Abstract
OBJECTIVES
Median arcuate ligament syndrome (MALS) is a vascular compression syndrome with symptoms that overlap chronic functional abdominal pain (CFAP). We report our experience treating MALS in a pediatric cohort previously diagnosed with CFAP.
PATIENTS AND METHODS
We prospectively evaluated 46 pediatric (<21 years of age) patients diagnosed with MALS at a tertiary care referral center from 2008 to 2012. All patients had previously been diagnosed with CFAP. Patients were evaluated for celiac artery compression by duplex ultrasound and diagnosis was confirmed by computed tomography. Quality of life (QOL) was determined by pre- and post-surgical administration of PedsQLtm questionnaire. The patients underwent laparoscopic release of the median arcuate ligament overlying the celiac artery which included surgical neurolysis. We examined the hemodynamic changes in parameters of the celiac artery and peri-operative QOL outcomes to determine correlation.
RESULTS
All patients had studies suggestive of MALS on duplex and computed tomography. 91% (n=42) positive for MALS were females. All patients underwent a technically satisfactory laparoscopic surgical release resulting in a significant improvement in blood flow through the celiac artery. There were no deaths and a total of 9 complications, 8 requiring a secondary procedure. 33 patients were administered QOL surveys. 18 patients completed the survey with 15 (83%) patients reporting overall improvement in the QOL. Overall, 31/46 patients (67%) reported improvement of symptoms since the time of surgery.
CONCLUSIONS
MALS was found to be more common in pediatric females than males. Laparoscopic release of the celiac artery can be performed safely in the pediatric population. Surgical release of the artery and resultant neurolysis resulted in significant improvement in the blood flow, symptoms, and overall QOL in this cohort. The overall improvement in QOL outcome measures after surgery leads us to conclude that MALS might be earlier diagnosed and possibly treated in patients with CFAP. We recommend a multidisciplinary team approach to care for these complex patients.
Keywords: celiac artery, vascular compression, functional abdominal pain
Introduction
The majority of chronic abdominal pain in children is thought to be functional (CFAP), that is, without demonstrable evidence of an underlying anatomic, metabolic, infectious, inflammatory, or neoplastic disorder.1, 2 Many of these difficult patients carry a host of symptom-based diagnoses, including functional dyspepsia, abdominal migraine, and especially, irritable bowel syndrome (IBS), all within the domain of what is now better known as functional gastrointestinal disorders (FGID). This classification was most recently updated as the Rome III criteria in 2006.1–6 The pathophysiology for FGID is poorly understood, but is thought to involve abnormalities in the enteric nervous system leading to dysregulation of brain-gut communication to explain altered bowel motility, visceral hypersensitivity, and stress-mediated effects in the pathogenesis of functional abdominal pain.1–4 IBS is perhaps the best example where annual direct and indirect management costs are estimated to be $8 and $25 billion respectively with evidence pointing to its origin in childhood of those with CFAP.1, 2, 7–11 As opposed to chronic abdominal pain where there is demonstrable pathology, i.e. celiac disease, inflammatory bowel disease, etc, for which there are established treatment strategies with mostly predictable outcomes, treatment for FGID remains unproven and published results mostly difficult to interpret.12–17
Median Arcuate Ligament Syndrome (MALS), also known as Celiac Artery Compression Syndrome, was first described by Harjola in 1963.18 The hallmark symptoms of post-prandial abdominal epigastric pain, nausea, occasional diarrhea and weight loss, overlap with those of CFAP. Although MALS has been advocated as an unusual cause of abdominal pain, the evidence has been based principally on anecdotal or small single-center retrospective analysis rather than level 1 or level 2 evidence.18–20 In anatomical terms, MALS is felt to be caused by a compressive anatomic relationship of the diaphragmatic crura to the celiac vessels leading to decreased flow, a steal phenomenon and resultant post-prandial abdominal pain.18, 21–23 Similarly it has been suggested that neurogenic compression may lead to the clinical symptoms.24 Only recently have advances in non-invasive, high definition duplex ultrasound scan and CT or MR angiography allowed vascular occlusive diseases such as MALS to be more readily diagnosed based on objective measurement of vessel flow velocity and alterations in vascular architecture. Leveraging these advances in diagnostic imaging in the context of an apparent non-coincidental overlap of GI symptoms between MALS and FGID, we prospectively evaluated MALS in 46 pediatric patients diagnosed with CFAP within the domain of FGID, and we report on the surgical outcomes and quality of life of these patients.
Patients and Methods
Patients
We evaluated 46 patients (42 females, 4 males; ages 8.6–20.5 yrs; median- 16.6; mean- 16.2±0.5 years) with diagnosis of CFAP with duplex US at a single tertiary care institution between August 2008 and January 2012. This was performed under an IRB approved protocol. A complete GI work up was performed by the patient’s gastroenterologist. Typical workup included a complete battery of studies shown in table 1. Study requirements included 1) diagnosis of FGID with chronic abdominal pain by a gastroenterologist and 2) thorough GI evaluation for abdominal pain with a minimum of upper endoscopy and abdominal/pelvic CT scan. Patients with chronic abdominal pain with demonstrable pathology were excluded. Informed consent was obtained from the patient or guardian (< 18 yrs.) as required by the University of Chicago Institutional Review Board (#16997A).
Table 1.
