Abstract
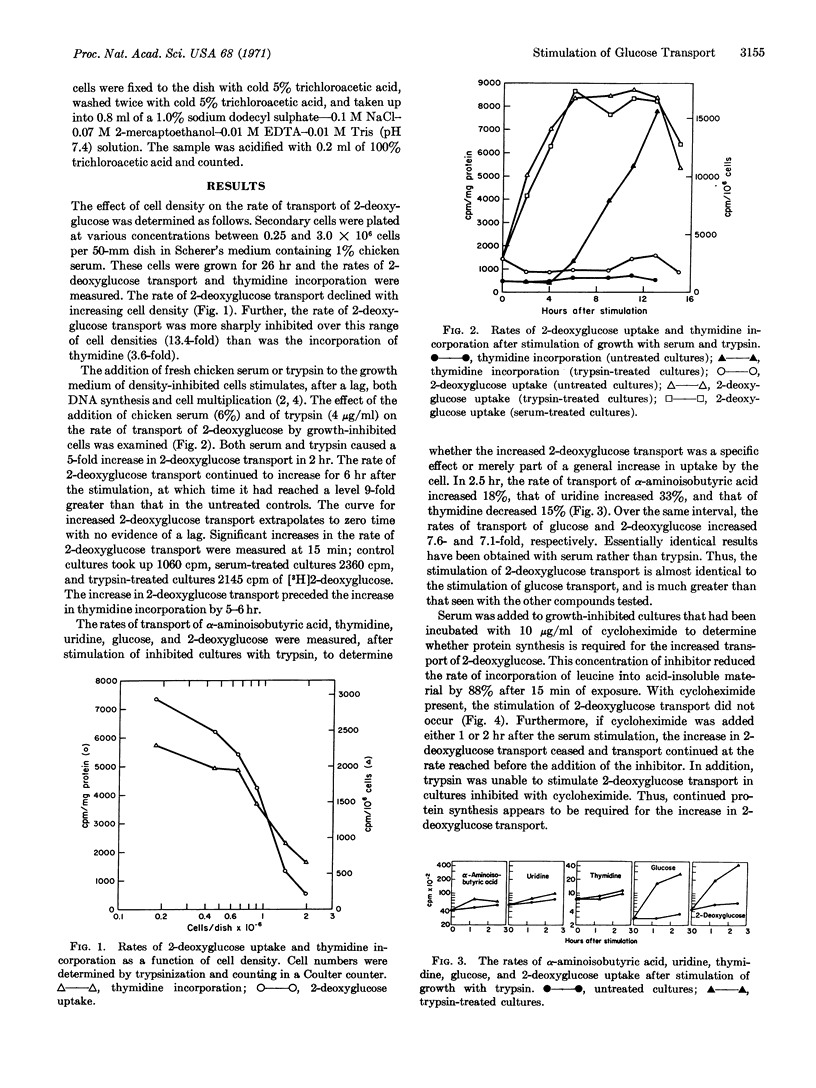
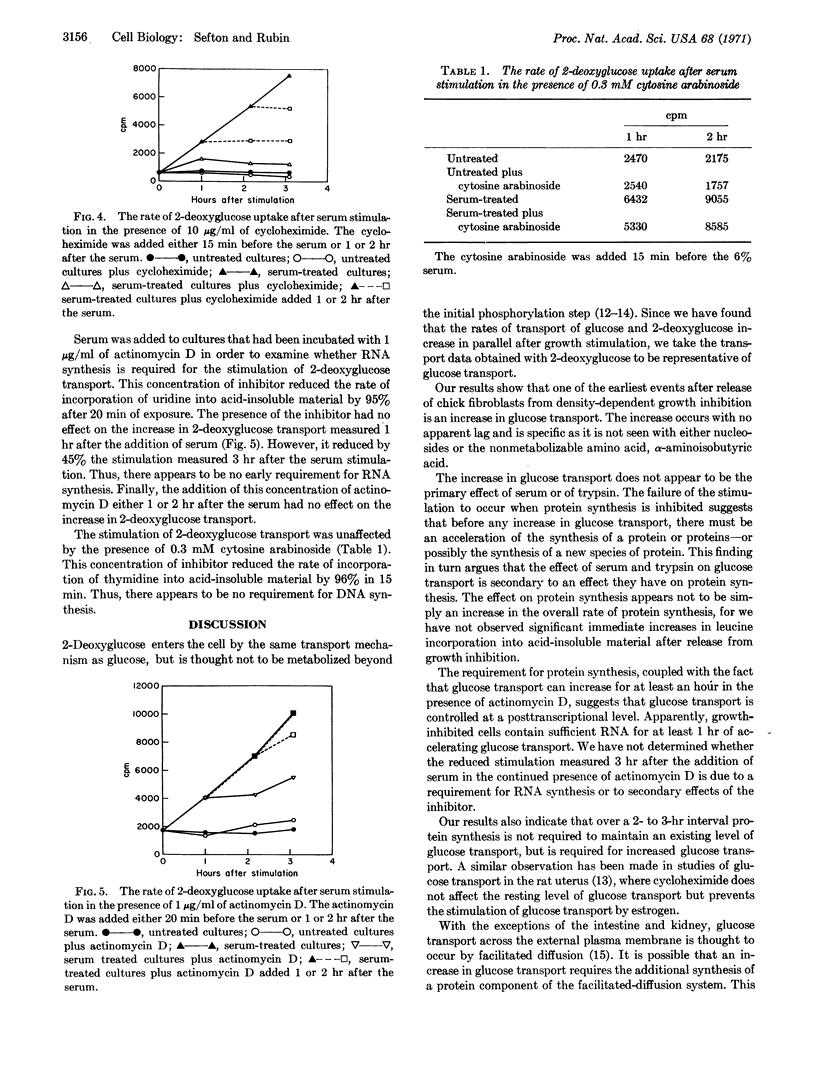
The rate of glucose transport in sparse, rapidly growing chick-embryo fibroblasts is much greater than that in density-inhibited cells. The addition of fresh chicken serum or trypsin to the medium of density-inhibited cells causes a large increase in the rate of glucose transport that is detectable 15 min after addition. The increase in glucose transport precedes the increase in DNA synthesis by 5-6 hr. Only small changes in rates of transport are seen with nucleosides or a nonmetabolizable amino acid. The increase in glucose transport requires protein synthesis but not RNA or DNA synthesis.
Keywords: serum, trypsin, cycloheximide, actinomycin D, 2-deoxyglucose
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr Local stimulation of growth in primary cultures of chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1969 Mar;62(3):906–911. doi: 10.1073/pnas.62.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Augl C., Gilden R. V. Evidence for a functional change in the plasma membrane of murine sarcoma virus-infected mouse embryo cells. Transport and transport-associated phosphorylation of 14C-2-deoxy-D-glucose. J Biol Chem. 1970 Feb 25;245(4):714–717. [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Rubin H., Colby C. Early release of growth inhibition in cells infected with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1968 Jun;60(2):482–488. doi: 10.1073/pnas.60.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Gorski J. Extrogen control of uterine glucose metabolism. An analysis based on the transport and phosphorylation of 2-deoxyglucose. J Biol Chem. 1968 Aug 25;243(16):4169–4174. [PubMed] [Google Scholar]
- Weber M. J., Rubin H. Uridine transport and RNA synthesis in growing and in density-inhibited animal cells. J Cell Physiol. 1971 Apr;77(2):157–168. doi: 10.1002/jcp.1040770205. [DOI] [PubMed] [Google Scholar]


