Abstract
Head and neck squamous cell carcinoma (HNSCC) has a proclivity for locoregional invasion. HNSCC mediates invasion in part through invadopodia-based proteolysis of the extracellular matrix (ECM). Activation of Src, Erk1/2, Abl and Arg downstream of epidermal growth factor receptor (EGFR) modulates invadopodia activity through phosphorylation of the actin regulatory protein cortactin. In MDA-MB-231 breast cancer cells, Abl and Arg function downstream of Src to phosphorylate cortactin, promoting invadopodia ECM degradation activity and thus assigning a pro-invasive role for Ableson kinases. We report that Abl kinases have an opposite, negative regulatory role in HNSCC where they suppress invadopodia and tumor invasion. Impairment of Abl expression or Abl kinase activity with imatinib mesylate enhanced HNSCC matrix degradation and 3D collagen invasion, functions that were impaired in MDA-MB-231. HNSCC lines with elevated EGFR and Src activation did not contain increased Abl or Arg kinase activity, suggesting Src could bypass Abl/Arg to phosphorylate cortactin and promote invadopodia ECM degradation. Src transformed Abl−/−/Arg−/− fibroblasts produced ECM degrading invadopodia containing pY421 cortactin, indicating that Abl/Arg are dispensable for invadopodia function in this system. Imatinib treated HNSCC cells had increased EGFR, Erk1/2 and Src activation, enhancing cortactin pY421 and pS405/418 required for invadopodia function. Imatinib stimulated shedding of the EGFR ligand heparin-binding EGF-like growth factor (HB-EGF) from HNSCC cells, where soluble HB-EGF enhanced invadopodia ECM degradation in HNSCC but not in MDA-MB-231. HNSCC cells treated with inhibitors of the EGFR invadopodia pathway indicated that EGFR and Src are required for invadopodia function. Collectively our results indicate that Abl kinases negatively regulate HNSCC invasive processes through suppression of an HB-EGF autocrine loop responsible for activating a EGFR-Src-cortactin cascade, in contrast to the invasion promoting functions of Abl kinases in breast and other cancer types. Our results provide mechanistic support for recent failed HNSCC clinical trials utilizing imatinib.
Keywords: Abl, imatinib mesylate, invadopodia, invasion, head and neck cancer, cortactin
Introduction
HNSCC is an aggressive disease characterized by extensive locoregional invasion and cervical lymph node metastasis (1,2). Overexpression of EGFR is common in HNSCC and correlates with enhanced invasion and nodal involvement (3–6). EGFR inhibition as adjuvant therapy in HNSCC increases survival, highlighting the importance of downstream EGFR signaling pathways in HNSCC progression (7). Downstream EGFR signaling cascades in HNSCC that promote invasion and metastasis utilize Src, Erk, PI3 kinase, Akt and STATs (8–11), supporting a role for EGFR-generated signals as important regulators of invasion promoting pathways in HNSCC.
Src kinase activation within the EGFR pathway is critical for driving tumor invasion (12,13). Elevated Src expression and activity is frequently found in HNSCC and other tumor types, where it has become a focus for targeted therapeutic design (14,15). While Src targeted drugs have been developed and demonstrate anti-invasive properties in preclinical studies (16–19), recent phase II trials demonstrate virtually no benefit for HNSCC patients with monotherapeutic Src inhibitor treatment (19,20). While combination therapy with receptor tyrosine kinase inhibitors increases efficacy (21–23), a clearer mechanistic understanding of how Src-based signaling governs HNSCC invasion is needed for the development of improved therapeutic strategies.
In carcinomas, Src activation results in the formation of invadopodia, actin-rich membraneous protrusions responsible for extracellular matrix (ECM) proteolysis, allowing tumor cells to infiltrate the stroma and vasculature (24,25). Src kinase activity regulates the cyclic development of non-degradative (pre-invadopodia) and active (mature) invadopodia (24,25). Maturation of pre-invadopodia involves recruitment and activation of matrix metalloproteinase MMP-14 to initiate ECM degradation (26–28).
Invadopodia maturation also involves increased F-actin polymerization responsible for driving plasma membrane protrusion (24,25,29). A prominent component of the invadopodia F-actin core is cortactin, an F-actin binding protein that activates the actin related protein (Arp)2/3 complex to stimulate branched actin polymerization (30–33). Cortactin binds neuronal Wiskott-Aldrich Syndrome protein (N-WASp), a second activator of Arp2/3 complex following phosphorylation of cortactin S405 and S418 by Erk1/2 (33–35). Cortactin modulates shifting of pre- to mature invadopodia through phosphorylation of two Src-targeted tyrosine residues (Y421 and Y470 in humans) (27,28,36). Y421 and Y470 phosphorylation results in SH2-dependent recruitment of the adaptor protein NCK1, which in turn binds and activates N-WASp to promote additional Arp2/3 activation (28,37–39). Along with tyrosine phosphorylation, cortactin domains that bind Arp2/3 and N-WASp are also required for invadopodia formation, collectively highlighting the importance of cortactin in invadopodia biogenesis and regulation (28,40,41).
The Abelson kinases Abl and Arg regulate actin cytoskeletal remodeling during motility and invasion (42–44). While Abl regulation of leukemic tumorigenesis is well established (45,46), Abl activity in solid tumors promotes multiple aspects of neoplastic progression, including increased invasion and metastasis (47–50). Activation of Abl and Arg downstream of EGFR and Src leads to direct cortactin phosphorylation at Y421 and Y470 responsible for invadopodia maturation in breast cancer and melanoma cell lines (51–55). In particular, Src-mediated activation of Arg and subsequent cortactin tyrosine phosphorylation has led to the proposal that Arg is the terminal kinase responsible for cortactin tyrosine phosphorylation required for invadopodia maturation (51,56).
Since invasive HNSCC typically contains elevated EGFR and Src activity, we postulated that downstream activation of Abl kinases may regulate invadopodia through cortactin phosphorylation in this tumor type. Paradoxically, we show that elimination of Abl expression results in enhanced invadopodia-based gelantinase activity in multiple HNSCC cells lines but not in MDA-MB-231 cells. Inhibition of Abl family kinase activity with imatinib mesylate (STI571; Gleevac) in HNSCC cells resulted in enhanced invadopodia maturation and cell invasion, whereas these processes were impaired in MDA-MB-231. Analysis of EGFR signaling indicates that EGFR and Src are hyperactivated in HNSCC compared to MDA-MB-231 cells. Introduction of active Src into Abl−/−/Arg−/− cells induced invadopodia formation, ECM matrix degradation and cortactin tyrosine phosphorylation, suggesting that elevated Src activity can bypass the requirement for Abl or Arg in invadopodia maturation. Imatinib treatment of HNSCC cells resulted in dose-dependent activation of EGFR, Src and Erk1/2, resulting in elevated cortactin tyrosine and serine phosphorylation absent in treated MDA-MB-231 cells. Imatinib enhanced production and shedding of the EGFR ligand HB-EGF in HNSCC cells, where soluble HB-EGF stimulated HNSCC ECM degradation. Inhibition of Src and Abl kinases with the dual specificity drug saracatinib suppressed EGFR activation and ECM degradation in HNSCC, suggesting that Src is responsible for mediating the pro-invasive signals resultant from imatinib-mediated Abl family kinase inactivation. Our results indicate that in HNSCC Abl kinases serve to suppress invadopodia formation and tumor cell invasion by downregulating autocrine HB-EGF activation of the EGFR-Src-cortactin signaling pathway, in contrast to the pro-invasive function of Abl and Arg in breast and other solid tumors. These results suggest that Abl kinase function in cancer invasion is context dependent, providing molecular insight into the mechanism behind the recent failure of clinical trials with imatinib in HNSCC patients (57).
