Abstract
Purpose of review
Stem cell therapy for intestinal diseases is an emerging area in clinical gastroenterology. We will review recent literature regarding mesenchymal stem cells (MSCs), which have been utilized in preclinical models and are now headed for clinical trials in several gastrointestinal diseases including inflammatory bowel disease.
Recent findings
Important studies over the last 2 years have made significant inroads into understanding the mechanisms of action of these cell types. The two major competing hypotheses are that MSCs home to areas of injury where they repair based on their stem cell activity or that MSCs act as a source of secreted factors that stimulate repair and inhibit inflammation.
Summary
Mesenchymal stem cells show promise for therapy in a number of intestinal diseases. Further understanding of their mechanism of action should improve our ability to use them therapeutically.
Keywords: inflammatory bowel disease, intestine, macrophage, mesenchymal stem cell, stem cell
Introduction
Cell-based therapies for inflammatory and auto-immune diseases are an emerging area of interest. Intestinal disease targets include inflammatory bowel disease, short gut syndrome and irradiation damage. A major reason why cellular therapy has become attractive for these diseases is that treatment must be multifocal and includes the ability to dampen inflammation, minimize opportunistic infection and stimulate repair. Mesenchymal stem cells (MSCs) have been utilized for this purpose due to their ability to differentiate into multiple cell types within a variety of organs as well as suppress immune functions. Early clinical trials with MSCs have yielded some exciting therapeutic potential, although the precise mechanism of their action is still unclear. Our opinion is that before their complex mechanisms as therapeutics can be fully understood, there must be more focus on the fate of MSCs after transplantation (i.e., location, clearance, and cellular properties). In this review, we will cover the studies in small animal models and relevant clinical studies published in the last 2 years and focus on the properties of transplanted cells as a therapy for intestinal diseases.
General properties of intravenously injected mesenchymal stem cells
MSCs are fibroblast-like cells that can differentiate into various cell types including adipocytes, osteoblasts, and chondroblasts [1]. Human MSCs have been isolated from a variety of tissues most typically by rapid adherence to tissue culture plastic. These cells contain a common immunophenotype of cell surface markers (e.g., positive for CD90, CD73, and CD105 and negative for CD45, CD34 and HLA-DR) as defined by the International Society for Cellular Therapy [2]. MSCs were initially considered for cell-based therapy because of their ability to home and differentiate at sites of injury as well as secrete paracrine mediators that stimulate repair and dampen inflammation [3]. Numerous studies show that intravenous MSC infusions are well tolerated from allogeneic or surprisingly even from xenogeneic donors in small animal models [4, 5]. One mechanism by which MSCs may avoid robust rejection is that they secrete human leukocyte antigen G (HLA-G), a natural inhibitor of ‘allograft’ rejection [6]. This nonclassical HLA class I molecule is best known for its expression in trophoblasts during pregnancy [which lack major histocompatibility complex (MHC) molecules] and protects the fetus from being attacked and rejected by maternal natural killer (NK) cells and T cells. Taken together, these studies have led some investigators to propose that human MSCs are ‘nonimmunogenic’ [7].
Despite the apparent lack of serious complications from MSC infusions however, recent studies show that MSCs are detected by the immune system. For example, NK cells, NKT cells and CD8+ T cells can clear MSCs injected into allogeneic mice [8]. This clearance mechanism includes memory as cells injected a second time are cleared faster. This response is really not surprising given MHC Class I mismatch in this experimental system. In another study, MSCs intracranially injected into allogeneic rhesus macaques stimulated inflammation that was cell-dose and MHC-mismatch dependent [9•]. Interestingly, MSCs upregulated MHC class I and class II molecules and showed less immunosuppressive ability after differentiation [10]. Future studies need to carefully evaluate the long-term immunosuppressive and immunoprivileged characteristics of MSCs, and whether immune recognition leads only to elimination of MSCs or to exacerbated clinical disease.
