Abstract
Sustained induction and activation of matrixins (matrix metalloproteinases or MMPs), and the destruction and deposition of extracellular matrix (ECM), are the hallmarks of cardiac fibrosis. The reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) is a unique membrane-anchored endogenous MMP inhibitor. We hypothesized that elevated angiotensin II (Ang II), which is associated with fibrosis in the heart, differentially regulates MMPs and RECK both in vivo and in vitro. Continuous infusion of Ang II into male C57Bl/6 mice for 2 weeks resulted in cardiac fibrosis, with increased expressions of MMPs 2, 7, 9 and 14, and of collagens Ia1 and IIIa1. The expression of RECK, however, was markedly suppressed. These effects were inhibited by co-treatment with the Ang II type 1 receptor (AT1) antagonist losartan. In vitro, Ang II suppressed RECK expression in adult mouse cardiac fibroblasts (CF) via AT1/Nox4-dependent ERK/Sp1 activation, but induced MMPs 2, 7 and 9 via NF-κB, AP-1 and/or Sp1 activation. Further, while forced expression of RECK inhibits, its knockdown potentiates Ang II-induced CF migration. Notably, RECK overexpression reduced Ang II-induced MMPs 2, 9 and 14 activation, but enhanced collagens Ia1 and IIIa1 expression and soluble collagen release. These results demonstrate for the first time that Ang II suppresses RECK, but induces MMPs both in vivo and in vitro, and RECK overexpression blunts Ang II induced MMP activation and CF migration in vitro. Strategies that upregulate RECK expression in vivo have the potential to attenuate sustained MMP expression, and blunt fibrosis and adverse remodeling in hypertensive heart diseases.
Keywords: Cardiac fibrosis, Adverse remodeling, RECK, MMP, Nox4
1. Introduction
The Renin-Angiotensin-Aldosterone system plays an important role in normal myocardial function. However, chronic elevation in angiotensin II (Ang-II) levels is associated with persistent hypertension, myocardial hypertrophy, fibrosis and adverse remodeling, which if untreated, can progress to heart failure. While the deleterious effects of chronically elevated Ang II are generally considered to be mediated through the Ang II receptor AT1, signaling through AT2 is thought to oppose AT1-dependent pro-inflammatory, pro-hypertrophic and pro-fibrotic effects [1]. In cardiac fibroblasts (CF), the principle cell type responsible for cardiac fibrosis [2–5], Ang II, via AT1, upregulates the expression levels of various matrixins (a.k.a. matrix metalloproteinases or MMPs) that promote extracellular matrix (ECM) degradation, resulting in increased CF migration and proliferation, ECM deposition, fibrosis and adverse remodeling [6].
To date, nearly 28 MMPs have been discovered, with several being expressed at very low or undetectable levels in the normal heart. However, following injury, their chronic activation contributes to the aberrant remodeling of ECM. Enhanced expression and/or activation of MMPs, such as the gelatinases MMP2 and MMP9, the transmembrane metalloprotease MT1-MMP (Membrane type 1 matrix metalloprotease or MMP14), and the matrilysin MMP7, promote breakdown of various ECM components that can result in fibroblast invasion and proliferation, and fibrosis.
The reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) is a unique membrane-anchored endogenous MMP inhibitor that contains several serine protease inhibitor-like domains [7]. It is expressed in several organs under physiological conditions, including heart [7]. Its expression is however suppressed in various tumors. In fact, many cancer and oncogene-transformed cells show little or no expression of RECK, which might be a contributory factor in their invasive potential and malignancy [8, 9]. Although RECK has been shown to inhibit the activation of MMPs 2, 7, 9 and 14 [7, 10–12], all of which are known to contribute causally to myocardial fibrosis and adverse remodeling, its role in CVD, especially in hypertensive heart disease, has not been described.
Since chronically elevated Ang II levels are associated with cardiac fibrosis and adverse remodeling, we investigated the effect of Ang II on the regulation of MMPs and RECK in vivo and in vitro. Our results show for the first time that Ang II/AT1-mediated cardiac fibrosis in a mouse model is characterized by increased MMPs 2, 7, 9, and 14 expression, but suppressed RECK. Further, Ang II stimulates MMPs 2, 9 and 14 expression in isolated cardiac fibroblasts via NF-κB, AP-1 and/or Sp1 activation, but suppresses RECK via ERK/Sp1-dependent signaling. Notably, while forced expression of RECK inhibits, its knockdown potentiates Ang II-induced CF migration. Strategies that upregulate RECK expression may have the potential to blunt fibrosis and adverse remodeling in hypertensive heart diseases.
2. Methods
2.1. Materials
The materials used are detailed in the Supplementary methods section.