Results
Abl expression suppresses invadopodia activity in HNSCC cells
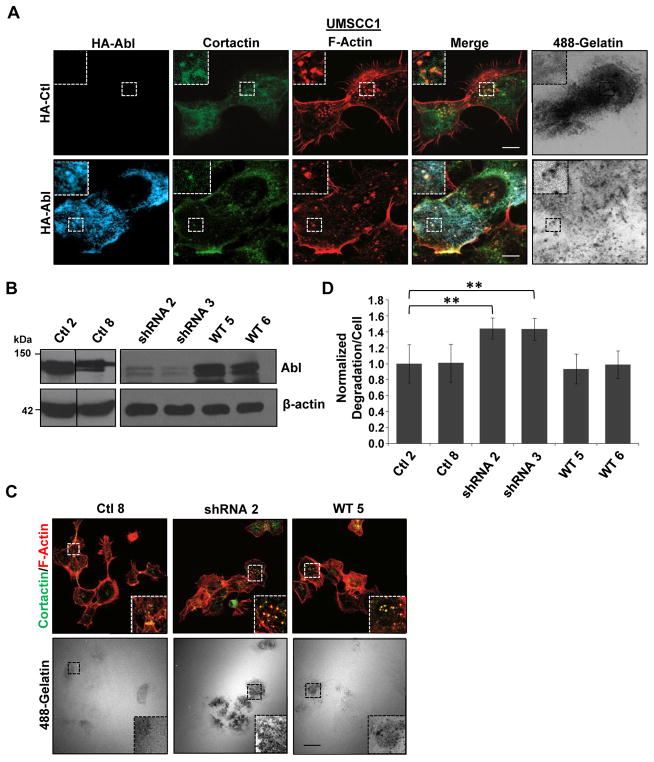
Since HNSCC cells form invadopodia (36,58) and Abl kinases mediate invadopodia function in other tumor types (51,53,55), we evaluated the role of Abl in HNSCC invadopodia formation and function. HA-tagged Abl localized within UMSCC1 invadopodia (Figure 1a) and in Src-expressing 1483 cells (Supplementary Figure 1a), implying a functional role. This was investigated by knockdown of Abl expression using RNA interference (RNAi). Stable UMSCC1 clones expressing an Abl specific short hairpin RNA (shRNA2 and 3) reduced Abl expression by 65% compared to controls (Ctl2 and 8) (Figure 1b). Expression of wildtype (WT) murine Abl in shRNA cells restored expression to endogenous levels (WT5 and 6). Abl knockdown resulted in a 44% increase in gelatin degradation compared to control (Ctl) and WT lines (Figure 1c–d) without affecting the number of cells degrading ECM or the number of invadopodia per cell (Supplementary Figure 1b–c). Abl knockdown in MDA-MB-231 cells did not impact matrix proteolysis (Supplementary Figure 2a–c), in agreement with previous results (51). These data suggest that Abl expression negatively regulates invadopodia function in HNSCC cells, as opposed its invadopodia promoting role in other tumor cell types (51,53,55).
Figure 1.
Abl expression inhibits invadopodia activity in HNSCC cells. (a) UMSCC1 cells transfected with empty vector (HA-Ctl) or HA-tagged Abl (HA-Abl) were plated on Oregon Green 488-gelatin coated coverslips (pseudocolored white) and incubated for 24 h. Cells were fixed and labeled with anti-HA (blue), anti-cortactin (green) and rhodamine-phalloidin (F-Actin; red). Boxed areas and corresponding insets denote regions of HA-Abl localization with invadopodia markers and regions of matrix degradation. (b) Western blot analysis of Abl expression. Cell lysates (100 μg) from UMSCC1 control (Ctl), shRNA Abl knockdown (shRNA) and wildtype (WT) Abl rescued shRNA cells. Parallel blots were probed for β-actin to confirm equal protein loading across all lines. Numbers denote different independent clones. (c) Representative confocal images of gelatin matrix degradation assays conducted with the indicated UMSCC1 clonal cell lines described in (b). Cells were plated on Oregon Green 488-gelatin coated coverslips (pseudocolored white) for 9 h, fixed and labeled with an anti-cortactin antibody (green) and rhodamine-phalloidin (F-Actin; red). The amount of gelatin degradation per cell area from four independent experiments was quantified in (d). Data are represented as mean ± C.I.; ** P≤0.01. Scale bars: 10 μm in (a), 30 μm in (c).
Imatinib treatment enhances HNSCC invadopodia activity
To determine if Abl kinase activity was responsible for the negative regulatory effects on invadopodia function in HNSCC cells, OSC19 and UMSCC1 cells were treated with the Abl family kinase inhibitor imatinib mesylate. Analysis of Crk phosphorylation confirmed partial inhibition of Abl kinase activity in imatinib-treated lines (Figure 2a). Imatinib treatment resulted in a dose-dependent increase in OSC19 and UMSCC1 ECM degradation, demonstrating a net 2.5-3.0-fold enhancement observed at the highest tolerated concentration (15 μM) (Figure 2b–c, 2e). In contrast, MDA-MB-231 cells treated with imatinib resulted in a 70% reduction in matrix proteolysis (Figure 2d–e). The effects on treated HNSCC and MDA-MB-231 cells is likely Abl family kinase specific, since the alternative imatinib target PDGFR is not expressed in these lines (Supplementary Figure 3). These data indicate that imatinib impairment of Abl family kinase activity in HNSCC relieves the inhibitory effect of Abl on invadopodia function, promoting ECM degradation.
Figure 2.
Targeted inhibition of Abl family kinases with imatinib has divergent affects on invadopodia activity in HNSCC and breast cancer cells. (a) OSC19, UMSCC1 (HNSCC) and MDA-MB-231 (breast cancer) cells were treated with the indicated concentrations of imatinib (STI571) for 24 h, lysed and 25 μg of cell lysate analyzed by Western blotting with anti-pY221 Crk to determine drug efficacy. The ratio of pY221 Crk phosphorylation to total Crk levels determined by densitometry is displayed between blots. Blots are representative from 3–4 independent experiments for each cell line. (b) OSC19, (c) UMSCC1, and (d) MDA-MB-231 cells were plated on Oregon Green 488-gelatin coated coverslips (pseudocolored white) in complete media for 1 h to allow attachment. Cells were then serum-starved and treated with GM6001 (10 μM) and either imatinib (STI571) or DMSO vehicle (0 μM) concomitantly for 12 h. After GM6001 washout, cells were incubated with complete media containing 10% FBS for 12 h in the presence or absence of imatinib. Cells were fixed and labeled with an anti-cortactin antibody (green) and rhodamine-phalloidin (F-Actin; red) and visualized using confocal microscopy. Representative images showing cortactin/F-Actin labeling and gelatin degradation for each line and indicated experimental condition are shown. Insets denote areas of invadopodia activity indicated by the presence of overlapping cortactin/F-Actin puncta that co-localize with dark regions of Oregon Green 488-gelatin clearing. Scale bars: 20 μm. (e) Quantification of Oregon Green 488-gelatin degradation for OSC19, UMSCC1 and MDA-MB-231 cells treated with the indicated imatinib (STI571) concentrations. Data from 3 independent experiments for each experimental condition are represented as mean ± C.I.; ** P≤0.01.
