Abstract
Innate immune effector mechanisms triggered by oncolytic viruses may contribute to the clearance of both infected and uninfected tumor cells in immunocompetent murine hosts. Here, we developed an in vitro tumor cell/bone marrow coculture assay and used it to dissect innate immune sensor and effector responses to intratumoral vesicular stomatitis virus (VSV). We found that the type III IFN interleukin-28 (IL-28) was induced by viral activation of innate immune-sensing cells, acting as a key mediator of VSV-mediated virotherapy of B16ova melanomas. Using tumor variants which differentially express the IL-28 receptor, we showed that IL-28 induced by VSV within the tumor microenvironment sensitizes tumor cells to natural killer cell recognition and activation. These results revealed new insights into the immunovirological mechanisms associated with oncolytic virotherapy in immune-competent hosts. Moreover, they defined a new class of tumor-associated mutation, such as acquired loss of responsiveness to IL-28 signaling, which confers insensitivity to oncolytic virotherapy through a mechanism independent of viral replication in vitro. Lastly, the findings suggested new strategies to manipulate immune signals that may enhance viral replication, along with antitumor immune activation, and improve the efficacy of oncolytic virotherapies.
Introduction
In theory, introduction of even low levels of a fully replication-competent oncolytic virus into a tumor will allow rapid spread of the virus, lysis of the tumor cells, and reductions in tumor burdens (1, 2). Through either natural, or engineered, selectivity for tumor cells, viral replication should be extinguished in normal cells (3–5). Many different oncolytic viruses have been successfully tested in preclinical and clinical models (6–10). However, such studies have, by necessity, often been carried out in models lacking fully functional immune systems (11–14). Therefore, the immune system has largely been perceived to inhibit this form of anticancer therapy by restricting viral replication, spread, and oncolysis (15–18). However, with the increasing availability of immune competent models of oncolytic virotherapy (19), it is becoming clear that the immune system may play a more positive role—both to prevent uncontrolled viral spread and toxicity (20) and as an effector of antitumor therapy (21–26).
We have previously shown that vesicular stomatitis virus (VSV) therapy of B16ova tumors in C57Bl/6 mice depends on host CD8+ and natural killer (NK) cells, confirming that viral replication is not the sole mediator of tumor regressions (27). More recently, we showed that it was not possible to detect progressive viral replication within B16ova tumors following intratumoral injection of VSV (26), probably because of a potent innate immune response at the site of the tumor. Moreover, a single-cycle virus was as effective as a fully replication-competent virus in generating antitumor therapy (21), although expression of the viral genome, at least for a single cycle, was required for therapy (21). Therefore, we hypothesized that VSV acts in this model to trigger a potent innate immune response within the tumor, which rapidly clears the virus and has both direct and indirect antitumor activity (21, 23).
The aim of the current study was to characterize the molecules and effector cells that mediate both the antiviral and antitumor innate response induced by VSV in the B16ova/C57Bl/6 model. Here, we identify interleukin-28 (IL-28), induced by viral activation of innate immune sensing cells, as a key mediator of antitumor therapy in this model. Using cell lines that are equally sensitive to VSV in vitro, we show that only tumors that can respond to IL-28 are sensitive to VSV-mediated therapy in vivo. Although our data here confirms that the innate response inhibits viral replication and spread (28–30), they also indicate that the same response is, in this case, probably the major mediator of the antitumor effects. Therefore, efforts to suppress the innate response to the virus need to be carefully targeted so that viral replication can be enhanced sufficiently to compensate for the loss of positive antitumor immune effects induced by the virus within the tumor.
Materials and Methods
Cell lines and viruses
B16(LIF) cells are murine melanoma cells (H2-Kb) periodically cultured in our laboratory over the previous 12 years. B16ova melanoma cells (H2-Kb), derived from a separate stock of B16 cells transduced with a cDNA encoding the chicken ovalbumin gene (31), were obtained from Dr. Esteban Celis (H. Lee Moffitt Cancer Center, Tampa, FL) and have been cultured periodically over the previous 7 years. Both cell lines were grown in DMEM (Life Technologies) supplemented with 10% (v/v) FCS (Life Technologies) and l-glutamine (Life Technologies). B16ova cells were maintained in G418 (5 mg/mL) to retain the ova gene. All cell lines were free of Mycoplasma infection.
VSV-GFP (Indiana serotype) was generated by cloning the green fluorescence protein cDNA into the plasmid pVSV-XN2 as described (32). Purification of viruses was by sucrose gradient centrifugation. Virus titers were measured by plaque assays on BHK-21 cells (32).
Bone marrow coculture
Bone marrow cells were removed from the femurs and tibias of mice by flushing with RPMI 1640 and were passed through a 100-µm filter to prepare single-cell suspensions. B16(LIF) or B16ova cells (1 × 105) were cocultured with 1 × 106 bone marrow cells for 24 hours at 37°C. Cocultures were then infected with VSV [multiplicity of infection (MOI), 0.1 with respect to the tumor cells] for 6 hours; cells were washed thrice with PBS before addition of anti-VSV neutralizing serum (titer of 1:2,560), recombinant IFN-α protein (R&D Systems), or anti–IL-28 antibody (R&D Systems). Twenty-four to 48 hours later, cell killing was assessed using light microscopy, crystal violet staining, or MTT assay (Cell Proliferation kit, Roche Diagnostics GmbH). Bone marrow cultures were depleted of immune subsets by coculture for 24 hours with anti-MAC3, anti–asialo-GM-1 (Cedarlane), anti-CD8 (Lyt2.4.3), anti-CD4 (GK1.5; Monoclonal Antibody Core Facility, Mayo Clinic), or anti–GR-1 antibodies (BioXcell) to deplete macrophages, NK, CD8 T cells, CD4 T cells, and granulocytic cells, respectively. Depletion of cells from bone marrow cultures was confirmed by fluorescence-activated cell sorting analysis to be in excess of 95% of the relevant cell type compared with nontreated control cultures.
Reverse transcription-PCR and mouse IL-28 receptor cloning
Total RNA was extracted from cells using Qiagen RNA extraction kits. Total cellular RNA (1 µg) was reverse transcribed in 20 µL using oligo(dT) as primer. A cDNA equivalent of 1 ng RNA was amplified by PCR using choice-taq DNA polymerase (Denville Scientific, Inc.). Full-length mouse IL-28 receptor (AK148133) was amplified from cDNA from B16ova cells using forward (5′-TGATCATGATCACAGGGACT-GAAATGTGGCGG-3′) and reverse (5′-GCGGCCGCGCACTC-TGGGATTCGGTCAA-3′) primers. The cDNA of IL-28R was inserted into the BamHI and NotI sites of the pSIN-CSGW-UbEm lenti-vector (Dr. Yasuhiro Ikeda, Mayo Clinic, Rochester, MN).