Guidelines for standard GI work-up in patients with possible MALS.
|
ESR: erythrocyte sedimentation rate. UGI: upper gastrointestinal. BMI: Body Mass Index.
Duplex Ultrasound Protocol
Prior to any intervention, all patients had a duplex ultrasound in our accredited Intersocietal Accreditation Commission (IAC) laboratory for vascular testing to fully evaluate the visceral vessels. All patients fasted overnight to minimize the amount of abdominal gas present at the time of study. All mesenteric duplex scans were performed with ATL HDI 3000 (Advanced Technology Laboratories, Bothel, Washington), ATL HDI 5000, or Acuson Sequoia 512 9 (Acuson Corp, Moutainview CA) ultrasound scanner with linear array 4–7 MHz or 5–10 MHz transducers. Similarly, all ultrasounds were performed by registered vascular technologists and were subsequently reviewed by board-certified vascular surgeons. The mesenteric arteries were examined in the supine position. The standard protocol for mesenteric duplex examination included the highest peak systolic and end diastolic velocities from the proximal, mid and distal superior mesenteric, proximal and distal celiac, proximal inferior mesenteric, proximal hepatic and proximal splenic arterial segments. Also, any pre or post-stenotic spectral waveforms were retained for confirmation of flow-reducing stenosis. Further, the patient was asked to take a deep inhalation and hold it while peak systolic velocities (PSV) were measured followed by similar measurements with deep exhalation. For the celiac, hepatic and splenic arteries, a PSV greater than 200cm/sec and an end diastolic velocity greater than 55cm/sec suggested a flow-reducing stenosis (>70%), the presence of a post-stenotic Doppler signal was then confirmed. Spectral broadening of the waveform may also be present throughout the cardiac cycle. Color flow depicting a luminal reduction and a color bruit in the same segment of the artery served as a complement to the velocity data and supported the presence of a stenosis. Further, demonstration of a decrease in PSV with deep inspiration was suggestive of MALS.
Computed Tomography Angiogram (CTA) Protocol
CTA of the abdomen was performed using Philips 64- and 256-slice scanners (Philips Healthcare, Andover, MA). Imaging was obtained at 64 × 0.625 mm collimation reconstructed at a slice thickness of 0.90 mm and a slice increment of 0.45 mm. Volume-rendered and maximum-intensity-projection images were constructed. The arterial phase was obtained in expiration to maximize evaluation for suspected median arcuate ligament syndrome. Delayed imaging was performed in deep inspiration. On average 110 cc of Omnipaque 350 (GE Healthcare, Waukesha, WI) contrast was administered intravenously. All CT images were interpreted by a single board certified radiologist (JL).
Operative Procedure and Follow-Up
46 patients diagnosed with MALS underwent a laparoscopic median arcuate ligament release with intra-operative duplex. Both Pediatric Surgeon and Vascular Surgeon were present and participated in each case. General anesthetic was administered with the option of an epidural for post-operative pain control. Initial laparoscopy was performed by the Pediatric Surgeon (DL, GM). The operative procedure was based on the initial report by Roayaie et al25 and modified for the pediatric population. Five 5 mm ports were placed in standard fashion: peri-umbilical port for camera placement; port in the right sub-costal region in the anterior axillary line for liver retraction; port in the right sub-costal region in the mid-clavicular line as an operating port; port in the left sub-costal region in the anterior axillary line for stomach retraction; and a second operating port in the left sub-costal region in the mid-clavicular line. Upon entry into the peritoneal cavity, a diagnostic laparoscopy was performed to rule out other causes of abdominal pain that may not have been evident on prior work up. The lesser sac was opened by dividing the gastro-hepatic ligament. The crural fibers of the diaphragm were exposed by mobilizing the esophagus laterally to visualize the “V” shaped configuration. The Vascular Surgeon (CS) then proceeded to take down the median arcuate ligament utilizing hook electrocautery. Progress was made in a cranial to caudal direction starting in the supra-celiac position and progressing along the anterior surface of the aorta exposing the proximal celiac and distally to the bifurcation. A complete dissection was performed by transecting all tissue overlying the celiac artery including the large nerve complexes as well as lymphatics to ensure a complete release of the vessel. We then proceeded to perform as complete a circumferential dissection of the celiac artery as possible. Upon complete exposure of the artery, we performed an intra-operative trans-abdominal duplex utilizing the same procedure and RVTs as previously described. Completion parameters included primarily loss of respiratory variation of the PSV with Valsalva maneuver (performed by Anesthesia providing a peak inspiratory pressure of 10 cmH20) and secondarily: significant reduction in the PSV and reduction in celiac/aortic PSV ratio. The patients were admitted post-operatively to the general floor. A clear liquid diet was initiated on post-operative day one and gradually advanced as tolerated to regular diet. Patients were discharged once tolerating regular diet, had adequate pain control with oral pain medication, and were adequately ambulating. Follow-up in clinic in 1–2 weeks after discharge was performed to monitor incisions. Repeat vascular duplex studies were performed at 6 months and one year post-operatively. Additionally, patients follow-up with psychiatry at 6 and 12 months post-operatively.
No Improvement Protocol
If after operative recovery the patient reports no improvement, follow up investigation is initiated. Repeat duplex ultrasound is performed, and if significantly increased velocities and continued respiratory variation are found, then angiography with possible angioplasty is offered to the patient. If repeat duplex reveals normalized hemodynamic parameters, repeat CTA is performed to evaluate for intra-abdominal pathology as a result of the surgery. If the CTA is normal, the patient is then offered a celiac plexus nerve block performed by anesthesia.