2.2. Infusion of Ang II and administration of losartan
This investigation conforms to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. All protocols were approved by the Institutional Animal Care and Use Committees of Tulane University, New Orleans, LA and the University of Texas Health Science Center, San Antonio. Male C57Bl/6 mice (~3 months old, and weighing ~25 g) were trained for systolic blood pressure (SBP) measurement by tail cuff plethysmography (CODA Noninvasive Blood Pressure System, Kent Scientific, Torrington, CT) without anesthesia [13, 14]. One group of mice was infused with 1.5 µg/kg/min of human Ang II for two weeks via subcutaneously implanted (midscapular region) Alzet miniosmotic pumps (n=8/group). Pumps were implanted under isofluorane anesthesia (5.0% for induction, and 2% for maintenance). A control group was implanted with sterile saline-filled pumps (n=6). A subset of mice receiving Ang II was co-treated with the AT1 antagonist losartan in drinking water (0.6 g/L). After blood pressure measurements, body weights were recorded, and the animals sacrificed. The hearts were rapidly excised, rinsed in ice-cold physiological saline, and weighed. The right ventricle and atria were trimmed away, and the left ventricle (LV) was weighed. The LV was cut into three pieces and two were snap-frozen in liquid N2 for not more than 3 days prior to analysis. The third piece was embedded in OCT for histo-morphometric analysis.
The dose of Ang II used in the present report is within the pathophysiological limits. Ang II was infused at 1.5 µg/kg/min for two weeks. While the basal levels of Ang II are ~0.25 pmol/ml, its infusion at 400 and 1,000 ng/kg/min has been shown to increase its systemic levels to approximately 0.5 and 0.8 pmol/ml, respectively [15]. These levels approximate 0.25 (basal), 0.5 and 0.8 ng/ml, respectively. Ang II at 1.5 µg/kg/min should equate to approximately 1.1 ng/ml. In patients with congestive heart failure and chronic kidney disease, Ang II levels are about 2–5 times above normal [16–19], and that based on the report of Gonzales-Villalobos et al. [15] the expected Ang II concentrations in our model should approximate to 4-fold normal in the mouse.
2.3. Assessment of cardiac remodeling
Since increased collagen synthesis and deposition is a significant feature of pathological cardiac remodeling, we quantified fibrosis by Picrosirius Red staining (8 µm cryosections) as previously described [13]. Myocardial hypertrophy served as confirmatory evidence of a response to Ang-II, and was analyzed by a ratio of heart weight to body weight.
2.4. Isolation of cardiac fibroblasts
Cardiac fibroblasts (CF) were isolated using collagenase digestion and differential centrifugation as we have described in our previous published reports [20–22] and detailed in Supplementary methods. CF were used between the second and third passages. At 70% confluency, the cells were made quiescent by incubating in medium containing 0.5% BSA (serum free) for 48 h. At the end of the experimental period, culture supernatants were collected and snap frozen. Cells were harvested, snap frozen, and stored at −80°C.
2.5. Detection of hydrogen peroxide by Amplex® Red assay
The quiescent CF were treated with Ang II (10−7M for 30 min). H2O2 production was measured as previously described [22] using a commercially available fluorescent Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes Inc./Life Technologies), according to the manufacturer's instructions. Fluorescence was recorded at 530 nm excitation and 590 nm emission wavelengths (CytoFluor II; Applied Biosystems, Foster City, CA). Standard curves were generated using known concentrations of H2O2. Studies were also performed after DPI pretreatment or Ad.siNox4 transduction.
2.6. Adeno and lenti viral transduction
Adenovirus containing the full-length mouse RECK ORF (GenBank accession # NM_016678.2) under control of the CMV promoter (Ad.RECK) was custom made at Vector Biolabs (Philadelphia, PA). Adenoviral vectors expressing siRNA against MMP2 (Ad.siMMP2) and MMP9 (Ad.siMMP9) were provided by Jasti S. Rao (University of Illinois College of Medicine, Peoria, IL). Ad.siGFP was used as a control. Lentival shRNA targeting RECK, p65, c-Jun (JUN), Sp1, and GFP were purchased from Sigma-Aldrich. Adeno and lentiviral infections are described in Supplementary methods.
2.7. Transcription factor activation
Nuclear extracts were prepared using the Panomics Nuclear Extraction kit according to the manufacturer's (Panomics/Affymetrix, Freemont, CA) instructions. Nuclear p-p65 (Ser536) p-c-Jun (Ser63) levels were analyzed by immunoblotting. Lamin A/C (nuclear) and GAPDH (cytoplasmic) served as loading and purity controls.