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57Bl/6 mice were purchased from The Jackson Laboratory at 6 to 8 weeks of age. IFN-α/β receptor knockout (KO) mice were a gift from Dr. Roberto Cattaneo (Mayo Clinic, Rochester, MN). To establish subcutaneous tumors, 5 × 105 tumor cells in 100 µL PBS were injected into the flank of mice. Viral injections (50 µL) were administered intratumorally at days 7, 9, and 11. Animals were examined daily and tumor sizes were measured thrice weekly using calipers. Animals were killed when tumor size was >1.0 × 1.0 cm in two perpendicular directions. NK cells were depleted by i.p. injections (0.1 mg/mouse) of anti-asialo-GM-1 (Cedarlane; ref. 27). For IL-28 depletion, 2 µg of anti-IL-28 antibody (R&D Systems) or IgG control (ChromPure Rat IgG; Jackson ImmunoResearch)per mouse was given at each injection of virus.
ELISA for IL-28
Supernatants were tested for IL-28 production by ELISA as directed by the manufacturer (PBL Interferonsource).
Flow cytometry analysis
Tumor-draining lymph nodes and tumors were dissociated to single-cell suspensions. Cells (1 × 106 ) were washed, resuspended in PBS containing 0.1% bovine serum albumin, and incubated with directly conjugated primary antibodies for 30 minutes at 4°C. Cells were washed and resuspended in 500 µL PBS containing 4% formaldehyde and analyzed by flow cytometry. Data were analyzed using the Flowjo software (Flowjo). Anti-CD11b FITC, anti-PDCA PE, anti-F4/ 80 APC, anti-GR1 PE-Cy7, anti-B220 Per-CP, and their respective isotype controls were purchased from BD Pharmingen.
NK cell isolation
NK cells were isolated from peripheral blood mononuclear cells using MACS-negative depletion kits following the manufacturer’s protocol (>90% purity; Miltenyi Biotec).
Statistics
Survival data were analyzed by log-rank test using Graph-Pad Prism 4 (GraphPad Software). In vitro experiments were analyzed using the JMP Software (SAS Institute, Inc.). Statistical significance was determined at the level of P < 0.05 (27).
Results
VSV-activated bone marrow cells are cytotoxic against B16ova tumor cells
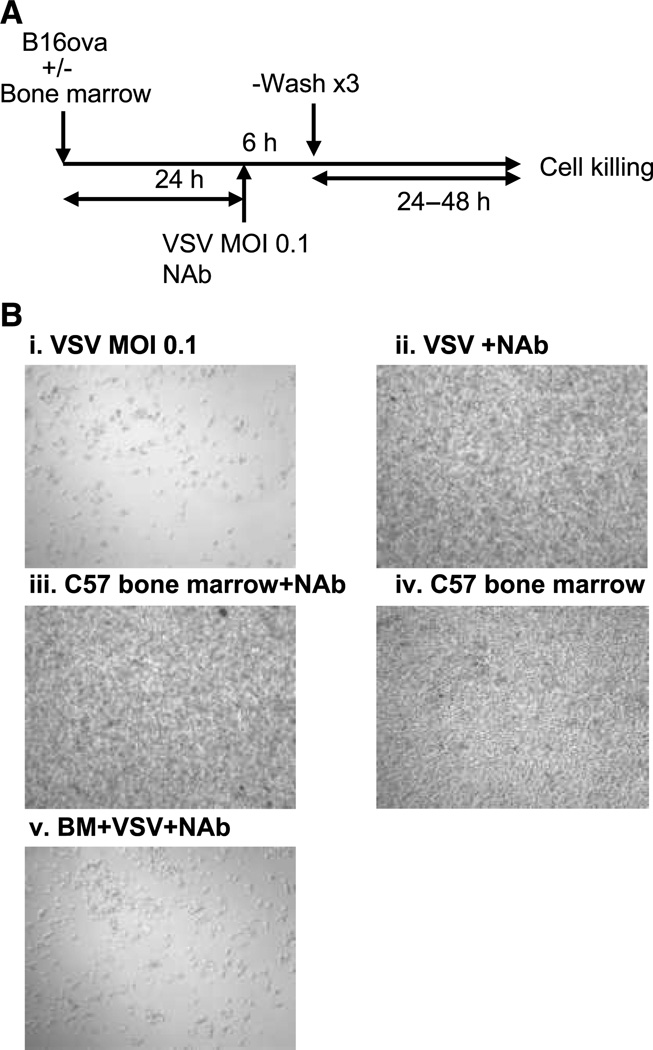
Our hypothesis was that following intratumoral injection of VSV, host-derived immune cells become activated by the virus to secrete cytokines, which subsequently recruit/ activate the same, or additional, antiviral and antitumor immune effector cells and mechanisms. To test this hypothesis, we established an assay in which host-derived bone marrow cells were cocultured with B16-derived tumor cells in the presence, or absence, of virus (Fig. 1A). Exposure of B16ova tumor cells to VSV, even at low MOI, induced rapid and extensive cytotoxicity (Fig. 1B, i), which could be inhibited by coculturing with anti-VSV neutralizing serum (Fig. 1B, ii). Coculture of B16ova cells with bone marrow cells from C57Bl/6 mice had no significant cytotoxic effects either with (Fig. 1B, iii) or without (Fig. 1B, iv) the neutralizing serum against VSV. In contrast, when cocultures of B16ova and C57-derived bone marrow cells were exposed to VSV in the presence of anti-VSV neutralizing serum, extensive cytotoxicity against the tumor cells was observed (Fig. 1B, v). These data indicated that the VSV-mediated activation of bone marrow cells induced bystander killing of B16ova tumor cells, distinct from direct viral-mediated oncolysis.
Figure 1.
VSV activates bone marrow cytotoxicity against B16ova. A, in vitro coculture between tumor cells and bone marrow cells; NAb: neutralizing anti-VSV immune serum. B, light microscopy of cocultures (from A) of B16ova cells 24 h following treatment with (i) VSV MOI 0.1 (no bone marrow), (ii) VSV+NAb (no bone marrow), (iii) bone marrow+NAb, (iv) bone marrow cells alone (no VSV or Nab), or (v) bone marrow (BM)+VSV+NAb.