Imatinib treatment stimulates HNSCC invasion
To determine if imatinib-enhanced HNSCC invadopodia activity corresponds with increased invasive behavior, 3D invasion assays were conducted using tumor spheroids embedded in collagen I. OSC19 and UMSCC1 cells treated with 10 μM imatinib resulted in enhanced invasion, whereas invasion of imatinib-treated MDA-MB-231 cells was inhibited after 24 h (Figure 3a–c). Average invaded distances were increased by 100 μm for OSC19 and 204 μm for UMSCC1 cells, while MDA-MB-231 invasion was suppressed by 47 μm (Figure 3d). These results indicate that imatinib treatment has opposing effects on tumor cell invasion, enhancing HNSCC cell invasion while impairing the invasiveness of MDA-MB-231.
Figure 3.
Imatinib enhances HNSCC invasion through 3D collagen matrices. (a) OSC19, (b) UMSCC1 and (c) MDA-MB-231 tumor cell spheroids pre-treated with 10 μM imatinib (STI571) or DMSO vehicle (0 μM) for 24 h were embedded in collagen I (0 Hr). Spheroids were further incubated in complete media without (0 μM) or with imatinib for an additional 24 h and invasion monitored by phase contrast microscopy. White circles indicate the maximum radial distance traveled by invaded cells. Scale bars: 200 μm. (d) Quantification of average invasive distance traveled by cells in each experimental condition (n ≥12 spheroids assayed per cell line and treatment). Data are represented as mean ± C.I.; ** P≤0.01; * P≤0.05.
Activated Src can bypass Abl and Arg to promote invadopodia formation
To address the contrasting invasive roles of Abl family kinases in HNSCC and MDA-MB-231 cells, protein levels and activation of known invadopodia kinases were evaluated. Western blotting demonstrated increased Abl expression in HNSCC lines compared to MDA-MB-231 cells, whereas Arg protein levels were equivalent (Figure 4a). In spite of increased Abl expression in HNSCC cells, Abl activation was not enhanced, as evidenced by similar Crk pY221 levels between HNSCC and MDA-MB-231 cells. However, OSC19 and UMSCC1 consistently displayed increased EGFR and Src activity compared to MDA-MB-231 (Figure 4a).
Figure 4.
Elevated Src activity bypasses Abl family kinase regulation of invadopodia function. (a) UMSCC1, OSC19 and MDA-MB-231 cells grown in complete media were analyzed by Western blotting of total cell lysates (25–50 μg) for relative basal activation of EGFR, Src and Abl family kinases with the indicated phosphorylation-specific antibodies. Lysates were immunoblotted in parallel for total EGFR, Src, Crk, Abl and Arg protein levels. (b) Abl−/−/Arg−/− double knockout (DKO) and wildtype (WT) mouse embryo fibroblasts (MEFs) were serum starved overnight, then stimulated with 100 ng/mL EGF for 15 min. Control and stimulated cells were lysed, and 50 μg of total protein assayed by immunoblotting with phosphorylation-specific antibodies against cortactin pY421 (Cort pY421) and total cortactin. The ratio of pY421 cortactin relative to total cortactin levels for each cell type and treatment are shown. (c) WT and DKO cells transiently expressing constitutive active Src (527F) were plated on Oregon Green 488-gelatin coated coverslips for 12 h, fixed, and immunolabeled with anti-cortactin-pY421 (blue), anti-cortactin (green) and rhodamine-phalloidin (F-Actin; red). Cells and matrix were imaged by confocal microscopy. Insets show co-localization of pY421 cortactin within invadopodia at areas corresponding with clearing of Oregon Green 488-gelatin (pseudocolored white). DKO cells expressing GFP-tagged wildtype (WT), 527F, kinase inactive (K295M) Src, co-expressing 527F Src and wildtype (WT) Abl or wildtype (WT) MEFs expressing 527F Src were assayed for the percentage of cells degrading the ECM (d) and for normalized matrix degradation per cell area (e). Data are represented as mean ± C.I. from 3 independent experiments. ** P≤0.01.
The increased Src activation in HNSCC cells suggested that active Src might directly regulate invadopodia activity independent of Abl and Arg. To test this, Abl−/−/Arg−/− mouse embryo fibroblasts (DKO) were used to simultaneously evaluate the role of Abl and Arg on invadopodia function. Lack of Abl and Arg expression in DKO cells was verified by immunoblotting (Supplementary Figure 4a). EGF stimulation of wildtype (WT) MEFs demonstrated a 3-fold increase in cortactin pY421 over non-stimulated controls, whereas stimulated DKO cells showed a 1.5 fold increase over basal levels (Figure 4b). While these results confirm that Abl and Arg contribute to cortactin tyrosine phosphorylation, they also indicate that additional cortactin tyrosine kinases are utilized downstream of EGFR. To determine if activated Src promotes invadopodia formation independent of Abl and Arg, WT and DKO cells transfected with activated Src (527F) were assayed for cortactin tyrosine phosphorylation, invadopodia formation and ECM proteolysis. DKO cells expressing 527F Src contained abundant invadopodia with pY421 cortactin that degraded ECM, similar to 527F Src transformed WT cells (Figure 4c). The ability of DKO cells to degrade gelatin matrix was dependent on Src kinase activity, since kinase inactive Src (K295M) failed to promote matrix proteolysis (Figure 4d). Expression of 527F Src in DKO cells resulted in an 86% increase in matrix degradation area compared to 527F Src expressing WT cells (Figure 4d–e, Supplementary Figure 4b). The amount of gelatin degradation per cell area in DKO 527F Src cells was inhibited by 60% following re-expression of WT Abl (Figure 4d–e), in agreement with increased matrix degradation by UMSCC1 cells with Abl knockdown (Figure 1c–d). Collectively these results indicate that Abl expression suppresses invadopodia activity in Src-transformed mouse embryo fibroblasts, and that Abl and Arg are not essential for cortactin tyrosine phosphorylation or invadopodia formation downstream of active Src.
Imatinib treatment enhances activation of the EGFR invadopodia signaling pathway in HNSCC
To determine the basis for the differential regulation of invadopodia activity and invasion when Abl/Arg activity is suppressed in HNSCC and MDA-MB-231, cells treated with imatinib were evaluated for activation of EGFR and downstream invadopodia signaling components. Increased EGFR activation was observed in imatinib-treated OSC19 and UMSCC1 cells, whereas no increase was observed in MDA-MB-231 (Figure 5a, Supplementary Figure 5a). A corresponding activation pattern was found for Src and Erk. Imatinib ablated Crk pY221 phosphorylation in MDA-MD-231, indicating that Abl/Arg-based signaling was inhibited. Crk pY221 phosphorylation was partially impaired in imatinib-treated HNSCC lines (Figure 5a) and was further downregulated when combined with the Src inhibitor SU6656 (59) (Supplementary Figure 5b), suggesting that Crk is targeted by Src and Abl kinases in HNSCC.