MSCs are classically isolated from bone marrow, though umbilical cord, adipose tissue, or recently the human gingiva has also been used as a source [11•, 12]. In mice, MSCs have been isolated from nearly every tissue including the intestine [12, 13]. During homeostasis, MSCs appear to be perivascular in location and may be pericytes [14]. Although many early studies used bone marrow MSCs, the need for more substantial quantities of these cells for clinical transplantation has driven studies of MSCs derived from anatomical locations more easily obtained in humans. In addition, more precise methods of isolation are in development. For example, Nestin+ CD45− cells have been identified as MSCs in the bone marrow, a finding that may allow for better isolation and purer populations in future studies [15••].
To date, the most common route of MSC transplantation in human and animal studies is intravenous injection. There are many reports that MSCs introduced in this manner become trapped in the lung, including our own observations after injecting colonic MSCs (Fig. 1).Arecent study actually quantified this phenomenon. When human MSCs were injected intravenously (i.v.) into mice, 99% of the cells disappeared from the blood in 5 min and the majority of cells were trapped as emboli in the lungs [16••]. A small percentage of MSCs (2–3%) reappeared in the blood up to 1 h after injection. The half-life of the trapped MSCs was approximately 24 h. The reason for MSC entrapment in the lungs was their large cell size [17•, 18]. A minor effect was MSC integrin α4 binding VCAM-1 on lung endothelium [17•]. These studies introduce the question about which cells are therapeutic after MSC infusion: the majority of cells that stay trapped in the lung, the rare cells that traffic to sites of injury, or both populations of cells. The effect of MSCs entrapped in the lung versus those that home to sites of injury is still unclear in most cases.
Figure 1. Mesenchymal stem cells injected intravenously are entrapped in the lung.
A section of lung from a mouse intravenously injected with 1×106 colonic MSCs. Emboli typically contain groups of cells with large nuclei (injected MSCs) and are frequently observed trapped in vascular spaces (arrow). MSC, mesenchymal stem cell.
Mesenchymal stem cell role as regenerative cells
MSCs have enormous potential as regenerative cells because of their stem cell properties. One well-established model for in-vivo homing and stem cell activity of injected MSCs is myocardial repair, whereby these cells home to damaged heart tissue and differentiate into cardiomyocytes [19]. However, it is unclear what role, if any, exogenously added MSCs play in the regeneration of intestinal epithelial or stromal supporting cells that undergo constant and rapid renewal [20]. Older studies (primarily using irradiation injury) showed that bone marrow derived stem cells can migrate into the intestines after various injuries and differentiate into stromal cells [20, 21]. These bone marrow transplants include MSCs that potentially are the source of the replacement stromal cells, suggesting that under the correct conditions, MSCs can perform this role. One recent paper found that MSCs underwent myogenic differentiation upon homing to the intestine during the repair phase of dextran sodium sulfate (DSS) colitis [22]. However, the degree to which differentiation played a role in the therapeutic benefits of systemically injected MSCs was unclear, because injection (and therefore entrapment in the lung) has not been decoupled from differentiation in this system.
Recent studies showed that systemically delivered MSCs can home and enter inflamed intestine; however, they appear to do so inefficiently. When human MSCs were injected i.v. into sublethally irradiated mice, only 0.13% of the cells in the intestine were donor-derived after 3 days [23•]. Additionally, these investigators noted that intestinal engraftment occurred in only 40% of mice despite clinical improvements in all treated mice, suggesting that engrafted cells in the intestine were not necessary for clinical benefits. A second study also supports low engraftment efficiency. Here, one million human MSCs injected i.v. into DSS-treated mice resulted in a transient engraftment in the intestine that peaked at day 2 postinjection and showed 15 transplanted MSCs/mg of tissue [24•]. Few cells were detected at day 6 postinjection. It is not clear from these studies that the degree to which MSC-derived stromal cells was present.