B16 variants show differential sensitivity to VSV-activated bone marrow cytotoxicity
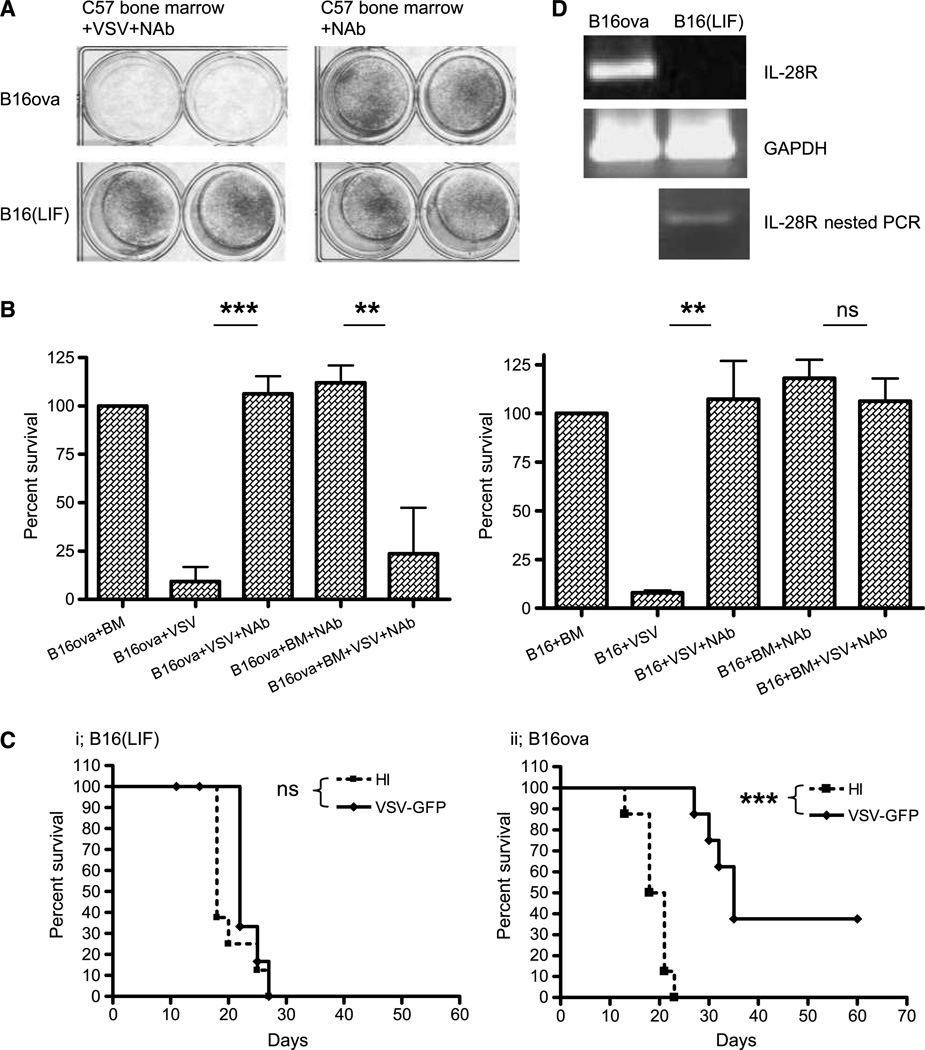
In contrast to B16ova cells, we observed that one of our B16-derived tumor cell lines, B16(LIF), was almost completely resistant to the cytotoxic effects of VSV-activated bone marrow cells (Fig. 2A and B). However, both B16ova and B16(LIF) cells showed almost equal sensitivity to cell-free VSV replication and cytolysis in vitro (Fig. 2B). Although B16ova and B16 (LIF) cells are both B16 derived, they have been passaged separately for several years in our laboratory (see Materials and Methods). Interestingly, the lack of sensitivity of B16(LIF) cells to VSV-activated bone marrow cell killing in vitro was mirrored by a consistent lack of efficacy of antitumor therapy following intratumoral injection of VSV in vivo (P = 0.18 compared with treatment with heat-inactivated VSV; Fig. 2C, i). In contrast, B16ova cells were sensitive both to VSV-activated bone marrow cell killing in vitro (Fig. 2A and B) and to intratumoral VSV treatment in vivo, in which we consistently observed significant prolongation of survival times over control treatments (P < 0.00001 compared with treatment with heat-inactivated VSV; Fig. 2C, ii).
Figure 2.
B16(LIF) cells are insensitive to VSV-activated bone marrow cytotoxicity. A and B, B16ova or B16(LIF) cells were cocultured with C57Bl/6 bone marrow with, or without, VSV as in Fig. 1A. Cell survival was assessed by crystal violet staining (A) or MTT assay (B). C, C57Bl/6 (8 mice/group) bearing 7-d subcutaneous (s.c.) B16(LIF) (i) or B16ova (ii) tumors were injected intratumorally every 2 d (three total) with 5 × 108 pfu of heat-inactivated virus (HI) or VSV. Survival with time is shown. D, cDNA from B16ova or B16(LIF) cells was screened for IL-28R. A weak signal was detected in B16(LIF) cells with 20 additional cycles. **, P < 0.01; ***, P < 0.001; ns; non significant.
To investigate further how B16ova and B16(LIF) cells may have diverged over several years in culture, we performed a gene chip analysis. Of the multiple genetic differences that exist between these two cell lines, we focused on genes associated with the innate immune response to viral infection. One such gene, the receptor for the type III IFN IL-28, was overexpressed in B16ova cells by a factor of 23-fold relative to its levels of expression in B16(LIF) cells, as confirmed by reverse transcription-PCR (Fig. 2D). We confirmed also that B16(LIF) cells do not express the IL-28R on infection with VSV at a MOI ranging from 0.1, 1.0, or 10.