Figure 5.
Imatinib activates the invadopodia kinase pathway in HNSCC cells. (a) OSC19, UMSCC1 and MDA-MB-231 cells grown in complete media were treated with DMSO vehicle (0 μM) or the indicated imatinib (STI571) concentrations for 24 h. Cells were lysed and 25–50 μg of total cell protein were assayed by immunoblotting for activation of EGFR (pY1068), Src (pY418), Erk (pErk) and Abl/Arg kinases (pY221 Crk). Parallel blots were probed for corresponding levels of each assayed protein. Ratios of phosphorylated/total protein are shown (represented as mean from ≥ 3 independent experiments). All blots are representative images. (b) Western blot analysis of cortactin S405 and S418 phosphorylation from cells treated and prepared in (a) with the indicated phosphorylation-specific antibodies. A representative blot was stripped and reprobed with anti-cortactin to verify equal loading. (c) OSC19, UMSCC1 and MDA-MB-231 cells treated as in (a) were lysed and cortactin was immunoprecipitated from 250 μg of cell extract. Immune complexes were assayed by Western blotting for cortactin Y421 phosphorylation (anti-Cort-pY421). Parallel blots with anti-pan-cortactin were conducted to verify cortactin immunoprecipitation.
Since Src and Erk regulate invadopodia in part by phosphorylating cortactin (28,36,40), cortactin tyrosine and serine phosphorylation was evaluated in imatinib-treated HNSCC and MDA-MB-231 cells. The Erk-targeted cortactin residues S405 and S418 demonstrated elevated phosphorylation in imatinib-treated OSC19 and UMSCC1 cells, corresponding with increased Erk 1/2 activation, while treated MDA-MB-231 cells did not demonstrate a substantial increase in cortactin phosphorylation (Figure 5b). Imatinib stimulated cortactin Y421 phosphorylation in HNSCC cells (Figure 5c), likely due to increased Src activation (Figure 5a). These data support activation of the EGFR-Src/Erk-cortactin pathway by imatinib in HNSCC cells that can bypass Abl/Arg inhibition to promote invadopodia activity and HNSCC invasion.
Imatinib stimulates HB-EGF synthesis and promotes HNSCC invadopodia activity
Imatinib treatment of multiple HNSCC lines results in synthesis and secretion of the EGFR ligand HB-EGF, enhancing EGFR activity (60). Lysates from imatinib treated OSC19 and UMSCC1 cells contained increased HB-EGF compared to controls, whereas HB-EGF levels in MDA-MB-231 cells were unaltered (Figure 6a). Conditioned media from imatinib-treated HNSCC cells contained increased soluble HB-EGF at levels 2.5-3.0 fold higher than from MDA-MB-231 cells (Figure 6b). Addition of recombinant HB-EGF to cells at concentrations equivalent to HB-EGF levels in imatinib-treated conditioned media enhanced ECM degradation activity by 86% in OSC19 and 30% in UMSCC1 cells, but did not increase invadopodia activity in MDA-MB-231 cells (Figure 6c–d). These results suggest that increased imatinib-induced HB-EGF expression and shedding by HNSCC cells produces an autocrine loop that stimulates EGFR activation responsible for enhancing invadopodia-mediated ECM proteolysis.
Figure 6.
Imatinib induced HB-EGF stimulates HNSCC invadopodia ECM degradation. (a) OSC19, UMSCC1 and MDA-MB-231 cells treated with DMSO (0 μM) or imatinib mesylate (STI571, 10 μM) for 24 h were lysed and 100 μg cell protein assayed by Western blot analysis for HB-EGF. Bracketed HB-EGF shows different HB-EGF post-translationally modified forms. Positive control (+ Ctl) recombinant HB-EGF ectodomain is denoted by an asterisk (*). A parallel blot was probed with anti-β-actin to confirm equal protein loading. (b) HB-EGF ELISA analysis of conditioned media from cells treated with the indicated imatinib (STI571) concentrations. Concentrations of cleaved, soluble HB-EGF were adjusted in accordance with total cellular protein levels after treatment from each cell line to allow cross comparison. (c) Soluble HB-EGF promotes HNSCC invadopodia ECM degradation. OSC19, UMSCC1, and MDA-MB-231 cells were plated on Oregon Green 488-gelatin coated coverslips in complete media for 2 h to allow attachment and stimulated with recombinant HB-EGF (0, 25, or 250 pg/mL) for 7 h. HB-EGF concentrations were calculated from average levels present in conditioned media of imatinib-treated cells. Cells were fixed and labeled with an anti-cortactin antibody (green) and rhodamine-phalloidin (F-Actin; red) and visualized using confocal microscopy. Insets denote areas of invadopodia activity. Scale bars: 20 μm. (d) Quantification of Oregon Green 488-gelatin degradation from OSC19, UMSCC1 and MDA-MB-231 cells treated with the indicated HB-EGF concentrations as in (c). Data are represented as mean ± C.I.; ** P≤0.01; *P ≤ 0.05.
Invadopodia ECM degradation promoted by impaired Abl kinase activity requires activation of EGFR and Src
To further confirm that Src regulates HNSCC invadopodia downstream of EGFR apart from Abl kinases, pharmacological agents targeting EGFR, Src, or simultaneous Abl/Src inhibition were evaluated for their impact on gelatin degradation in HNSCC. UMSCC1 cells treated with the EGFR inhibitor gefitinib at 5 μM reduced EGFR activation by 60% (Supplementary Figure 6a) and matrix degradation by 50% (Figure 7a). Similar results were obtained with SU6656, where 15 μM treatment resulted in a 65% decrease of the Src-targeted Y410 in p130CAS (Supplementary Figure 6b) and a 50% reduction in matrix degradation (Figure 7b). Treatment of UMSCC1 cells with the dual Abl kinase/Src inhibitor saracatinib, (61,62) at 1 μM inhibited EGFR activity by 55%, Src activity by 93%, and Abl/Arg activity by 97% (Supplementary Figure 6c). ECM proteolysis by was impaired by 80% (Figure 7c). Furthermore, the enhanced ECM degradation activity in UMSCC1 cells with Abl knockdown (Figure 1b–d) was abrogated with 10 μM SU6656 (Figure 7d, Supplementary Figure 6d). These data indicate that the enhanced matrix degradation activity promoted by targeted Abl kinase inhibition in HNSCC requires intact EGFR-Src signaling. Similar results were obtained with saracatinib in OSC19 and MDA-MB-231 cells (Supplementary Figure 7a–c).
Figure 7.
Targeted inhibition of the EGFR-Src pathway impairs HNSCC invadopodia activity. UMSCC1 cells treated with the indicated concentrations of gefitinib (a), SU6656 (b) and saracatinib (c) for 24 h were fixed and evaluated for Oregon Green 488-gelatin degradation by fluorescence microscopy with rhodamine-phalloidin and anti-cortactin antibodies. (d) UMSCC1 control (Ctl 8), shRNA Abl knockdown (shRNA 3) and Abl-rescued shRNA cells (WT 6) were treated with vehicle (0 μM) or SU6656 (10 μM) and assayed for effects on Oregon Green 488-gelatin proteolysis as above. Cells and matrix were imaged by confocal microscopy and the level of matrix degradation quantified for each treatment condition. Data are represented as mean ± C.I.; ** P≤0.01; *P≤0.05.