Quality of Life (QOL)
Assessment of surgical treatment outcome was determined by pre- and post-surgical administration of PedsQLtm questionnaires.26 This validated questionnaire was administered during both the pre and post-operative periods. This validated survey addresses problems with health and activities (including pain), feelings, interpersonal interactions, and work/studies. We began using the survey in June of 2009. For all patients the PedsQLtm questionnaires were provided at the initial pre-operative visit. Instructions were provided by a team member and collected on the day of surgery or in the pre-operative period. For patients less than 18 years of age, an additional, corresponding parental questionnaire was used to validate the child’s score. The reported score represents an average of the parental and child score. The same questionnaires were then administered at or around the 6month-1 year follow up clinic visits. Scores were based on physical, emotional, social and school functioning and converted to a 0–100 scale with higher scores indicating better QOL. Furthermore, all patients’ charts were reviewed for patient reported documentation of improvement of symptoms in the postoperative period. Patients who did not return the survey, received follow-up e-mails that included the QOL survey. All patients received follow up e-mails and a general question as to whether they experienced improvement in symptoms since the time of surgery.
Statistics
Microsoft Excel software (Seattle, WA) was used for analysis. A two tailed t-test for independent samples was used to compare means. For the quality of life analysis, a paired t-test was performed. A p-value < 0.05 was considered significant.
Results
Forty-six patients labeled with CFAP by a gastroenterologist were diagnosed with MALS by duplex ultrasound. Representative images are shown in Figure 1A and B. Forty-two (91%) of the patients were female and the median age was 16.5 years (8.5–20.5). These patients were found to have duplex criteria consistent with celiac artery compression. Hemodynamic parameters of the 46 patients revealed: mean PSV 381 cm/s (228–1050 cm/s), end diastolic velocity (EDV) 141 cm/s (26–620 cm/s). With deep inspiration the mean PSV dropped to 197 cm/s (80–427 cm/s) (p<0.001) reflecting an average decrease in velocity of 180 cm/s (Table 2). Patients with a positive duplex (PSV>200cm/s, EDV>55cm/s, ΔPSV with inspiration >60 cm/s) then underwent CTA to confirm findings prior to surgery (Figure 1C and D) or provided CTA or magnetic resonant angiography (MRA) from the referring institution. These imaging studies confirmed celiac compression prior to surgery and provided more anatomic detail for operative planning. Duplex US correlated with CTA or MRA in 93% (43/46) of cases. After multi-disciplinary consensus, patients underwent laparoscopic surgery that resulted in a significant improvement in the hemodynamic parameters (Table 2). With respect to anatomy, there were no intra-operative vascular anomalies missed by CTA. In the three cases in which CTA did not correlate with Duplex US, all three met inclusion criteria based on the Duplex US. Two of the three patients had non-significant (<20%) stenosis of the origin of the celiac artery. The third patient had no evidence of compression. All results were discussed with patients and families as well as the multi-disciplinary team and surgery was offered to these patients. Two of the three patients have reported improvement in symptoms with one of the patients with a documented 7% increase in the BMI but with occasional crampiness. The second patient reported significant improvement of her symptoms at six months. The third patient who had a positive duplex and negative CTA reported a decrease in QOL from 45 to 39. The patient wanted to pursue alternative therapies elsewhere.
Figure 1.
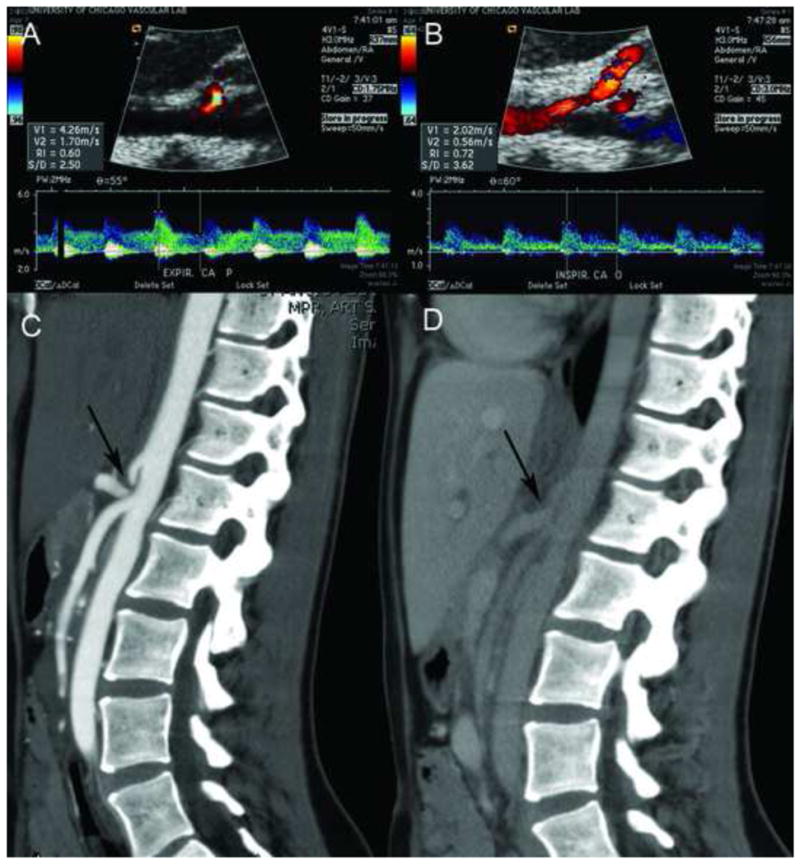
Ultrasound with B-mode imaging showing the Aorta in logitudinal-section and arrow pointing at the origin of the celiac artery during expiration (A) with corresponding increase in peak systolic velocity to 426 cm/s. Figure 1B: The Aorta in logitudinal-section with the origin of the celiac artery during inspiration with a corresponding decrease in peak systolic velocity to 202 cm/s. Figure 1C: CT-Angiogram in sagittal view showing the origin of the celiac artery arising from the Aorta with a “J-Hook” configuration (arrow) during expiration and (D) during deep inspiration. Note the increase of the lumen size with deep inspiration (arrow) in D. The origin of the superior mesenteric artery is normal in appearance.