IL-28, as well as type I IFNs, mediate VSV-activated bone marrow cytotoxicity
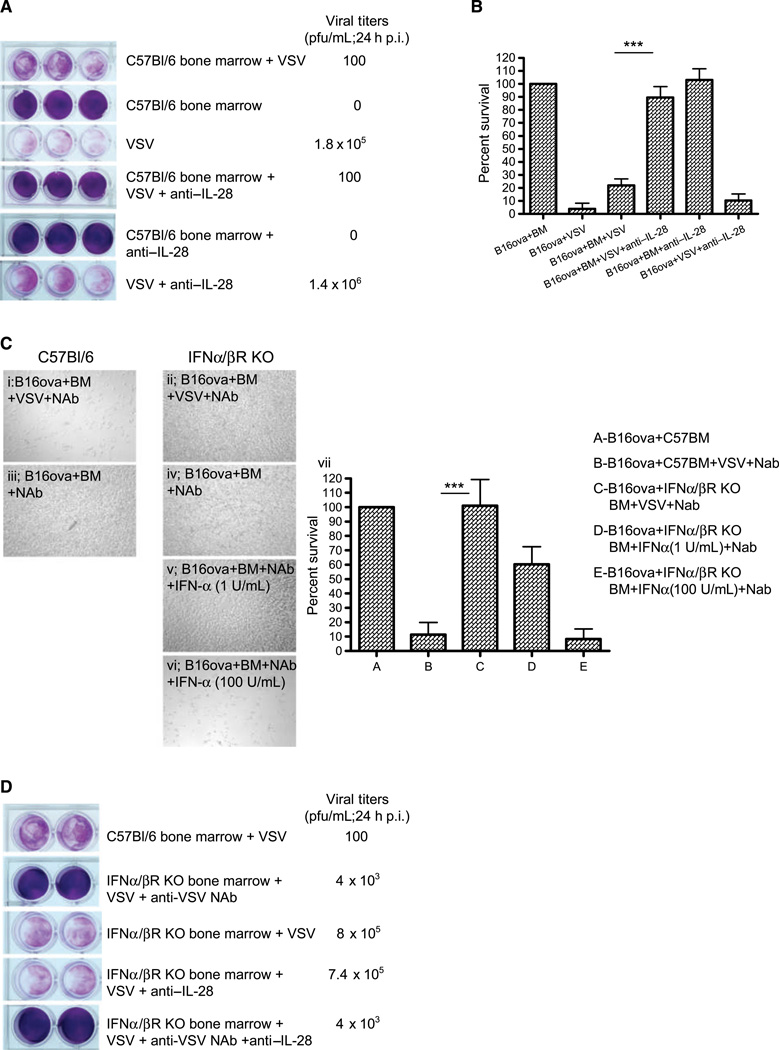
We hypothesized that differences in the expression of the IL-28R between the B16ova and B16(LIF) lines may mediate the differential responses of these cell lines to both VSV-activated bone marrow cytotoxicity in vitro as well as VSV-mediated antitumor therapy in vivo. Consistent with this hypothesis, antibody-mediated blockade of IL-28 signaling abrogated the VSV-activated bone marrow–mediated killing of B16ova cells in vitro (Fig. 3A and B). We also observed that when the bone marrow cytotoxicity assay was repeated, even in the absence of neutralizing VSV serum, components of normal C57Bl/6 bone marrow suppressed viral replication through the B16ova cultures [mean titers reduced from 1.8 × 105 plaque-forming unit (pfu)/mL with no bone marrow cells to ~100 pfu/mL in the presence of bone marrow cells; Fig. 3A]. However, although blockade of IL-28 prevented VSV-activated bone marrow–mediated cytotoxicity against B16ova cells, it did not reduce VSV replication/spread through B16ova cultures even in the presence or absence of bone marrow cells (Fig. 3A).
Figure 3.
VSV-activated bone marrow cytotoxicity depends upon type I and III IFNs. A, B16ova cells were cocultured with bone marrow cells as in Fig. 1A, with or without anti-IL-28 antibody (15 ug/mL) upon infection with VSV. Twenty-four hours later, supernatants were harvested for viral titer and cell viability was assessed by crystal violet staining (A) and MTT assay (B). C, light microscopy of cocultures of B16ova cells with either C57Bl/6-derived bone marrow (i and iii) or bone marrow from IFN-α/βR KO mice (ii, iv, v, and vi) 48 h following treatment with VSV (MOI 0.1) +NAb (I and ii) or bone marrow+Nab (no VSV; iii and iv), or with bone marrow from IFN-α/βR KO mice +Nab (no VSV) with added IFN-α at 1 (v) or 100 (vi) U/mL. MTT assay of these results is shown in vii. D, the experiment in A was repeated with coculture of B16ova with C57Bl/6- or IFN-α/βR KO-derived bone marrow, with VSV +/− anti-IL-28 antibody. Twenty-four hours after VSV, supernatants were harvested for viral titer and cell viability was assessed by crystal violet. ***, P < 0.001.
Type I IFNs (IFN α/β) exert potent antiviral effects against VSV (11, 14, 33, 34). To identify the factor in bone marrow cocultures that inhibited viral spread/replication through highly permissive B16ova cells, the assays of Fig. 3A and B were repeated using bone marrow cells from IFN α/β receptor KO mice. The inability of bone marrow cells to respond to IFN α/β completely abolished VSV-activated cytotoxicity against B16ova cells in vitro (Fig. 3C, i and ii). In addition, although IFN-α was not itself directly cytotoxic to B16ova tumor cells at 1 or 100 U/mL (data not shown), addition of IFN-α to IFN α/β receptor KO bone marrow/tumor cell cocultures replaced the requirement for VSV to induce in vitro cytotoxicity against B16ova tumor cells (Fig. 3C, v and vi). Finally, IFN α/β receptor KO bone marrow was unable to suppress VSV spread and replication in B16ova cocultures (Fig. 3D) and blockade of IL-28 in these cultures had no inhibitory effect on viral replication (Fig. 3D).
Taken together, these data indicate that type I IFNs are critical for both the VSV-activated bone marrow cytotoxicity against B16ova cells and for the suppression of VSV replication through these cultures; in contrast, although the type III IFN IL-28 mediates VSV-activated bone marrow cytotoxicity against B16ova, it does not exert significant inhibitory effects on viral replication and spread.