Discussion
The effects of Abl-based signaling in solid tumors are currently controversial. Several studies have determined that Abl family kinase activity directly contributes to enhancing tumor proliferation, invasion and metastasis in breast and melanoma cell lines (47,48,51,53,55). Abl family kinases also positively modulate tumorgenesis in gastric tumors (50) and non-small cell lung carcinoma (49). On the other hand, recent reports indicate that suppression of Abl kinase activity by imatinib increases breast tumor growth (86), invasion (63) and inhibits epithelial-to-mesenchymal transition (64). Imatinib also enhances thyroid cancer cell invasion (65). While these tumor stimulating findings have been attributed to use of mutationally modified Abl constructs or non-physiological levels of imatinib (53), our results comparing HNSCC lines with MDA-MB-231 cells indicate that Abl kinase inhibition by RNAi or clinically relevant imatinib concentrations yields opposite outcomes on tumor cell invasive events, suggesting that Abl kinases function to suppress HNSCC invasion by reducing invadopodia matrix degradation activity driven through the EGFR-Src-cortactin pathway.
Src activation is central to invadopodia formation and maturation, where phosphorylation of numerous downstream cytoskeletal proteins is required for invadopodia biogenesis and ECM proteolytic activity (24,25). Cortactin phosphorylation downstream of Src is involved in all stages of invadopodia formation (28,36,37,41). Src, Abl and Arg directly phosphorylate cortactin (52,66,67), indicating that these kinases can promote cortactin-based Arp2/3 nucleation activity indirectly via NCK1 and N-WASp. Src can also phosphorylate and activate Abl and Arg downstream of EGFR and other growth factor receptors (54,68) and recent work has shown that removal of Abl or Arg by RNAi prevents invadopodia formation in Src transformed fibroblasts, MDA-MB-231 breast cancer and melanoma cell lines (51,53,55). While these studies indicate that Abl and Arg are the key cortactin kinases responsible for cortactin tyrosine phosphorylation in invadopodia (Figure 8a), we show that EGF stimulation of Abl/Arg-null (DKO) fibroblasts enhances cortactin tyrosine phosphorylation, indicating that Src and/or other EGFR-activated cortactin targeting kinases phosphorylate cortactin apart from Abl or Arg. Src-transformed Abl/Arg-null fibroblasts retain the ability to degrade ECM and contain tyrosine phosphorylated cortactin within invadopodia, demonstrating that Abl family kinases are not essential in this system for invadopodia function driven by cortactin tyrosine phosphorylation. Abl re-expression in DKO cells impaired Src-generated ECM degradation, confirming an inhibitory role for Abl in invadopodia maturation similar to that observed in HNSCC cells. These results indicate that elevated levels of Src activity commonly present in HNSCC lines (36) or due to ectopic expression of active Src forms can circumvent the requirement for Abl or Arg in regulating invadopodia activity through cortactin phosphorylation (Figure 8b).
Figure 8.

Schematic diagram of EGFR-invadopodia signaling pathways altered by Abl inhibition in HNSCC. (a) The current invadopodia pathway depicting EGFR activation to cortactin phosphorylation as primarily determined in breast cancer cell lines. EGFR activation increases Src and Erk1/2 kinase activity, where Src stimulates Abl/Arg activation resulting in direct Abl/Arg cortactin tyrosine phosphorylation, while Erk1/2 directly phosphorylates cortactin S405/418. (b) In HNSCC, inhibition of Abl/Arg results in increased activation of EGFR through elevated HB-EGF synthesis and shedding, resulting in autocrine EGFR hyperstimulation. This leads to elevated Src activity that bypasses Abl/Arg and directly phosphorylates cortactin. Erk1/2 activation is also increased under these conditions, where the combined effect of enhanced Src and Erk1/2 activation increases cortactin phosphorylation and correlates with elevated HNSCC-mediated ECM degradation and invasion.
Imatinib-stimulated HNSCC invadopodia activity and invasion is likely due to increased activation of EGFR and associated downstream signaling, since imatinib treatment enhances EGFR, Src and Erk activation in HNSCC lines while having minimal impact on EGFR signaling in MDA-MB-231 cells. Imatinib treatment of HNSCC cells results in increased cortactin phosphorylation at Src-targeted Y421 and Erk-targeted S405/S418, phosphorylation events present within invadopodia required for ECM matrix degradation activity (40,69,70). As in other HNSCC lines (60), we observed that imatinib treatment increases synthesis and release of the EGFR ligand HB-EGF from OSC19 and UMSCC1 cells at concentrations 10 fold higher than MDA-MB-231 cells. Application of HB-EGF at imatinib treated conditioned media concentrations enhanced invadopodia activity in HNSCC but not in MDA-MB-231 cells. This suggests that HNSCC cells lacking Abl or treated with imatinib generate an autocrine loop, where increased HB-EGF synthesis and shedding in turn binds and activates EGFR to stimulate Src and Erk activation, leading to elevated cortactin phosphorylation and enhanced invadopodia ECM degradation (Figure 8b). HB-EGF induction of HNSCC invadopodia activity likely occurs in part through stimulation and secretion of MMP9 (71,72), which localizes with UMSCC1 invadopodia at sites of ECM degradation (73). While our data are congruent with these findings, it is possible that other EGFR ligands may also be upregulated by Abl kinase suppression. The increased level of EGFR overexpression in HNSCC cells would render this tumor type more responsive to soluble HB-EGF than cell types that contain lower EGFR levels (e.g., MDA-MB-231), which may be the underlying reason for the differential response to Abl knockdown, imatinib and HB-EGF in our analyzed cell lines. Whether such a scenario applies to other EGFR overexpressing cancers and/or tumor types that display pro-invasive behavior in response to Abl kinase suppression will be important to determine.
How Abl kinase inhibition promotes HB-EGF synthesis and shedding in HNSCC is unclear. In addition to cytoskeletal regulation, Abl is a nuclear kinase and work in Abl-null and imatinib-treated fibroblasts indicates that Abl functions to suppress NF-κB activity through stabilization of the NF-κB regulator HDAC1 (74). Elevated NF-κB activity results in increased HB-EGF expression and EGFR activation (75), providing a link between Abl activation and negative regulation of HB-EGF expression. High HB-EGF expression is linked to poor clinical prognosis in HNSCC (76) and low Abl expression in HNSCC correlates with late stage tumors with poor outcome (77), supporting a potential connection between Abl activity and HB-EGF levels in driving HNSCC progression.