Although MSC engraftment in moderately damaged intestine appears to be an inefficient process, recent efforts are aimed at increasing this efficiency. Recent studies have made inroads into understanding MSC homing. Cellular homing to the bone marrow requires E-selectin ligands; however, MSCs were shown to lack the proper sugar modifications on CD44 to bind E-selectin [25]. After modification of CD44 ex vivo using a selective fucosyltransferase, MSCs were able to enter the bone more efficiently. Although an exciting proof of principle, the use of ex-vivo enzymes is limited to glycosylated proteins already present on MSCs and may prove more difficult for targeting MSCs to other locations such as intestines. A second method was to genetically modify MSCs [26]. Using a model of murine myocardial infarction MSCs were engineered to overexpress the chemokine receptor CXCR4. This receptor increased their recruitment to ischemic heart tissue that expressed the cognate ligand CXCL12. This study has implications for human intestinal diseases, because CXCL12 is expressed in the intestine and CXCR4-expressing cells are recruited during inflammation in IBD [27]. One study found that culturing MSCs under sublethal hypoxic conditions increased adhesion, survival, and chemotaxis toward CXCL12 in vivo [28•]. The increased chemotaxis was due to upregulation of CXCR4 through the AKT/PI3K pathway. Taken together, these studies suggest that simply pretreating cultured MSCs in hypoxic conditions may increase cell survival and the in-vivo migration to inflammatory tissues expressing CXCL12, including the intestines. Additionally, these studies highlight the importance of setting strict guidelines for how MSCs will be grown in culture, as culture conditions can alter MSC properties.
One other approach to improve MSC engraftment in the context of intestinal inflammation was to coat MSCs with antibodies [29•]. MSCs were treated with palmitated protein G and then coated with anti-VCAM-1 or MAd-CAM-1 antibodies. When injected into DSS-treated mice, an increase in VCAM-1-coated MSC engraftment occurred in the colon. Both MAdCAM-1-coated and VCAM-1-coated MSCs significantly increased survival after DSS compared to no treatment, MSCs alone, or MSCs co-injected with free MAdCAM-1 or VCAM-1 antibodies. Future studies will need to determine whether engraftment in the intestine increases the regenerative cells (MSCs), increases the regenerative properties (paracrine factors), or is unrelated to the therapeutic effects observed after MSC transplant.
Intravenously injected mesenchymal stem cells as local mediators of regeneration
Two hallmarks of MSCs are their immunosuppressive and anti-inflammatory functions in vitro and in vivo. MSCs can suppress a wide array of both innate and adaptive immune cells as well as activate regulatory T cells by direct and indirect pathways [7]. MSCs from xenogeneic, allogeneic and syngeneic sources isolated frombone marrow, colonic, gingival, and adipose tissues all have shown a surprisingly similar level of clinical success for many different types of injury. Despite this seemingly interchangeable feature of MSCs from different sources, MSCs isolated from a wide variety of mouse organs are not identical as determined by cell surface molecules or their efficiency at differentiation [13]. One recent study has found that MSCs isolated from human adipose tissue had a higher expression of chemokine receptors when compared to MSCs from human bone marrow [30]. One future need is more comprehensive studies that make direct comparisons of MSCs from varying sources.
Regarding the mechanism of the MSC effects in damaged or diseased tissue, the emerging paradigm is that immunomodulation is critical, perhaps more important than their multipotent stem cell capabilities. MSCs secrete many growth factors including VEGF, IGF-1, and EGF as well as immunomodulatory molecules such as prostaglandin E2 (PGE2), TGF-β and IL-10. Recently, the anti-inflammatory protein tumor necrosis factor (TNF)-stimulated gene 6 (TSG-6) was identified by microarray analysis in human MSCs trapped in the lung after injection into mice with induced myocardial infarction [16••]. TSG-6 is upregulated by pro-inflammatory cytokines and in turn inhibits leukocyte migration potentially through alteration of CD44 interacting with hyaluronic acid [31]. We predict that additional immunosuppressive factors will be discovered depending on the source of MSCs and the cause and location of injury. Discovery of the important soluble factors produced by MSCs could potentially lead to therapies where the factors are produced in high quantity and injected without the cells. For a recent example, recombinant TSG-6 was added by intraocular injection after corneal chemical and mechanical injury and was shown to reduce inflammation [32]. Also, MSC supernatant has been shown to enhance skin wound healing in part by increasing angiogenesis and macrophage recruitment [33].