GR1+ cells sense VSV infection
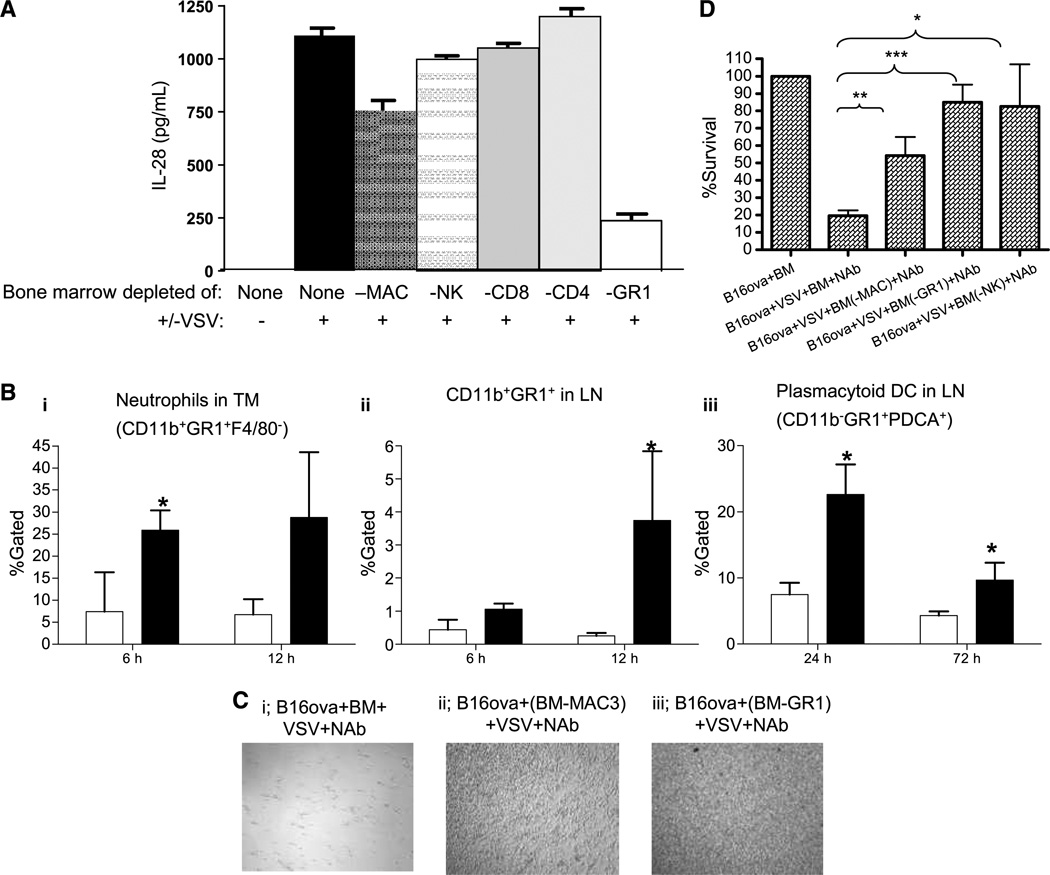
We confirmed that IL-28 is produced by normal C57Bl/6 bone marrow cultures, but only when activated by VSV (Fig. 4A). Depletion of the bone marrow cultures of both macrophages and GR1+ cells reproducibly significantly reduced the levels of VSV-activated IL-28 secretion (P < 0.01 for macrophages and P < 0.001 for GR-1+ cells compared with no depletion; Fig. 4A). To determine the source of the IL-28 observed in Fig. 4A, this experiment was repeated in the absence of cocultured B16ova tumor cells. Within 48 hours of infection of the bone marrow cell cultures (no added B16ova), levels of IL-28 detected by ELISA were very similar (~1,100 pg/mL/106 bone marrow cells) to those seen in the presence of cocultured B16ova cells. In addition, the dependence of IL-28 production upon both macrophages (Mac+) and GR-1+ cells was identical to that seen on coculture with tumor cells. These data confirm that the IL-28 produced by the B16 tumor cell/GR-1+ bone marrow cultures upon exposure to VSV is derived predominantly from the bone marrow cells, rather than from either the tumor cells, directly or as a result of a factor induced by the tumor cells upon VSV infection that then acts on the bone marrow cells.
Figure 4.
GR1+ cells in bone marrow sense VSV. A, bone marrow from C57Bl/6 mice was left undepleted (none) or depleted of macrophages, NK, CD8, CD4, or GR1+ cells and cocultured with B16ova cells +/− VSV (MOI 0.1) as in Fig. 1A. Forty-eight hours after addition of VSV, supernatants were assayed for IL-28. B, C57Bl/6 (3 mice/group) bearing B16ova tumors were injected intratumorally with 5 × 108 pfu of VSV (filled column) or PBS (opened column). Tumors (i) and draining lymph node (LN; ii and iii) were harvested at times shown and analyzed for (i) neutrophils (CD11b+GR1+F4/80−), CD11b+GR1+ (ii), or plasmacytoid dendritic cells (CD11b+GR1+PDCA+; iii). Infiltrates in tumors injected intratumorally with heat-inactivated VSV as a negative control were directly comparable with those tumors treated with PBS (data not shown). TM, tumor; DC, dendritic cells. C, C57Bl/6 bone marrow was left undepleted (i) or depleted of macrophages (ii) or GR1+ cells (iii), and cocultured with B16ova with VSV (MOI, 0.1) and neutralizing anti-VSV antibody (NAb) as in Fig. 1A. Forty-eight hours after the addition of VSV, cell survival was visualized or quantified by MTT assay (D). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
VSV activation of bone marrow cells to produce IL-28 was also dependent on type I IFN signaling because bone marrow from IFN α/β KO mice did not secrete IL-28 upon exposure to VSV (<100 pg/mL in the assay of Fig. 4A; data not shown). To relate these in vitro data with the in vivo mechanisms that mediate the treatment of B16ova tumors (Fig. 2C), we investigated which effector cells respond to VSV. Intratumoral VSV induced significant infiltration into the tumor by neutrophils (CD11b+;GR1+;F4/80−) within 6 hours of virus injection (Fig. 4B, i). In addition, both CD11b+;GR1+ cells and plasmacytoid dendritic cells (CD11b−;GR1+; PDCA+; Fig. 4B, ii and iii) accumulated in tumor-draining lymph nodes over longer periods (12–72 h) following virus injection. Consistent with our observations of the importance of GR1+ cells in sensing VSV infection through the production of IL-28 in vitro (Fig. 4A) and of their recruitment in vivo (Fig. 4B), depletion of GR1+ cells significantly inhibited the in vitro cytotoxicity of bone marrow cultures against B16ova tumor cells (P = 0.0004 compared with no depletion; Fig. 4C and D). Although we did not observe reproducible increases in macrophage infiltration into VSV-injected B16ova tumors in vivo (data not shown), depletion of Mac3+ cells from bone marrow cultures also inhibited VSV-activated bone marrow– induced cytotoxicity against B16ova cells in vitro (P = 0.005 compared with no depletion; Fig. 4C, i and ii, and D). Depletion of CD4, CD8, or B220 cells from bone marrow cultures had no significant effect on the VSV-activated bone marrow cytotoxicity against B16ova cells in vitro (data not shown).