2.8. mRNA expression
Total RNA was isolated using the TRIzol method, treated with DNase, and 1 µg of DNA-free total RNA reverse transcribed using the Quantitect cDNA Synthesis Kit (Qiagen). MMP2, MMP9, RECK, collagen Ia1, and collagen IIIa1 mRNA expression levels were analyzed by RT-qPCR using TaqMan® probes and Eppendorf Realplex4 system [20–23]. Data are shown as fold change (2−ΔΔCt). All data were normalized to corresponding 18S rRNA, and expressed as fold change relative to untreated controls.
2.9. Immunoblotting, immune complex kinase assay, and Biotrak activity assays
Extraction of whole cell lysates, membrane, cytoplasmic and nuclear protein extracts, immunoprecipitation, immunoblotting, chemiluminescence, and densitometry were performed as previously described [20, 21, 23]. ERK enzyme activity was analyzed by immune complex kinase assays using whole cell homogenates and a commercially available colorimetric assay kit (Cell Signaling Technology, Inc.).
MMP2 and MMP9 activities in concentrated culture supernatants containing 1 µg of total protein were analyzed using the MMP2 (RPN2631) and MMP9 (#RPN2634) activity assay kits (GE Healthcare Biosciences, Piscataway, NJ) according to the manufacturer’s instructions. MMP14 activity in Ang II-treated CF cell extracts was determined by the MT1-MMP Biotrak assay kit (RPN2637). CF were washed with ice-cold PBS and lysed for 15 minutes in MMP assay buffer supplemented with 0.25% (v/v) Triton X-100. MMP14 levels were also quantified by immunoblotting using the membrane fractions. GAPDH in cytoplasmic extracts served as a loading control.
2.10. Soluble collagen release
The effect of Ang II on the secretion of soluble collagen was determined as previously described [22]by the Sircol Collagen assay (Biocolor, Newtownabbey, N. Ireland). The assay is based on the specific binding of the anionic dye Sirus red, to the basic amino acid residues of collagen. Cell numbers were quantified by CyQuant assay as previously described [24], and collagen levels were normalized to cell numbers,
2.11. Cell migration
CF migration was quantified as described previously using BioCoat™ Matrigel™ invasion chambers and 8.0-µm pore polyethylene terephthalate membranes with a thin layer of Matrigel™ basement membrane matrix [20, 22]. Cultured CF were trypsinized and suspended in RPMI + 0.5% bovine serum albumin, and 1 ml containing 2.0 × 105 cells/ml was layered on the coated insert filters. Cells were stimulated with Ang II (10−7 M). The lower chamber contained 10% serum. Plates were incubated at 37 °C for 12 h. Membranes were washed with PBS, and non-invading cells on the upper surface were removed using cotton swabs. CF invading into and through the Matrigel™ matrix were quantified by MTT assay. Numbers of CF migrating in response to Ang II were normalized to those of untreated cells and expressed as fold change from untreated.
2.12. Cell death analysis
To determine whether transduction of viral vectors, pharmacological inhibitors or over-expression of mutant proteins affected cell viability, cell death was analyzed using the Cell Death Detection ELISAPLUStrypan blue dye exclusion, and microscopic visualization of cell shape and for cells floating in the media.
2.13. Statistical analysis
Comparisons between controls and various treatments were performed by analysis of variance with post hoc Dunnett's t tests. All assays were performed at least three times, and the error bars in the figures indicate the S.E.
3. Results
3.1. Ang II differentially regulates MMPs and RECK in vivo
The continuous infusion of mice with Ang II for 2 weeks resulted in increased systolic blood pressure and cardiac hypertrophy (Supplementary Fig. 1). Further, Ang II increased both interstitial and perivascular fibrosis (Fig. 1A). The levels of expression of the ECM protein genes collagen types Ia1 and IIIa1 were enhanced (Fig. 1B). Moreover, Ang II infusion was associated with increased activation of MMPs 2 and 9, and increased levels of MMP7 and 14 in myocardial homogenates (Fig. 1C). In marked contrast, the mRNA and protein expression of RECK, the negative regulator of MMPs, was significantly reduced (Fig. 1D, E). Co-treatment with the AT1 antagonist losartan reversed these changes. Thus the cardiac fibrosis that results from Ang II infusion is characterized by the increased expression of matrix degrading enzymes, reinforced by the reduced expression of their natural inhibitor RECK (Fig. 1).
Fig. 1. Ang II/AT1-induced myocardial fibrosis is characterized by increased MMPs and suppressed RECK expression.