MSCs may also play immunomodulatory roles through contact-dependent mechanisms. One such contact-dependent-mechanism is the inhibitory signaling through PD-L1 on MSCs and its receptor PD-1 on activated lymphocytes and myeloid cells. This pathway is well known to play a central role in limiting inflammation by inhibiting activated lymphocytes [34]. In a mouse model of systemic lupus erythematosus, IFN-γ primed MSCs inhibited B cell proliferation and differentiation into plasma cells in a cell-contact-dependent mechanism involving PD-1–PD-L1 interactions [35]. IFN-γ increases PD-L1 on MSCs in vitro; therefore, it is possible that MSCs inhibit activated lymphocytes in vivo through this pathway. Because many intestinal diseases such as IBD are thought to involve overactivation of T cells, MSCs may provide additional benefit if they can contact lymphocytes in the diseased area. There may be other cell-contact-dependent mechanisms MSCs can utilize in vitro, but more research is needed to understand the contribution of systemically secreted factors, locally secreted factors, and cell–contact interactions.
Local injection of mesenchymal stem cells
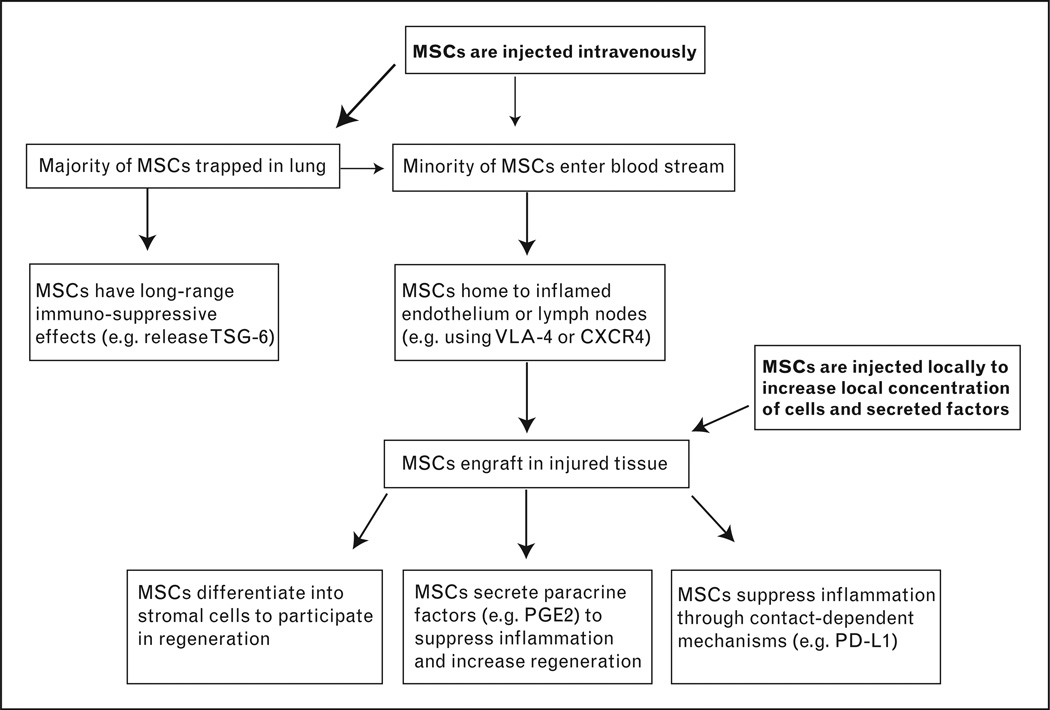
Although intravenous injection is the most widely used method for transplantingMSCs, local injection or topical application of MSCs may be more useful under certain situations. Local injection may be more beneficial, because it removes the need for cell homing to the injured site and therefore more cells may participate in healing and repair (Fig. 2). MSCs have been injected into the colonic submucosa after rats injured with TNBS and were shown to decrease lesion size compared to injected PBS or bone marrow cells [36]. MSCs have also been injected into the submucosa of rats after ischemia–reperfusion injuries and shown to decrease pathology scores and bacterial translocation [37]. Additionally, these investigators showed an engraftment efficiency of approximately 20%, providing evidence that local injection may be a reasonable method to increase MSC engraftment. Another successful local MSC injection was found in mice injured with irradiation. In this study, transplanted mice had increased survival and histological scores [38]. One final exciting use of locally applied MSCs was seen in a phase II clinical trial for treatment of complex perianal fistulas [39]. Patients were treated with fibrin glue or fibrin glue plus adipose-derived MSCs. Seventy-one percent of the patients in the +MSC group demonstrated clinical healing as compared to 16% with glue alone. These recent advances in local applications of MSCs should encourage more research and clinical trials using novel introduction of MSCs.