Pathway analysis with inhibitors of invadopodia signaling components confirmed that EGFR and Src activation in HNSCC is central to driving invadopodia-based ECM degradation. Simultaneous inhibition of Src and Abl kinases decreased matrix degradation by UMSCC1 and OSC19 cells, reinforcing the point that the elevated invadopodia activity in HNSCC resultant from Abl kinase inhibition requires concurrent Src activation. While preclinical studies on HNSCC lines has shown that saracatinib and the related inhibitor dasatinib impair cell invasion and display potent anti-tumor effects (9,17), phase II trials in HNSCC patients with either drug as a single agent yielded no benefit in spite of apparent Src inhibition (78,79). In contrast, a phase II trial with imatinib administered to HNSCC and NSCLC patients was closed early due to lack of efficacy and antagonistic effects, with a patient subset displaying a worse clinical outcome in response to imatinib (57). While not directly evaluated, these results support a role for Src inhibition in counteracting the pro-invasive effects of Abl kinase that would result in increased patient tumor progression. Although some response has been achieved with imatinib in solid tumors with combination approaches (78,80–82), our results further emphasize the importance of careful patient selection and exclusion criteria for using imatinib or other Abl kinase inhibitors in HNSCC and other solid tumors that display similar characteristics.
We have determined that Abl kinases have a context-dependent role in regulating invadopodia function and tumor invasion. In HNSCC cells Abl serves to suppress invadopodia ECM degradation and tumor invasion by preventing HB-EGF synthesis and extracellular shedding, where it is capable of activating the EGFR/Src/cortactin signaling pathway to accelerate invadopodia-based ECM degradation and tumor cell invasion. This is in contrast to MDA-MB-231 cells, a cell line commonly used to analyze invadopodia and invasive signaling, where Abl kinase inhibition prevents invadopodia activity and impairs invasiveness. These results stress the need for further mechanistic insight into the signaling processes that regulate the pro- and anti-oncogenic roles of Abl in solid tumors in order to prevent detrimental affects of imatinib treatment in ongoing and future patient trials.
Material and Methods
Plasmid constructs
The lentiviral vector pLL5.0 (83) was used for Abl knockdown by subcloning a shRNA targeting human Abl oligonucleotide, (5′GCTCCGGGTCTTAGGCTAT3′; (84)) with HpaI and XhoI sites. For Abl knockdown-rescue experiments, the resulting vector was modified to encode a 6X-HA epitope tag using BamHI and SbfI sites. Human Abl cDNA was PCR amplified from pMSCV-puro Abl (85) and subcloned into EcoRI and BamHI digested pLL5.0 6X-HA. Src-GFP constructs (WT, 527F, and 295M) were used as described (36).
Cell culture, lentiviral infection and transfection
UMSCC1, OSC19 and 1483 cells were maintained as described (36). Abl−/−/Arg−/−, wild-type MEFs, NIH3T3 and HEK 293T/17 cells were cultured as before (86). MDA-MB-231 cells were cultured in alpha Minimum Essential Media (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan UT), and 1% penicillin-streptomycin.
UMSCC1 cells stably infected with pLL5.0 6X-HA, pLL5.0 6X-HA Abl shRNA or pLL5.0 co-expressing Abl shRNA and HA-tagged Abl were generated by puromycin selection following standard methods. 1483, Abl−/−/Arg−/− or wild type MEF cells transiently expressing CMV-Src or HA-Abl constructs were transfected with the Nucleofector I device (Amaxa Biosystems, Berlin, Germany).
Western blotting, antibodies and immunoprecipitation
Western blotting of cell lysates was conducted as described (73). Antibodies used were: anti-Src clone GD11 (1:1000; EMD Millipore, Billerica, MA), anti-pY418 Src (1:1000; Invitrogen, Carlsbad, CA), anti-p130CAS (1:1000; BD Biosciences, San Jose, CA), anti-pY410 p130CAS (1:1000; Cell Signaling Technology, Danvers, MA), anti-cortactin clone 4F11(1 μg/ml, (86)), anti-pY421 cortactin (1:500; BD Biosciences), anti-pS405 cortactin (1:2000; (70)), anti-pS418 cortactin (1:500; (70)), anti-ERK1/2 clone C-14 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-pERK1/2 (1:1000; Santa Cruz), anti-β-actin (1:10,000; EMD), anti-HA (1:200; Covance, Berkley, CA), anti-Crk (1:1000; BD), anti-pY221 Crk (1:1000; Cell Signaling), anti-PDGFR (1:200; Cell Signaling), anti-EGFR (1:1000, BD), anti-pY1068 EGFR (1:1000; BD), anti-Arg (1:500; EMD), anti-HB-EGF (1:200; EMD), and anti-Abl clone 8E9 (1:500; BD). Blots were quantified as described (73).
Immunoprecipitation was conducted from cells lysed in RIPA buffer (69). Clarified lysates (250 μg) were incubated with 5 μg anti-cortactin antibody 4F11 for 2 h at 4 °C. Immune complexes were captured by incubation with 30 μL Protein A/G beads (ThermoFisher Scientific, Rockford, IL) for 1 h, washed with RIPA and analyzed by Western blotting.
Invadopodia matrix degradation assays and fluorescence microscopy
Cells were plated on Oregon Green 488-conjugated gelatin (Invitrogen, Grand Island, NY) coated coverslips (27,87). In cases of inhibitor treatment, cells were allowed to attach for 1 h, then serum starved for 12 h in the presence of 10 μM GM6001 (Sigma) and either imatinib mesylate (LGM Pharmaceuticals, Boca Raton, FL), saracatnib (AstraZeneca, Alderley Park, Cheshire, United Kingdom), gefitinib (AstraZeneca) or SU6656 (EMD). Serum-free media was replaced with complete media containing 10% FBS and kinase inhibitors for 12 h. Cells were rinsed in PBS, fixed in fresh 4% paraformaldehyde and labeled as described (36). Primary antibodies used were anti-cortactin clone 4F11 (1:500), anti-cortactin EP1922Y (1:500; Novus Biologicals, Littleton, CO), anti-cort-pY421 (1:500), anti-Src GD11 (1:500), anti-Abl clone 8E9 (1:200), anti-GFP (1:500; Invitrogen), and anti-HA (1:200). Primary antibodies were visualized using Alexa Fluor 405 and 647 conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:2000; Invitrogen). F-actin was visualized using rhodamine-conjugated phalloidin (1:500; Invitrogen). Cells were mounted with ProLong Gold (Invitrogen) and images were acquired with a Zeiss LSM510 confocal microscope using AIM software (Carl Zeiss MicroImaging, Thornwood, NY). Gelatin degradation was quantified as described previously (87). In brief, ≥ 90 transiently transfected and ≥ 300 lentiviral infected or inhibitor-treated cells evaluated for each condition. For therapeutic treatments and RNAi stable cell lines, the area of degradation and cell area was determined by analyzing the intensity of degraded gelatin or F-actin respectively in an entire field of view utilizing ImageJ software. For transient transfections, the area of degradation and cell area was determined by Image J software on an individual cell basis. The number of invadopodia per cell (n ≥ 50) and number of cells degrading matrix (n ≥ 100) were determined or each independent experiment (n = ≥ 3) (36).