Table 2.
Hemodynamic characteristics of patients screened with duplex ultrasound.
| MEAN PSV (cm/s) | MEAN PSV WITH INSP. (cm/s) | MEAN CHANGE IN PSV WITH RESPIRATION (cm/s) | MEAN PSV:AORTIC RATIO | |
|---|---|---|---|---|
| Pre-op | 381±23* | 197±11* | 180±16* | 2.2±0.14* |
| Intra-op | 212±8 | 222±12 | 4±5 | 1.2±0.05 |
| Post-op | 235±11 | 180±7 | 46±9 | 1.4±0.06 |
p<0.001 when pre-operative parameters were compared with post-operative parameters.
(PSV: Peak systolic velocity; EDV: end diastolic velocity; Insp: Inspiration; cm/s: centimeter/second)
QOL surveys were distributed to thirty-three patients in the pre-operative and post-operative periods. A total of 18 patients completed both the pre and post-op QOL forms. Due to a combination of factors including the late initiation of survey administration in the study group after the first few patients had already undergone surgery as well as poor patient compliance led to the low number of total patients that had completed both pre- and post-operative QOL surveys. The average total pre-op score was 57.9 and the average total post-op score was 76.7 with a median follow up of 11.8 months (6.5–24.3 months. This improvement in QOL was significant with a p-value of 7×10−5 (Figure 2). Overall, 15/18 (83%) patients who completed the questionnaire had improvement of the QOL. Because of the 55% return rate on the QOL (18 of the 33 patients given the survey), we reviewed the charts of the remaining patients and via e-mail contact asked specifically if they had an improvement of their quality of life since surgery. Overall, 31/46 patients (67%) responded that they had improvement since the time of surgery. Of those who had no improvement, 7 have been classified by gastroenterologists and are undergoing treatment for FGID and 8 failed to respond to follow up requests. When the groups were divided into those patients that reported improvement from the surgery and those who did not, the post operative duplex revealed PSV (229±14 vs. 248±29; p=0.38), PSV with inspiration (179±10 vs. 182±15; p=0.9) and ratio of Celiac/Aortic PSV (1.3±0.1 vs. 1.6±0.1; p=0.09), ΔPSV (39±10 vs. 59±22; p=0.3). We found no significant hemodynamic variable that was predictive of improvement or no improvement when we compared the pre-operative hemodynamics (Table 3) and the post-operative hemodynamics (Table 4). The QOL questionnaire corresponded well with documentation of the patient stated quality of life. The median follow up time for the entire cohort was 8.9 months (range; 1.5–34.7 months).
Figure 2.
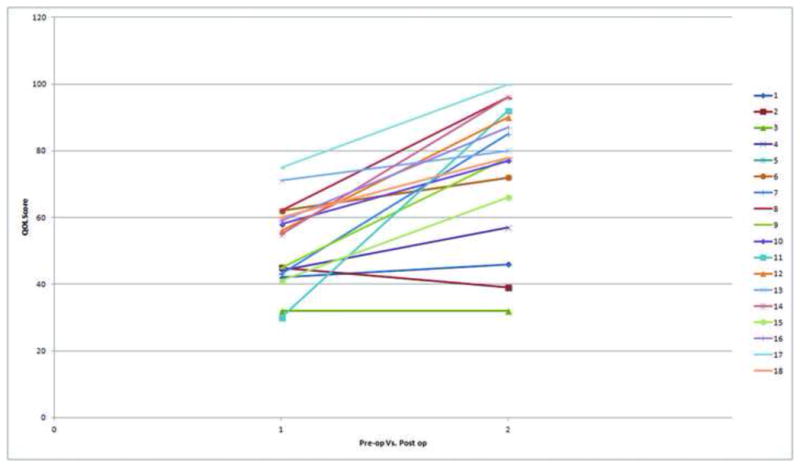
Individual patient QOL results when compared pre-operatively to post operatively. Of the 18 patients that had completed both the pre and post-operative questionnaire, 15 patients self-reported improvement after surgery. This improvement in QOL was significant with a p-value of 7×10−5
Table 3.