Figure 2. Schematic of the locations and mechanisms of transplanted mesenchymal stem cells.
Most MSCs that are injected intravenously become trapped in the lung, where they are capable of performing systemic immunomodulation. The few MSCs that escape the lung can enter circulation and engraft in inflamed tissue to act as local regenerative or immunomodulatory cells. Future research is needed to clarify the importance of each mechanism so that future therapies may become more targeted. For example, local injections of MSCs, in-vitro manipulation of MSCs, and injection ofMSCsupernatants may all provide better clinical outcomes depending on the specific disease or tissue that is targeted. MSC, mesenchymal stem cell.
Mesenchymal stem cells can modulate the activation state of macrophages
MSCs can interact with immune cells in order to dampen specific responses to injury and inflammation, as described in more detail elsewhere [4, 7, 40]. Macrophages are critical cells in many intestinal inflammatory diseases, because they can be responsible for both initiating and resolving inflammation and can communicate with all the major effector cells of immune system. Macrophages can also stimulate the epithelial progenitor niche during injury in order to help maintain the epithelial barrier [41, 42]. Although MSCs secrete many molecules capable of modulating macrophages [i.e., indoleamine 2,3-dioxygenase (IDO), TGF-β, and PGE2], the exact mechanisms are still an active area of research.
An in-vivo model using cecal ligation puncture highlights the importance of PGE2 released by MSCs toward inhibition of classic macrophage activation. Bone marrow MSCs injected i.v. were activated by TLR ligands or TNFα to stimulate production of PGE2, which in turn lead to IL-10 production by macrophages [43••]. Given the short range of action of PGE2 and the fact that most injected MSCs were in close proximity to lung macrophages, it is likely that the lung macrophages may have been the primary target of MSC-produced PGE2. Thus, questions remain as to whether i.v. injected MSCs can program anti-inflammatory macrophages through PGE2 in the injured intestine, where relatively few MSCs are found in close proximity with macrophages. It is worthwhile to note that PGE2 released from MSCs likely can have other effects, such as maintaining epithelial integrity in the intestine during injury [44]. In a separate study, human gingival MSCs polarized macrophages to produce IL-10 in vitro and in a skin wound healing model [45]. However, polarization required IL-6 and granulocyte–macrophage colony-stimulating factor and was only partially dependent on PGE2 and was independent of IL-10. Differences in the species studied, sources of MSCs or macrophages, in-vitro conditions, or in-vivo models all could explain why there are diverse mechanisms for how MSCs interact with macrophages. This point is highlighted by a recent study which shows that human MSCs use IDO to suppress T cell proliferation in vitro, whereas murine MSCs used nitric oxide [46]. More work is needed to clarify the effects of MSCs on specific cells in different intestinal disease models to determine if MSCs must home or if their secreted factors can have systemic effects.
Conclusion
MSCs are an emerging area of interest, with more than 1500 papers published in the last 2 years, compared to approximately 600 published from 2004 to 2006. Most of the original studies describing these cells involve injecting MSCs in an attempt to repair damage or inflammation. Recent studies in the last 2 years have critically examined homing, paracrine, and systemic mechanisms of these cells, with an increased focus on where these cells exert their therapeutic properties. Future studies should answer the critical question as to the mechanism by which MSCs exert an impact on intestinal diseases.
Acknowledgements
We would like to thank Monica Walker for providing the image of MSCs trapped in the lung.