Taken together, these data indicate that the VSV-mediated activation of both macrophages and GR1+ cells within bone marrow cultures induces IL-28 and cytotoxicity against B16ova tumor cells in vitro, consistent with our in vivo observations that VSV activates the recruitment of GR1+ cells to both B16ova tumors and tumor-draining lymph node in vivo.
Expression of IL-28R by B16ova sensitizes B16ova cells for NK recognition
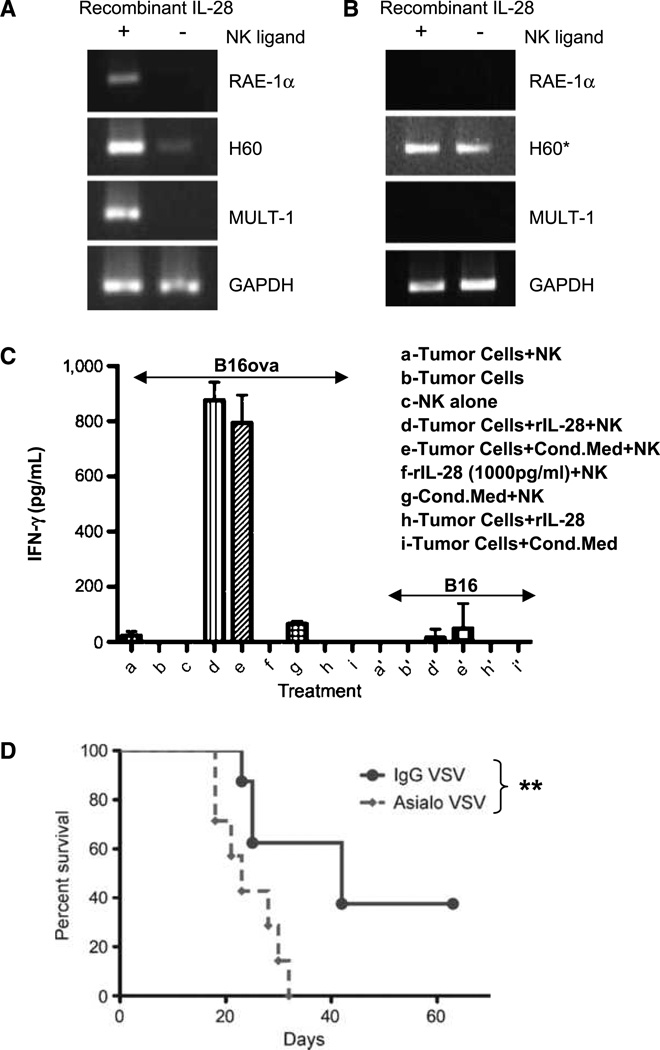
Although NK1.1 cells were not a reproducible source of IL-28 from VSV-activated bone marrow cultures (Fig. 4A), depletion of these cells from bone marrow cultures abolished cytotoxicity against B16ova tumor cells (Fig. 4D) almost to the same extent as blockade of IL-28 (Fig. 3A and B). Therefore, we investigated whether there was a mechanistic link between VSV-induced, IL-28 expression and tumor cell sensitivity to NK cells. Whereas IL-28 induced expression of at least three different NK cell ligands on B16ova cells (Fig. 5A), it had no effect on the expression of NK ligands on B16(LIF) cells (Fig. 5B). In addition, B16ova cells treated with either recombinant IL-28 or with conditioned medium from VSV-activated bone marrow cultures activated IFN-γ production from NK cells (Fig. 5C). In contrast, B16(LIF) cells were not effective targets for NK cells, even in the presence of IL-28 (Fig. 5C). Consistent with these in vitro findings, VSV-mediated therapy of B16ova tumors in mice depleted of NK cells was significantly decreased compared with that seen in VSV-treated, nondepleted animals (P = 0.01; Fig. 5D).
Figure 5.
IL-28 activates the NK recognition of B16ova. A and B, B16ova (A) or B16(LIF) (B) were cultured +/− recombinant IL-28 for 24 h and cDNA was screened for expression of NK ligands RAE, H60, and MULT-1. *, signal detected upon additional 20 cycles. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. C, NK cells were cocultured with B16ova (a–i) or B16(LIF) (a’-i’) +/− IL-28 or 48-h conditioned medium from C57Bl/6 bone marrow cells exposed to VSV (MOI, 0.1). Twenty-four hours later, supernatants were assayed for IFN-γ. D, mice (n = 8/group) bearing 4d B16ova s.c. tumors were depleted of NK cells by anti–asialo-GM-1 antibody or mock depleted (control IgG). VSV was injected intratumorally on days 7, 9, and 11. **, P < 0.01.
Taken together, these data show that IL-28 treatment of B16ova cells induces both the expression of NK ligands and NK cell activation, and that these responses correlate very well with both VSV-activated, IL-28-dependent bone marrow cytotoxicity against B16ova in vitro, as well as the NK dependence of VSV-mediated therapy of B16ova tumors in vivo.