3-D spheroid invasion assays
Cells were labeled with Vybrant® DiI (Invitrogen). 96 well plates were coated with 100 μL of 1.5% noble agar (BD Biosciences, Sparks, MD) in Dulbecco’s PBS. 1 × 103 (OSC19), 5 × 103 (UMSCC1), or 2.5 × 103 (MDA-MB-231) labeled cells were plated into individual wells for 48 h to form spheroids. Two spheroids were transferred to a microcentrifuge tube and centrifuged at 1000 × g for 3 min. The media was aspirated and replaced with 500 μL of 2 mg/mL rat tail collagen I (BD). The spheroid mixture was transferred to an individual well of 24-well plate pre-coated with 400 μL solidified 2 mg/mL collagen I. Plates were incubated for 1 h at 37 °C then overlayed with 1 mL of complete media. Spheroid invasion was visualized by fluorescence microscopy (Zeiss, Axiovert 200M) to establish the central z-axis (0 h) and imaged at 0 and 24 h by phase contrast microscopy. Spheroids were pretreated for 24 h and maintained in media with DMSO vehicle or 10 μM imatinib. Maximal radial distances for invaded cells was calculated using Axiovision 4.6 software (Zeiss).
HB-EGF ELISA assays
HB-EGF specific enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s protocol (Abcam, Cambridge, MA). Cells were treated with imatinib (10μM) or DMSO for 12 h, washed with PBS and incubated for 24 h in serum-free media with imatinib or DMSO. Conditioned media was concentrated to 500 μL, and 100 μL of media incubated overnight at 4°C in HB-EGF antibody-coated microplate strips. Absorbance values were obtained at 450 nm with a Biotek Synergy H1 Hybrid Reader (Winooski, VT). Standard curves were generated and results normalized to total cellular protein concentration for comparison across different cell lines.
Statistical analysis
Differences in mean values between groups were evaluated using Students t-test and significance was determined at P < 0.05. Scale bars represent confidence intervals (C.I.).
Supplementary Material
Acknowledgments
Funding Support: National Institute of Health grants R01 DE014578, P20 RR16440 and the West Virginia University Mary Babb Randolph Cancer Center
We thank Bruce Mayer (University of Connecticut) for Abl constructs, Anthony Koleske (Yale University) for Abl−/−/Arg−/− fibroblasts, Silja Wessler (Paul-Ehrlich Institute) for the Abl shRNA construct, Elena Pugacheva (West Virginia University) for MDA-MB-231LN cells, Jim Bear (University of North Carolina) for pLL5.0 and advice on spheroid assay development. We thank Mark Auble and Barbara Frederick for technical assistance. Saracatinib and gefitinib were provided by AstraZeneca. Supported by NIH grants R01 DE014578, P20 RR16440 (to SAW) and the West Virginia University Mary Babb Randolph Cancer Center. The West Virginia University Microscopy Imaging Facility (supported by the Mary Babb Randolph Cancer, NIH grants P20 RR16440 and P30 RR032138/GM103488) is gratefully acknowledged.
Footnotes
Conflict of Interest
The authors declare they have no competing financial interests in relation to the work described.
References
- 1.Pryor DI, Solomon B, Porceddu SV. The emerging era of personalized therapy in squamous cell carcinoma of the head and neck. Asia Pac J Clin Oncol. 2011 Sep;7(3):236–251. doi: 10.1111/j.1743-7563.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011 Jan;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 3.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006 Jun 10;24(17):2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 4.Hama T, Yuza Y, Saito Y, O-uchi J, Kondo S, Okabe M, et al. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist. 2009 Sep;14(9):900–908. doi: 10.1634/theoncologist.2009-0058. [DOI] [PubMed] [Google Scholar]
- 5.Hama T, Yuza Y, Suda T, Saito Y, Norizoe C, Kato T, et al. Functional mutation analysis of EGFR family genes and corresponding lymph node metastases in head and neck squamous cell carcinoma. Clin Exp Metastasis. 2012 Jan;29(1):19–25. doi: 10.1007/s10585-011-9425-5. [DOI] [PubMed] [Google Scholar]
- 6.Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: Molecular bases for EGFR-targeted therapy. Pathology - Research and Practice. 2011 Jun 15;207(6):337–342. doi: 10.1016/j.prp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010 Sep;15(3):355–373. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nor JE. Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia. 2009 Jun;11(6):583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, Freilino M, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008 Jul 1;14(13):4284–4291. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JL, Qu XJ, Russell PJ, Goldstein D. Interferon-alpha promotes the anti-proliferative effect of Erlotinib (OSI-774) on human colon cancer cell lines. Cancer Lett. 2005 Jul 8;225(1):61–74. doi: 10.1016/j.canlet.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Huang YT, Hwang JJ, Lee LT, Liebow C, Lee PP, Ke FC, et al. Inhibitory effects of a luteinizing hormone-releasing hormone agonist on basal and epidermal growth factor-induced cell proliferation and metastasis-associated properties in human epidermoid carcinoma A431 cells. Int J Cancer. 2002 Jun 1;99(4):505–513. doi: 10.1002/ijc.10373. [DOI] [PubMed] [Google Scholar]
- 12.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010 Apr;223(1):14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 13.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009 Oct;6(10):587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 14.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003 Dec;22(4):337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 15.Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, et al. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007 Mar 15;67(6):2800–2808. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- 16.Chen YS, Wu MJ, Huang CY, Lin SC, Chuang TH, Yu CC, et al. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS One. 2011;6(11):e28053. doi: 10.1371/journal.pone.0028053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 18.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009 Mar 1;69(5):1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;3;33(3):122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010 Jul 15;16(14):3526–3532. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler DL, Iida M, Kruser TJ, Nechrebecki MM, Dunn EF, Armstrong EA, et al. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009 Apr;8(8):696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, Antonia S, et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2010 Mar 10;28(8):1387–1394. doi: 10.1200/JCO.2009.25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer. 2012 Jan 1;118(1):63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011 Jun 23;12(7):413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011 Nov 10;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 26.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009 Sep 1;122(Pt 17):3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 27.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006 Mar 15;66(6):3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 28.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009 Aug 24;186(4):571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011 Oct;23(5):597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Ren G, Crampton MS, Yap AS. Cortactin: Coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell Motil Cytoskeleton. 2009 Oct;66(10):865–873. doi: 10.1002/cm.20380. [DOI] [PubMed] [Google Scholar]
- 31.Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009 Jun;28(1–2):137–149. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 32.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011 Mar-Apr;5(2):187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, et al. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001 Mar 6;11(5):370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 34.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008 Sep;65(9):687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004 Jun;24(12):5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley LC, Ammer AG, Hayes KE, Martin KH, Machida K, Jia L, et al. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J Cell Sci. 2010 Nov 15;123(Pt 22):3923–3932. doi: 10.1242/jcs.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010 Nov 1;123(Pt 21):3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005 Jan 31;168(3):441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008 Feb 1;121(Pt 3):369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 41.Webb BA, Jia L, Eves R, Mak AS. Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur J Cell Biol. 2007 Apr;86(4):189–206. doi: 10.1016/j.ejcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal. 2010 Sep;14;3(139):re6. doi: 10.1126/scisignal.3139re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009 Oct 1;122(Pt 19):3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plattner R, Pendergast AM. Activation and signaling of the Abl tyrosine kinase: bidirectional link with phosphoinositide signaling. Cell Cycle. 2003 Jul-Aug;2(4):273–274. [PubMed] [Google Scholar]