Duplex characteristics of patients screened with Duplex in the pre-operative period divided into those that had improvement and those that did not.
| MEAN PSV (cm/s) | MEAN PSV WITH INSP. (cm/s) | MEAN CHANGE IN PSV WITH RESPIRATION (cm/s) | MEAN PSV:AORTIC RATIO | |
|---|---|---|---|---|
| Improvement (n=31) | 387±33 | 203±15 | 185±22 | 2.2±0.1 |
| No improvement (n=15) | 369±27 | 186±14 | 171±20 | 2.2±0.2 |
PSV: Peak systolic velocity; EDV: end diastolic velocity; Insp: Inspiration; CM/S: centimeter/second)
Table 4.
Duplex characteristics of patients screened with Duplex in the post-operative period divided into those that had improvement and those that did not.
| MEAN PSV (cm/s) | MEAN PSV WITH INSP. (cm/s) | MEAN CHANGE IN PSV WITH RESPIRATION (cm/s) | MEAN PSV:AORTIC RATIO | |
|---|---|---|---|---|
| Improvement (n=31) | 229±14 | 179±10 | 39±10 | 1.3±0.1 |
| No improvement (n=15) | 248±26 | 182±15 | 59±22 | 1.6±0.1 |
PSV: Peak systolic velocity; EDV: end diastolic velocity; Insp: Inspiration; CM/S: centimeter/second)
Overall, morbidity and mortality was low. There were no deaths associated with the procedure and a total of nine complications. In this pediatric cohort, we had no early conversions to an open procedure and no blood transfusions. Two patients had a feeling of fullness in their chest that required esophageal dilation. This was early in the experience and we surmised was due to our initial practice of completely dividing the crura from the esophageal hiatus to the aortic hiatus. We then re-approximated the crural fibers with interrupted stitches. In all subsequent patients, we performed minimal esophageal dissection with a focus on the aortic hiatus. The supra-celiac aorta was exposed and there was no need for re-approximation of crural fibers. We performed circumferential dissection of the celiac artery because of the possibility of circumferential ligamentous bands. Unlike Roayaie,25 we performed lysis of both the right and left crus to optimize circumferential dissection of the celiac artery. No patient had significant post-operative reflux. One patient had a chemical pancreatitis with elevation in amylase and lipase that resulted in a delay in resumption of the diet. The etiology of this was unclear though we feel this is likely due to retraction of the pancreas caudally to optimize dissection of the celiac artery. Six patients underwent other procedures for continued abdominal pain and nausea. The procedures included two patients that underwent a celiac plexus nerve block; two patients underwent angiography with angioplasty; one patient had an open aorto-celiac bypass; and one patient had a local block at the umbilical port incision. Overall, 4 of these patients still reported no improvement, and the patient who underwent the aorto-celiac bypass reported significant improvement in symptoms.
Discussion
If one subscribed to the traditional biomedical concept of disease where a single biological entity can account for every clinical condition, FGID, i.e., IBS, abdominal migraine, functional dyspepsia in the clinical spectrum of CFAP would be akin to fiction1–5. In the opposite spectrum, diseases with demonstrable pathology such as Celiac or Crohn’s disease are better described with, in most-cases, well defined treatment strategies and mostly predictable outcomes. Yet mounting evidence has determined that in most cases, chronic abdominal pain is functional and FGID has emerged as an important, clearly relevant class of disorders in medicine since its inception in Rome in 1988 and most recently, the Rome III criteria in 2006 1–5. The Rome Criteria based on the principle of symptom-based diagnostic criteria has helped physicians make positive diagnoses of functional bowel disorders, and better understand the pathophysiology of these disorders. The Rome criteria has helped spearhead the development of new pharmacological agents to treat altered motility, visceral hypersensitivity, and stress-mediated effects thought to be important mechanisms in the pathogenesis of FGID. Although many of these treatment trials are promising, definitive statements concerning therapeutic efficacy are still limited in large part due to yet to be established guidelines for clinical trial research. Yet billions of dollars per year continue to be spent on frequent physician visits, costly medications, tests and procedures, not to mention the accumulation of lost days at school and work for those with CFAP and their caregivers.1, 2 This does not even take into account the effects on these patients’ lives for which no price can be placed.
Celiac compression or MALS, similar to FGID occurs typically in young females with CFAP and share similar symptoms of post-prandial, mid-epigastric pain, and other constitutional symptoms such as nausea, vomiting, and occasional diarrhea. Some 40 years after it was first described, MALS remains a controversial and vexing condition. The link between symptoms and narrowing of the celiac axis, celiac nerve plexus, or both remains unknown. Most suggest an ischemic origin where occlusion of the axis would lead to intestinal angina. Alternative hypotheses include a steal phenomena resulting in a water-shed effect on the celiac plexus, thus producing pain; and/or disruption of neuro-enteric pain pathways affecting visceral hypersensitivity mediated through the celiac ganglion, the latter perhaps similar to the pathogenesis of FGID. Critics of MALS have suggested that occlusion of both the celiac and superior mesenteric artery would be necessary to produce classic intestinal angina and have pointed out that the few reports of long-term follow-up after celiac decompression have often been disappointing.27
The bulk of MALS literature has been case reports including one from our own institution in 1994 of MALS in a set of 27-year old monozygotic twin females with diagnosis of chronic abdominal pain.28 What is striking is that two key features of MALS, young female predominance and its associated GI symptoms, overlap with those of CFAP.1, 2 Given this overlap, we hypothesized that perhaps a subset of patients, most likely young females with chronic abdominal pain previously diagnosed with FGID may actually have MALS instead. The two conditions may not be mutually exclusive as evidenced by the known co-existence of FGID with GI diseases of demonstrable pathology, like Crohn’s. Our initial duplex scans of these patients with CFAP revealed a cohort with a positive screen for MALS. Substantiating the diagnosis was a significant decrease in PSV with deep inhalation where downward excursion of the diaphragm to decompress the celiac axis correlated with improvement in velocities. Importantly, 67% of the patients had overall improvement of symptoms after they had undergone a release of the celiac artery, raising a question about the initial diagnosis of CFAP.