IL-28/IL-28R expression mediates VSV therapy
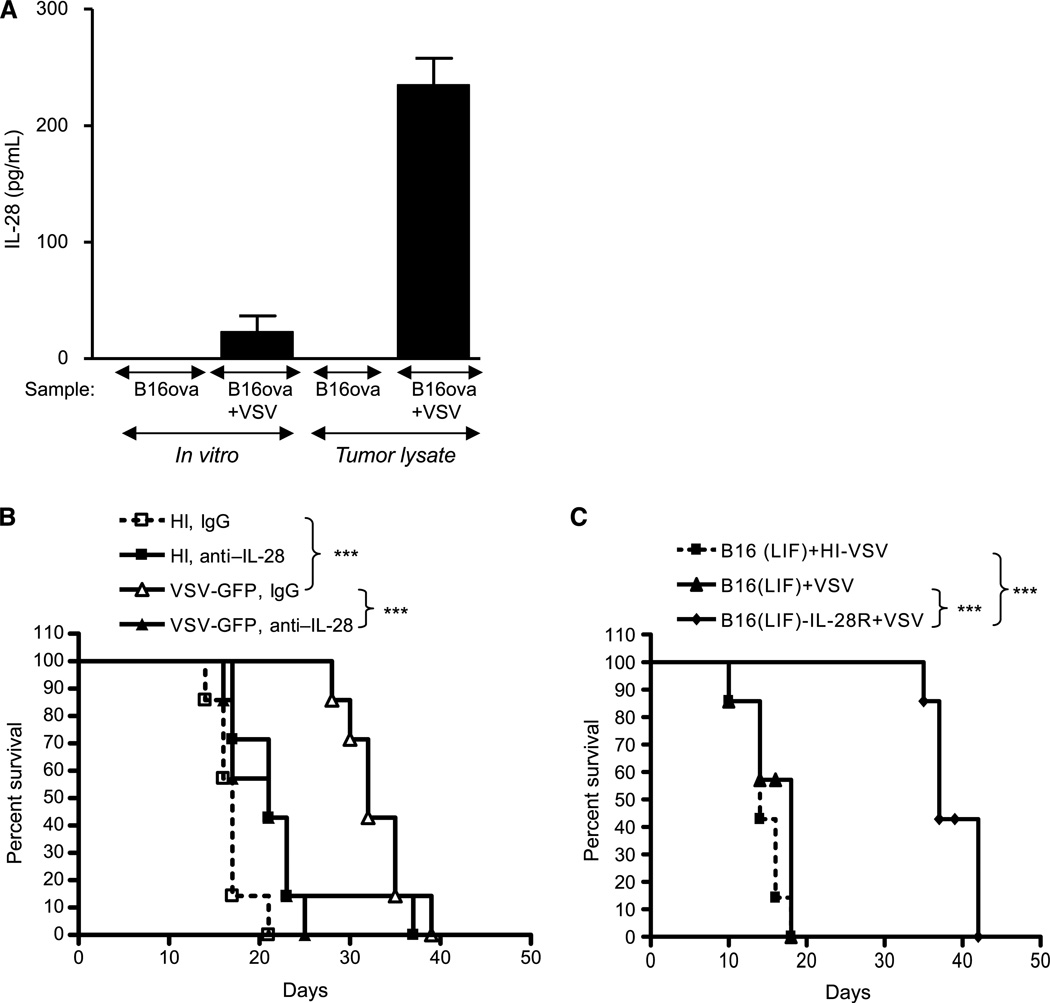
Finally, we tested the role of IL-28 in the VSV treatment of tumors in vivo. B16ova tumor lysates expressed IL-28 only following exposure to VSV in vivo (Fig. 6A). When VSV-induced intratumoral IL-28 was neutralized by antibody blockade, antitumor efficacy was lost and was no better than control treatments (P = 0.0002 VSV+ control IgG versus VSV +anti–IL-28Ab; Fig. 6B). Our hypothesis from the gene chip analysis was that B16(LIF) tumors are not amenable to treatment with VSV in vivo (Fig. 2C) because of a lack of expression of the IL-28R (Fig. 2D). To test this hypothesis, we transduced B16(LIF) cells with a lentiviral vector expressing the IL-28R. Unlike parental B16(LIF) cells, B16(LIF)-IL-28R populations were as effective at activating NK cells as were B16ova cells, following treatment with IL-28 in the assay of Fig. 5C (inducing >700 pg/mL of IFN-γ from cocultured NK cells when treated with IL-28). As usual, intratumoral injection of VSV into established subcutaneous B16(LIF) tumors was not significantly more effective than control treatments (Fig. 6C). However, although nontreated B16(LIF)-IL-28R tumors grew at an identical rate to B16(LIF) tumors (data not shown), VSV treatment of B16(LIF)-IL-28R tumors induced highly significant delays of tumor growth [P = 0.0008 compared with VSV-treated B16(LIF)] and extended the survival times of mice to levels similar to those seen with VSV treatment of B16ova tumors (Fig. 2C and Fig. 6B). Therefore, blockade of VSV-induced IL-28 expression in vivo inhibits VSV-mediated antitumor therapy of B16ova tumors and, by restoring the expression of the IL-28R to B16(LIF) cells, this VSV-insensitive tumor was converted into one in which VSV has antitumor efficacy.
Figure 6.
IL-28 signaling mediates the VSV therapy of B16ova tumors. A, freeze thaw lysates of in vitro cultured B16ova or B16ova infected with VSV (MOI, 0.1) 24 h previously or of 7d–established B16ova tumors from mice treated intratumorally with either PBS or VSV (5 × 108 pfu) 24 h previously were assayed for IL-28. B, C57Bl/6 mice (n = 8/group) bearing 7d–established subcutaneous B16ova tumors were injected intratumorally at days 7, 9, and 11 with 5 × 108 pfu of heat-inactivated virus (HI) or VSV along with anti–IL-28 or control antibody. C, C57Bl/6 mice (n = 8/group) bearing 7d–established B16(LIF) or B16(LIF)-IL-28R tumors were injected intratumorally with 5 × 108 pfu of heat-inactivated VSV (HI-VSV) or VSV on days 7, 9, and 11. ***, P < 0.001.
Discussion
Our data are consistent with a model in which the innate immune response to intratumorally injected VSV mediates therapy in the B16ova/C57Bl/6 immune-competent model, in large part through the activity of the type III IFN IL-28. These findings are consistent with our previous reports that the therapeutic efficacy of oncolytic VSV in this model requires host-derived cells such as NK cells (27); is not dependent on multiple rounds of virus replication; and is associated with a rapidly evolving, and resolving, innate response to virus in the tumor (21, 23).
We show here that a subset of bone marrow–derived cells sense virus presence/infection by secreting type I and III IFNs. Additional immune subsets then respond by clearing infected as well as uninfected bystander cells, thereby suppressing ongoing viral infection. We believe that this cytotoxicity against tumor cells is largely responsible for the antitumor effects of VSV treatment of B16ova tumors (Fig. 2C).
The B16ova and B16(LIF) B16–derived cell lines have been cultured separately for several years but show almost identical sensitivity to VSV in vitro (Fig. 2). However, although we consistently achieve therapy of B16ova tumors with intratumoral VSV, B16(LIF) tumors are insensitive to VSV-mediated therapy (Fig. 2C). Using gene chip analysis, we identified a possible molecule, the receptor for IL-28, which might explain this difference (Fig. 2D).
IL-28, or IFN-λ, a newly identified class two cytokine receptor ligand (35–37), is produced in response to viral infection and has antiviral activity (35–37). Although IL-28 signaling pathways are similar to those of IFN type I (38), IL-28 interacts with a different receptor complex constituted by the IL-10 receptor β (IL-10Rβ) and IL-28Rα chains (35, 36). IL-28 also has an antitumor activity in many tumor models (39–41).