- 45.Ernst T, Hochhaus A. Chronic myeloid leukemia: clinical impact of BCR-ABL1 mutations and other lesions associated with disease progression. Semin Oncol. 2012 Feb;39(1):58–66. doi: 10.1053/j.seminoncol.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Gu M, Mi Y, Qiu L, Bian S, Wang J. Clinical characteristics and outcomes of mixed phenotype acute leukemia with Philadelphia chromosome positive and/or bcr-abl positive in adult. Int J Hematol. 2011 Dec;94(6):552–555. doi: 10.1007/s12185-011-0953-1. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006 Jun 1;66(11):5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 48.Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008 Feb 14;27(8):1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- 49.Lin J, Sun T, Ji L, Deng W, Roth J, Minna J, et al. Oncogenic activation of c-Abl in non-small cell lung cancer cells lacking FUS1 expression: inhibition of c-Abl by the tumor suppressor gene product Fus1. Oncogene. 2007 Oct 25;26(49):6989–6996. doi: 10.1038/sj.onc.1210500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furlan A, Stagni V, Hussain A, Richelme S, Conti F, Prodosmo A, et al. Abl interconnects oncogenic Met and p53 core pathways in cancer cells. Cell Death Differ. 2011 Oct;18(10):1608–1616. doi: 10.1038/cdd.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011 Mar 1;71(5):1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009 May 4;185(3):503–519. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganguly SS, Fiore LS, Sims JT, Friend JW, Srinivasan D, Thacker MA, et al. c-Abl and Arg are activated in human primary melanomas, promote melanoma cell invasion via distinct pathways, and drive metastatic progression. Oncogene. 2011 Sep 5; doi: 10.1038/onc.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999 Sep 15;13(18):2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem. 2010 Dec 17;285(51):40201–40211. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibony-Benyamini H, Gil-Henn H. Invadopodia: The leading force. Eur J Cell Biol. 2012 May 24; doi: 10.1016/j.ejcb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Tsao AS, Liu S, Fujimoto J, Wistuba II, Lee JJ, Marom EM, et al. Phase II trials of imatinib mesylate and docetaxel in patients with metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. J Thorac Oncol. 2011 Dec;6(12):2104–2111. doi: 10.1097/JTO.0b013e31822e7256. [DOI] [PubMed] [Google Scholar]
- 58.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007 May 1;67(9):4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 59.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000 Dec;20(23):9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson FM, Saigal B, Donato NJ. Induction of heparin-binding EGF-like growth factor and activation of EGF receptor in imatinib mesylate-treated squamous carcinoma cells. J Cell Physiol. 2005 Nov;205(2):218–227. doi: 10.1002/jcp.20383. [DOI] [PubMed] [Google Scholar]
- 61.Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006 Nov 2;49(22):6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 62.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006 Mar 1;12(5):1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 63.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006 Aug;8(8):815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 64.Allington TM, Galliher-Beckley AJ, Schiemann WP. Activated Abl kinase inhibits oncogenic transforming growth factor-beta signaling and tumorigenesis in mammary tumors. FASEB J. 2009 Dec;23(12):4231–4243. doi: 10.1096/fj.09-138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frasca F, Vigneri P, Vella V, Vigneri R, Wang JY. Tyrosine kinase inhibitor STI571 enhances thyroid cancer cell motile response to Hepatocyte Growth Factor. Oncogene. 2001 Jun 28;20(29):3845–3856. doi: 10.1038/sj.onc.1204531. [DOI] [PubMed] [Google Scholar]
- 66.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998 Oct 2;273(40):25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 67.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007 Mar 6;17(5):445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 68.Plattner R, Koleske AJ, Kazlauskas A, Pendergast AM. Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol. 2004 Mar;24(6):2573–2583. doi: 10.1128/MCB.24.6.2573-2583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, et al. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003 Aug;14(8):3216–3229. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS One. 2010 Nov 4;5(11):e13847. doi: 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O-charoenrat P, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, Eccles SA. Epidermal growth factor-like ligands differentially up-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res. 2000 Feb 15;60(4):1121–1128. [PubMed] [Google Scholar]
- 72.Ohnishi Y, Inoue H, Furukawa M, Kakudo K, Nozaki M. Heparin-binding epidermal growth factor-like growth factor is a potent regulator of invasion activity in oral squamous cell carcinoma. Oncol Rep. 2012 Apr;27(4):954–958. doi: 10.3892/or.2011.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ammer AG, Kelley LC, Hayes KE, Evans JV, Lopez-Skinner LA, Martin KH, et al. Saracatinib Impairs Head and Neck Squamous Cell Carcinoma Invasion by Disrupting Invadopodia Function. J Cancer Sci Ther. 2009 Nov 30;1(2):52–61. doi: 10.4172/1948-5956.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liberatore RA, Goff SP, Nunes I. NF-kappaB activity is constitutively elevated in cAbl null fibroblasts. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17823–17828. doi: 10.1073/pnas.0905935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Liu R, Lee SW, Sloss CM, Couget J, Cusack JC. Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene. 2007 Mar 29;26(14):2006–2016. doi: 10.1038/sj.onc.1209999. [DOI] [PubMed] [Google Scholar]
- 76.Hatakeyama H, Cheng H, Wirth P, Counsell A, Marcrom SR, Wood CB, et al. Regulation of heparin-binding EGF-like growth factor by miR-212 and acquired cetuximab-resistance in head and neck squamous cell carcinoma. PLoS One. 2010 Sep 13;5(9):e12702. doi: 10.1371/journal.pone.0012702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yanagawa T, Harada H, Iwasa S, Tabuchi K, Omura K, Suzuki H, et al. c-Abl expression in oral squamous cell carcinomas. Oral Oncol. 2000 Jan;36(1):89–94. doi: 10.1016/s1368-8375(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 78.Fury MG, Baxi S, Shen R, Kelly KW, Lipson BL, Carlson D, et al. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) Anticancer Res. 2011 Jan;31(1):249–253. [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks HD, Glisson BS, Bekele BN, Johnson FM, Ginsberg LE, El-Naggar A, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011 May 15;117(10):2112–2119. doi: 10.1002/cncr.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montero JC, Seoane S, Ocana A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011 Sep 1;17(17):5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- 81.Lara PN, Jr, Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, et al. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009 Mar;20(3):179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gucalp A, Sparano JA, Caravelli J, Santamauro J, Patil S, Abbruzzi A, et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin Breast Cancer. 2011 Oct;11(5):306–311. doi: 10.1016/j.clbc.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai L, Makhov AM, Schafer DA, Bear JE. Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell. 2008 Sep 5;134(5):828–842. doi: 10.1016/j.cell.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poppe M, Feller SM, Romer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007 May 24;26(24):3462–3472. doi: 10.1038/sj.onc.1210139. [DOI] [PubMed] [Google Scholar]
- 85.Antoku S, Saksela K, Rivera GM, Mayer BJ. A crucial role in cell spreading for the interaction of Abl PxxP motifs with Crk and Nck adaptors. J Cell Sci. 2008 Sep 15;121(Pt 18):3071–3082. doi: 10.1242/jcs.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, et al. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006 Aug 15;66(16):8017–8025. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- 87.Martin KH, Hayes KE, Walk EL, Ammer AG, Markwell SM, Weed SA. Quantitative Measurement of Invadopodia-mediated Extracellular Matrix Proteolysis in Single and Multicellular Contexts. J Vis Exp. 2012 Aug 27;(66):pii: 4119. doi: 10.3791/4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.