For control purposes to highlight celiac pathology, normal PSV were demonstrated in the aorta, and neighboring truncal vessels. While surgery in this area has considerable associated risks including injury to the aorta, bowel, pancreas, esophagus, stomach, portal structures and liver, our approach has shown minimal morbidity and mortality. Surgery was only considered complete upon objective normalization of respiratory variation and/or normalization of the flow velocities often requiring tedious dissection. In our cohort of patients, we were able to significantly improve all hemodynamic variables with our laparoscopic approach. This correlated with an objectively improved quality of life based on total PedsQLtm scores. Because our response rate to PedsQLtm was only 55% we wanted to objectively document success or failure so medical records were reviewed and attempts at contacting the patients were made. If the last medical record documented continued pain and the patient was unreachable, we documented this as no improvement. With this protocol, we found patient reported improvement since the time of surgery in 31 of the 46 patients or 67% of patients. Although a success rate of 67% seems low, when considered within the context of these patients previously being labeled with FGID, we feel that there may be a surgical benefit with the release of the celiac artery in an appropriately selected patient population of FGID.
As to the causes for failure to improve, there may be several. First, it is possible that these patients are truly CFAP with concomitant MALS. Second, there may be a component of nerve compression that was inadequately released at the time of surgery. For these patients, celiac plexus block was used as a last resort. Unfortunately, no improvement was seen in the two patients who had continued pain and normalization of velocities. Whether this was due to a failed celiac plexus block or CFAP is unclear. Thirdly, we feel that there is a strong correlation between chronic physical pain and psychological pain. For this reason, we have made psychiatric and chronic pain screening and follow up a requirement for all patients. If there are any concerns in the pre-operative setting that may indicate psychiatric therapy as a benefit, we try to initiate this as soon as possible prior to surgery. We hope to report on this pathway in the near future. Finally, there is a possibility of fixed residual stenoses after external decompression that results in continued ischemia. These patients were found to have increased velocities on repeat duplex post-operatively. The idea that the laparoscopic approach in adolescents is limited by the inability to reconstruct the artery was highlighted by Said et al29 and we have adapted the approach of surgical decompression followed by either endovascular or open reconstruction has highlighted by Rosborough.30 No patient had an intra-operative conversion for bleeding. A total of three patients in our study underwent adjunct vascular procedures: one patient underwent balloon angioplasty for a residual web with excellent results and resolution of pain; the second patient had a web with a kink and was recommended to have a vascular reconstruction in conjunction with ongoing psychiatric counseling and this case continues; the third patient underwent a celiac reconstruction and reports resolution of symptoms. We are encouraged by our results that the majority of our adolescent patients have improved pain and QOL with just the laparoscopic release alone without adjunct vascular reconstruction. The excellent results reported by Roseborough30 reported that nine vascular interventions were performed in six of the 15 patients. Given the self reported 85% improvement and the 67% survey reported improvement of QOL in our study, we feel that three vascular interventions out of 45 patients may reflect that earlier intervention during adolescence may result in less fixed stenoses and a decreased need for adjunct vascular reconstruction. We are cautious in stating this because this study was not designed to look at fixed stenoses and we did note a less than perfect compliance rate. Furthermore, in two of these patients, there was arterial kinking that resulted post release and would not be addressed by endovascular approaches.
As our experience grew, we noted trends and developed concerns about the compliance rate to the PedsQLtm survey as well as the recurring fact that these patients were labeled as “difficult patients”. We felt that there may be several reasons why the compliance was low: first, the broad demographic location of many patients meant relying on patients to return surveys; second, for those patients that had no improvement, there was no benefit in filling out the survey; third, for those patients that had improvement, they could now move on and were not thinking about the survey; and finally, the age group itself makes compliance challenging.
Given the midrange compliance, high complexity of the patients, the young age, and the multi-factorial etiologies of pain, we have since assembled a multidisciplinary team to address all the needs of the patient. We have assembled experts in child Psychiatry and eating disorders; pain management; GI; Radiology (both CT and interventional); Pediatric and Vascular surgery. The multidisciplinary team approach has provided a major advantage in our ability to select patients who are appropriate candidates for surgery. Reilly et al. 31 published there series of 51 patients and demonstrated sustained relief in patients with post-prandial pain, age between 40–60, and weight loss of 20 pounds or more. These parameters unfortunately are not all transferrable to the adolescent population. We did observe that patients with post-prandial pain tended to have improvement in the pain compared to patients with nausea. To overcome this difficulty in determining who will benefit from an operation, we have come to rely on the input opinion from each member of the team. If any member feels that surgery is not indicated, or should be delayed we will not proceed down a surgical pathway until we have addressed any confounding issue. We carry this approach into the operating room where both the pediatric surgeon and vascular surgeon are present. Initial laparoscopy and dissection of the diaphragmatic crura are performed by the pediatric surgeon and the aortic and celiac dissection is performed by the vascular surgeon. Given the complexity of this disease process, we have become hyper-vigilant about safety and feel that we need to be ready to convert to open immediately; therefore, we recommend this combined service approach. We have found that this multidisciplinary approach throughout the course of the clinical pathway is patient centered and comprehensive with respect to evaluation and execution of the treatment plan.
