.1038/modpathol.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu B, Kong LL, Matthews RT, Viapiano MS. The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J Biol Chem. 2008;283:24848–59. doi: 10.1074/jbc.M801433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camaj P, Seeliger H, Ischenko I, Krebs S, Blum H, De Toni EN, et al. EFEMP1 binds the EGF receptor and activates MAPK and Akt pathways in pancreatic carcinoma cells. Biol Chem. 2009;390:1293–302. doi: 10.1515/BC.2009.140. [DOI] [PubMed] [Google Scholar]
- 33.Hwang CF, Chien CY, Huang SC, Yin YF, Huang CC, Fang FM, et al. Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol. 2010;222:367–79. doi: 10.1002/path.2776. [DOI] [PubMed] [Google Scholar]
- 34.Palfi S, Swanson KR, De BS, Chretien F, Oliveira R, Gherardi RK, et al. Correlation of in vitro infiltration with glioma histological type in organotypic brain slices. BrJCancer. 2004;91:745–52. doi: 10.1038/sj.bjc.6602048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–63. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 36.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME. Molecular targets of glioma invasion. Cell MolLife Sci. 2007;64:458–78. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Cheng L, Guryanova OA, Wu Q, Bao S. Cancer stem cells in glioblastoma--molecular signaling and therapeutic targeting. Protein Cell. 2010;1:638–55. doi: 10.1007/s13238-010-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. JNatlCancer Inst. 2007;99:1583–93. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 40.Viapiano MS, Lawler SE. Glioma invasion: Mechanisms and Therapeutic Challenges. In: Van Meir E, editor. CNS Cancer: Models, Prognostic Factors and Targets. New Jersey: Humana Press; 2009. pp. 1219–52. [Google Scholar]
- 41.Song EL, Hou YP, Yu SP, Chen SG, Huang JT, Luo T, et al. EFEMP1 expression promotes angiogenesis and accelerates the growth of cervical cancer in vivo. Gynecol Oncol. 2011;121:174–80. doi: 10.1016/j.ygyno.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Pioli PD, Siegel E, Zhang Q, Nelson J, Chaturbedi A, et al. EFEMP1 suppresses malignant glioma growth and exerts its action within the tumor extracellular compartment. Mol Cancer. 2011;10:123. doi: 10.1186/1476-4598-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjolund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer. 2005;41:2620–9. doi: 10.1016/j.ejca.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Chen T, Zhang J, Mao Q, Li S, Xiong W, et al. Notch1 promotes glioma cell migration and invasion by stimulating beta-catenin and NF-kappaB signaling via AKT activation. Cancer Sci. 2012;103:181–90. doi: 10.1111/j.1349-7006.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- 45.Raghu H, Gondi CS, Dinh DH, Gujrati M, Rao JS. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch -1 receptor. Mol Cancer. 2011;10:130. doi: 10.1186/1476-4598-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulleman E, Quarto M, Vernell R, Masserdotti G, Colli E, Kros JM, et al. A role for the transcription factor HEY1 in glioblastoma. J Cell Mol Med. 2009;13:136–46. doi: 10.1111/j.1582-4934.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuo GW, Kohls CD, He BC, Chen L, Zhang W, Shi Q, et al. The CCN proteins: important signaling mediators in stem cell differentiation and tumorigenesis. Histol Histopathol. 2010;25:795–806. doi: 10.14670/hh-25.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evdokimova V, Tognon C, Ng T, Sorensen PH. Reduced proliferation and enhanced migration: two sides of the same coin? Molecular mechanisms of metastatic progression by YB-1. Cell Cycle. 2009;8:2901–6. doi: 10.4161/cc.8.18.9537. [DOI] [PubMed] [Google Scholar]
- 49.Geffers I, Serth K, Chapman G, Jaekel R, Schuster-Gossler K, Cordes R, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol. 2007;178:465–76. doi: 10.1083/jcb.200702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.