National Institutes of Health Grants DK071619 and DK07161-90251.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010;2010:9. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 6.Selmani Z, Naji A, Gaiffe E, et al. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87(9 Suppl):S62–S66. doi: 10.1097/TP.0b013e3181a2a4b3. [DOI] [PubMed] [Google Scholar]
- 7.Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 9. Isakova IA, Dufour J, Lanclos C, et al. Cell-dose-dependent increases in circulating levels of immune effector cells in rhesus macaques following intracranial injection of allogeneic MSCs. Exp Hematol. 2010;38:957e1–967e1. doi: 10.1016/j.exphem.2010.06.011. This article demonstrates that MSCs do not escape immune recognition in primates.
- 10.Yuan T, Zhang L, Feng L, et al. Chondrogenic differentiation and immunological properties of mesenchymal stem cells in collagen type I hydrogel. Biotechnol Prog. 2010 doi: 10.1002/btpr.484. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. This article characterizes human gingival MSCs as immunosuppressive cells.
- 12.Walker MR, Brown SL, Riehl TE, et al. Growth factor regulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) expression in colonic mesenchymal stem cells. J Biol Chem. 2009;285:5026–5039. doi: 10.1074/jbc.M109.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all postnatal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 14.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 15. Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. This article describes MSCs in the bone marrow as nestin-positive cells and as HSC niche cells.
- 16. Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. This article quantifies the entrapment of MSCs in the lungs and describes a novel MSC factor capable of systemic immunosuppression.
- 17. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. This article describes the entrapment of stem cells in the lungs based on cell size and adhesion molecules.
- 18.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Schuleri KH, Boyle AJ, Hare JM. Mesenchymal stem cells for cardiac regenerative therapy. Handb Exp Pharmacol. 2007;180:195–218. doi: 10.1007/978-3-540-68976-8_9. [DOI] [PubMed] [Google Scholar]
- 20.Brittan M, Hunt T, Jeffery R, et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komori M, Tsuji S, Tsujii M, et al. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Arimura Y, Yabana T, et al. Myogenic lineage differentiated mesenchymal stem cells enhance recovery from dextran sulfate sodiuminduced colitis in the rat. J Gastroenterol. 2010 doi: 10.1007/s00535-010-0320-7. [DOI] [PubMed] [Google Scholar]
- 23. Semont A, Mouiseddine M, Francois A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952–961. doi: 10.1038/cdd.2009.187. This article quantifies the level of engraftment after MSC therapy and provides evidence that engraftment is not necessary to observe clinical improvement.
- 24. Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. This article quantifies the engraftment of MSCs in the spleen, MLN and intestine over 1–6 days after injection.
- 25.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 27.Dotan I, Werner L, Vigodman S, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583–592. doi: 10.1002/ibd.21106. [DOI] [PubMed] [Google Scholar]
- 28. Liu H, Xue W, Ge G, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun. 2010 doi: 10.1016/j.bbrc.2010.09.076. This article describes a simple method to increase MSC homing toward CXCL12.
- 29. Ko IK, Kim BG, Awadallah A, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–1372. doi: 10.1038/mt.2010.54. This article describes the novel approach of coating MSCs with antibodies that recognize inflamed endothelium to increase homing.
- 30.Ahmadian Kia N, Bahrami AR, Ebrahimi M, et al. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9446-6. [DOI] [PubMed] [Google Scholar]
- 31.Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34(Pt 3):446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- 32.Oh JY, Roddy GW, Choi H, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H, Chen X. Immunoregulatory role of B7-H1 in chronicity of inflammatory responses. Cell Mol Immunol. 2006;3:179–187. [PMC free article] [PubMed] [Google Scholar]
- 35.Schena F, Gambini C, Gregorio A, et al. Interferon-gamma-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62:2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi Y, Tsuji S, Tsujii M, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Qu L, Li Y, et al. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2009 doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Kudo K, Liu Y, Takahashi K, et al. Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res (Tokyo) 2010;51:73–79. doi: 10.1269/jrr.09091. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 41.Pull SL, Doherty JM, Mills JC, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seno H, Miyoshi H, Brown SL, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. This article provides a convincing mechanism whereby MSCs can activate an antiinflammatory state in macrophages in vivo.
- 44.Brown SL, Riehl TE, Walker MR, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]