This report suffers from several limitations inherent to the descriptive study design. There is an inherent selection bias as we are a tertiary care hospital and found that many of the patients sought us out because of the persistence of pain and lack of a specific diagnosis. Because of this self-referral pattern, we were somewhat limited in follow up. To the best of our ability we attempted QOL follow up and patient reported improvement of abdominal pain, but as such are limited in the long term weight gain, pain medication use and use of GI medications. Furthermore, we realize that despite our best attempts, 28 of the patients did not complete the full questionnaires which results in selection bias.
We realize that radiation exposure in this young population is of great concern. The gold standard for diagnosis of MALS is invasive angiography with measurements of pressure gradients. However, we felt that the combination of duplex ultrasound and a CTA with inspiratory and expiratory phases would give us the required data to support the duplex ultrasound. We initially chose CTA because magnetic resonant angiography has a tendency to have inadequate spatial resolution and movement artifacts.32, 33 Additionally, there is very little literature on the use of MRA in the pediatric population particularly in visualization of the celiac artery. The literature that is available, does note that despite the fact that there is no exposure to ionizing radiation, there are risks from contrast agents as well as the frequent requirement of sedation or general anesthesia.34 Although we still use CTA, we have been moving toward more frequent utilization of MRA. We also realize that longer follow-up is necessary to fully understand the ramifications of the surgery.
Overall, we believe that our results demonstrate an overlooked diagnosis of MALS in pediatric females with CFAP. The finding of young female predominance appears non-coincidental and especially relevant considering that this is also a main feature of FGID. We are encouraged by our early surgical results after ligament release. Whether the clinical improvement seen is due to restoration of adequate celiac blood flow, positive alteration of yet undefined neuro-enteric circuitry affecting visceral hypersensitivity through neurolysis, or both, remains an interesting question. Perhaps long term follow-up of a large pool of MALS patients with FGID analyzing changes in symptom clustering patterns before and after surgery would help answer this question, and, more importantly, help gain better understanding of the pathophysiology of FGID leading to more targeted treatment trials.
In conclusion, MALS was more commonly diagnosed in pediatric females than males. Laparoscopic release of the median arcuate ligament overlying the celiac artery can be performed safely in the pediatric population with the release of the artery and resultant neurolysis, resulting in significant improvement in the hemodynamics, symptoms, and overall QOL in this cohort. The overall improvement in QOL outcome measures after surgery leads us to conclude that MALS might be earlier diagnosed and possibly treated in patients with CFAP. In the previously thought group of children with the diagnosis of CFAP, MALS may be a treatable etiology with minimally invasive treatment. We thus advocate increased awareness of the diagnosis of MALS, appropriate screening for patients with possible MALS, a multidisciplinary team approach for the care and selection of surgical candidates with MALS, and continued study of these patients following surgery to better determine long-term effects.
Acknowledgments
Diane Nilsson, University of Chicago Medicine; Cynthia Chou MD, Lake Forest Pediatrics Associates; University of Chicago Medical Center Vascular Lab. The Quality of life study described in this paper was carried out using the PedsQLtm, developed by Dr. James W. Varni.
Dr. Skelly is funded by NIH K-08-HL091053 as well by the American Vascular Association/American College of Surgeons and NHLBI Jointly Sponsored Mentored Clinical Scientist Development Award. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or the NIH
We particularly would like to express our gratitude to the late Dr. Donald Liu who not only was one of the innovators of this technique and the MALS program at the University of Chicago, but also a valued mentor, colleague, and friend.