B16(LIF) cells express very low levels of IL-28R (Fig. 2D), are not killed by VSV-activated bone marrow cells in vitro (Fig. 2A and B), do not respond to IL-28 by inducing NK ligands (Fig. 5B), cannot activate NK cells in vitro (Fig. 5C), and are not sensitive to VSV in vivo (Fig. 2C). In contrast, B16ova cells express high levels of IL-28R (Fig. 2D), are sensitive to VSV-activated bone marrow cytotoxicity (Fig. 1–Fig. 3), upregulate NK ligands, activate NK cells in response to IL-28 (Fig. 5), and are sensitive to intratumoral VSV (Fig. 2C). These data explain our previous findings that NK cells mediate the VSV therapy of B16ova tumors (27). Importantly, neutralization of VSV-induced IL-28 abrogated VSV therapy in vivo (Fig. 6A and B). Finally, a VSV-insensitive B16(LIF) tumor was converted into a VSV-responsive tumor by overexpressing the IL-28R in the B16(LIF) parental cells (Fig. 6C).
These data show that innate immune signaling through VSV-induced IL-28, rather than viral replication/oncolysis, is a critical mediator of antitumor therapy with this oncolytic virus. Future efforts aimed at suppressing the innate response as an adjunct to virotherapy should, therefore, take into account that important immune adjuvant benefits may be lost in the process.
Our data also suggest a hierarchy of control and separation of function between the type I and type III IFNs in the response to VSV. Bone marrow from IFN-α/βR KO mice did not express IL-28 in response to VSV, suggesting that the type I IFN response is an upstream controller of the type III response. In addition, coculture of B16ova cells with bone marrow cells of IFN-α/βR KO mice failed to suppress VSV replication in vitro (Fig. 3D). Similarly, VSV injected into B16ova tumors in IFN-α/βR KO mice led to viral replication, dissemination, and rapid fatal toxicity. Thus, type I IFNs are highly inhibitory to spread/replication of VSV, as previously described (14, 28, 42). However, blockade of IL-28 in vitro neither increased viral replication (Fig. 3A) nor did it induce viral dissemination/toxicity in vivo (Fig. 6B), consistent with reports that IL-28R KO mice survive VSV infections as well as wild-type mice (43). However, IL-28 was critically important for VSV-mediated antitumor therapy (Fig. 6). Ank and colleagues (43) have previously shown that expression, or otherwise, of the IL-28R is a critical determinant of the ability of cells to acquire sensitivity to the antiviral effects of IL-28. Our results here extend this concept into the fields of both tumor immunology and oncolytic virotherapy, and suggest that screening for IL-28R expression may be a critical prognostic feature for predicting effective responses to oncolytic virotherapy.
We have shown that GR1+ cells sense intratumoral VSV by secreting IL-28 (Fig. 4A) to trigger antitumor effector mechanisms, including the activation of NK cell recognition of tumor cells (Fig. 5). GR1+ plasmacytoid dendritic cells secrete IFNs (44–47), which regulate the acute cellular response to viral infection with VSV. Consistent with this, abrogation of VSV-mediated activation of cytotoxicity against B16ova cells in bone marrow cultures depleted of GR1+ cells (Fig. 4C) was rescued by the addition of IFN-α.
Finally, our results show that screening tumor cells for their in vitro sensitivity to an oncolytic virus may not reflect their sensitivities in vivo, such as the case with B16ova and B16(LIF) cells (Fig. 2). This will facilitate the evaluation of patient tumors, on an individualized basis, to predict whether oncolytic virotherapy is likely to be effective.
In summary, we show here that viral-mediated activation of innate immune signaling is critical for the VSV therapy of B16ova tumors in immune-competent mice. These studies open new avenues to understand how oncolytic viruses interact with the host immune system and how it may be possible selectively to manipulate key immune signals to enhance both viral replication and antitumor immune activation to improve efficacy of oncolytic virotherapies.
Acknowledgments
We thank Toni Higgins for the expert secretarial assistance.
Grant Support
The Richard M. Schulze Family Foundation, the Mayo Foundation, and by NIH grants CA107082, CA130878, and CA132734.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 2.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 3.Everts B, van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005;12:141–161. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]
- 4.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 5.Sinkovics JG, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz) 2008;56(Suppl 1):3–59s. doi: 10.1007/s00005-008-0047-9. [DOI] [PubMed] [Google Scholar]
- 6.Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 7.Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 8.Hadaschik BA, Zhang K, So AI, et al. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 2008;68:4506–4510. doi: 10.1158/0008-5472.CAN-08-0238. [DOI] [PubMed] [Google Scholar]
- 9.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic viro-therapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 10.Stanford MM, McFadden G. Myxoma virus and oncolytic viro-therapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- 11.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 12.Grote D, Russell SJ, Cornu TI, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immuno-deficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 13.Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 14.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 15.Breitbach CJ, Paterson JM, Lemay CG, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 16.Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracel-lular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TL, Abdelbary H, Arguello M, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wein LM, Wu JT, Kirn DH. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 2003;63:1317–1324. [PubMed] [Google Scholar]
- 19.Thomas MA, Spencer JF, La Regina MC, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 20.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galivo F, Diaz RM, Wongthida P, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2009 doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grote D, Cattaneo R, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulo-cyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- 23.Prestwich RJ, Errington F, Diaz RM, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DL, Fraser NW. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol Ther. 2003;8:543–51. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 25.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 26.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 27.Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 28.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 29.Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma viro-therapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- 30.Wakimoto H, Ikeda K, Abe T, et al. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol Ther: J Am Soc Gene Ther. 2002;5:275–282. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 31.Linardakis E, Bateman A, Phan V, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- 32.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balachandran S, Roberts PC, Kipperman T, et al. α/β interferons potentiate virus-induced apoptosis through activation of the FADD/ Caspase-8 death signaling pathway. J Virol. 2000;74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 35.Kotenko SV, Gallagher G, Baurin VV, et al. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 37.Vilcek J. Novel interferons. Nat Immunol. 2003;4:8–9. doi: 10.1038/ni0103-8. [DOI] [PubMed] [Google Scholar]
- 38.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-λ 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 39.Lasfar A, Lewis-Antes A, Smirnov SV, et al. Characterization of the mouse IFN-λ ligand-receptor system: IFN-λs exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 40.Numasaki M, Tagawa M, Iwata F, et al. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 41.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-λ in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 42.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 43.Ank N, Iversen MB, Bartholdy C, et al. An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 44.Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 45.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells-virus experts of innate immunity. Semin Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 47.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacy-toid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]