Footnotes
Conflict of Interest Declarations: The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosures: The authors have no financial conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Lehmann HP, Boyle JT, Gerson WT, Hyams JS, Squires RH, Walker LS. Clinical Report: Chronic Abdominal Pain in Children. Pediatrics. 2005;115:812–815. [Google Scholar]
- 2.Di Lorenzo C, Colletti RB, Lehmann HP, et al. Chronic Abdominal Pain in Children: A Technical Report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Ped Gastroenterol Nutr. 2005;40:249–261. doi: 10.1097/01.mpg.0000154661.39488.ac. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II: The functional gastrointestinal disorders. Diagnosis, pathophysiology and treatment: a multinational consensus. 2. McLean Virginia: Degnon Associates; 2000. [Google Scholar]
- 4.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WG. The road to Rome. Gastroenterology. 2006;130:1552–1556. doi: 10.1053/j.gastro.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 7.Koloski NA, Talley NJ, Boyce PM. Predictors of health care seeking for irritiable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychological factors. Am J Gastroenterol. 2001;96:1340–1349. doi: 10.1111/j.1572-0241.2001.03789.x. [DOI] [PubMed] [Google Scholar]
- 8.Saito YA, Schoenfeld P, Locke GRI. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 9.Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use and costs: a US managed care perspective. Am J Gastroenterol. 2003;98:600–607. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjecets with irritable bowel syndrome. Gastroenterology. 1995;109:1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 11.Martin R, Barron JJ, Zacker C. Irritable syndrome: toward a cost-effective management approach. Am J Manag Care. 201(7):S268–275. [PubMed] [Google Scholar]
- 12.Lembo A, Weber HC, Farraye FA. Alosetron in irritable bowel syndrome: strategies for the use in a common gastrointestinal disorder. Drugs. 2003;63:1895–1905. doi: 10.2165/00003495-200363180-00002. [DOI] [PubMed] [Google Scholar]
- 13.Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil. 2005;17:643–653. doi: 10.1111/j.1365-2982.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 14.Sagami Y, Shimada Y, Tayama J, et al. Effect of corticotrophin-releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138:125–128. doi: 10.1067/mpd.2001.109606. [DOI] [PubMed] [Google Scholar]
- 16.See MC, Birnbaum AH, Schechter CB, Goldenberg MM, Benkov KJ. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci. 2001;46:985–992. doi: 10.1023/a:1010793408132. [DOI] [PubMed] [Google Scholar]
- 17.Symon DN, Russell G. Double-blind, placebo-controlled trial of pizotifen syrup in the treatment of abdominal migraine. Arch Dis Child. 1995;72:48–50. doi: 10.1136/adc.72.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harjola PT. A rare obstruction of the coeliac artery. Ann Chir et Gynaec. 1963;52:547. [PubMed] [Google Scholar]
- 19.Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965;95(3):731–744. doi: 10.2214/ajr.95.3.731. [DOI] [PubMed] [Google Scholar]
- 20.Harjola PT, Lahtiharju A. Celiac axis syndrome: Abdominal angina caused by external compression of the celiac artery. Am J Surg. 1968;115:864–869. doi: 10.1016/0002-9610(68)90537-0. [DOI] [PubMed] [Google Scholar]
- 21.Stanley JC, Fry WJ. Median arcuate ligament syndrome. Arch Surg. 1971 Aug;103(2):252–258. doi: 10.1001/archsurg.1971.01350080168026. [DOI] [PubMed] [Google Scholar]
- 22.Loukas M, Pinyard J, Vaid S, Kinsella C, Tariq A, Tubbs RS. Clinical anatomy of celiac artery compression syndrome: a review. Clinical Anatomy. 2007;20:612–617. doi: 10.1002/ca.20473. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965 Nov;95(3):731–744. doi: 10.2214/ajr.95.3.731. [DOI] [PubMed] [Google Scholar]
- 24.Watson WC. Coeliac-axis compression. Lancet. 1977 Sep 10;2(8037):561–562. doi: 10.1016/s0140-6736(77)90699-7. [DOI] [PubMed] [Google Scholar]
- 25.Roayaie S, Jossart G, Gitlitz D, Lamparello P, Hollier L, Gagner M. Laparoscopic release of celiac artery compression syndrome facilitated by laparoscopic ultrasound scanning to confirm restoration of flow. J Vasc Surg. 2000 Oct;32(4):814–817. doi: 10.1067/mva.2000.107574. [DOI] [PubMed] [Google Scholar]
- 26.Varni JW, Seid M, Rode CA. The PedsQL™: Measurement Model for the Pediatric Quality of Life Inventory. Medical Care. 1999;37(2):126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Evans W. Long-term evaluation of the celiac band syndrome. Surgery. 1974;76:867–871. [PubMed] [Google Scholar]
- 28.Bech F, Loesberg A, Rosenblum J, Glagov S, Gewertz B. Median arcuate ligament compression in monozygotic twins. J Vasc Surg. 1994;19(5):934–938. doi: 10.1016/s0741-5214(94)70021-4. [DOI] [PubMed] [Google Scholar]
- 29.Said SM, Zarroug AE, Gloviczki P, Shields RC. Pediatric median arcuate ligament syndrome: first report of familial pattern and transperitoneal laparoscopic release. J Pediatr Surg. 2010 Dec;45(12):E17–E20. doi: 10.1016/j.jpedsurg.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Roseborough GS. Laparoscopic management of celiac artery compression syndrome. J Vasc Surg. 2009 Jul;50(1):124–133. doi: 10.1016/j.jvs.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 31.Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK. Late results following operative repair for celiac artery compression syndrome. J Vasc Surg. 1985 Jan;2(1):79–91. [PubMed] [Google Scholar]
- 32.Dellegrottaglie S, Sanz J, Rajagopalan S. Technology insight: Clinical role of magnetic resonance angiography in the diagnosis and management of renal artery stenosis. Nat Clin Pract Cardiovasc Med. 2006 Jun;3(6):329–338. doi: 10.1038/ncpcardio0556. [DOI] [PubMed] [Google Scholar]
- 33.Leiner T, Schoenberg SO. Current status of renal artery magnetic resonance imaging: theoretical and practical considerations and the potential role of blood-pool contrast agents. Eur Radiol. 2007 Mar;17 (Suppl 2):B13–17. [PubMed] [Google Scholar]
- 34.Grist TM, Thornton FJ. Magnetic resonance angiography in children: technique, indications, and imaging findings. Pediatr Radiol. 2005 Jan;35(1):26–39. doi: 10.1007/s00247-004-1350-1. [DOI] [PubMed] [Google Scholar]