A, Ang II infusion increases cardiac fibrosis via AT1. Male C57Bl/6 mice were continuously infused with Ang II (1.5 µg/kg body wt/min) for 14 days via miniosmotic pumps. Saline served as a control. One group of mice receiving Ang II was co-treated with the AT1 antagonist losartan in drinking water (0.6 g/L). Collagen deposition was analyzed by Picrosirius Red staining of cryosections (8 µm), and photographed at 100× magnification (n=6). B, Ang II increases ECM protein gene expression. Left ventricular (LV) tissue from mice described in A was analyzed for collagens type Ia1 and IIIa1 by RT-qPCR. Expression of 18S rRNA served as an invariant control. *P < 0.01 vs. saline, †P < 0.05 vs. Ang II (n = 6/group). C, Ang II induces MMPs expression. LV tissue from mice described in A was analyzed for MMP2 and 9 activation by immunoblotting using antibodies that detect both pro and active forms (left hand panel). MMPs 7 and 14 expressions were also analyzed by immunoblotting (right panel) (n=2–4). D, E, Ang II suppresses RECK expression. LV tissue from mice described in A were analyzed for RECK mRNA expression by RT-qPCR (D) and protein levels by immunoblotting (E; n=2–4). D*P < at least 0.01 vs. saline, †P < 0.05 vs. Ang II (n = 6/group).
3.2. Ang II-induced cardiac fibroblast (CF) migration is mediated by MMPs 2 and 9, but inhibited by RECK
Fibroblasts are the major non-muscle cell type in the heart responsible for ECM regulation and remodeling, with migration of CF into injured tissue playing a significant role in the remodeling process [2–5]. Since myocardial RECK expression was significantly reduced in vivo following Ang II infusion (Fig. 1D, E), we investigated the role of RECK on CF migration using Boyden chamber invasion assays. Ang II induced a significant increase in CF migration that was significantly inhibited by losartan (Fig. 2A). Notably, overexpression of RECK by adenoviral transduction markedly inhibited Ang II-induced CF migration (Fig. 2B). In contrast, knockdown of RECK using lentiviral shRNA potentiated CF migration (Fig. 2B). Neither RECK overexpression nor its knockdown affected cell viability (data not shown). In contrast to RECK, knockdown of MMP2 or MMP9 attenuated Ang II-induced CF migration (Fig. 2C). These results indicate that RECK and MMPs differentially regulate Ang II-stimulated CF migration (Fig. 2).
Fig. 2. Ang II-induced cardiac fibroblast migration is differentially regulated by RECK and MMPs.
A, Ang II stimulates CF migration via AT1. At 70% confluency, CF were made quiescent by incubating in medium supplemented with 0.5% BSA for 48h. The quiescent CF were trypsinized, re-suspended in medium containing 0.5% BSA, layered on Matrigel™ basement membrane matrix-coated filters, incubated with or without losartan (10 µM for 1 h) and then with Ang II (10−7M for 12 h). The lower chamber contained media with 10% serum. Cells migrating to the other side of the membrane were quantified using MTT assay. B, Forced expression of RECK inhibits, but its knockdown potentiates Ang II-induced CF migration. CF transduced with Ad.RECK (moi 40 for 24 h) or lentiviral RECK shRNA (moi 0.5 for 48 h) were made quiescent, layered on Matrigel™ basement membrane matrix-coated filters, incubated with Ang II (10−7M) for 12 h, and analyzed for migration by MTT assay. Knockdown of RECK was confirmed by immunoblotting (inset). Akt served as an off target (n=3). *P < 0.001 vs. untreated; †P < 0.01 vs. Ang II, §P < 0.05 vs. Ang II (n=6). C, Ang II induces CF migration via MMP2 and MMP9. CF transduced with adenoviral MMP2 or MMP9 siRNA (moi 100 for 48 h) were analyzed for migration as in B. Knockdown of MMP2 and MMP9 was confirmed by immunoblotting as shown on the right (n=3). *P < 0.001 vs. untreated; †P < at least 0.05 vs. Ang II (n=6).
3.3. Ang II induces CF migration through Nox4 NADPH oxidase-dependent ERK activation
The MAP kinase ERK plays a critical role in Ang II-induced signal transduction and responses, including Ang II-induced CF proliferation [25, 26]. Addition of Ang II to CF induced ERK enzyme activity in a time-dependent manner, as determined by a phosphorylation assay using Elk-1 as a substrate (Fig. 3A). Further, since Ang II is a potent pro-oxidant, and NADPH oxidases are often the major contributors of ROS generation, we investigated the role of Nox4, the dominant NADPH oxidase isotype in CF [27], in H2O2 production and ERK activation. Pre-treatment with the Nox inhibitor DPI, or knockdown of Nox4 with Ad.siRNA each inhibited Ang II-induced H2O2 production (Fig. 3B) and ERK activation (Fig. 3C). Pretreatment with the ERK inhibitor PD98059, but not the p38MAPK inhibitor SB203580 or the JNK inhibitor SP600125, also inhibited Ang II-induced ERK activation (Fig. 3D). Further, PD98059 attenuated Ang II-induced CF migration (Fig. 3E), as did knockdown of Nox4 (Fig. 3F). These results indicate that Ang II stimulates CF migration via Nox4/ROS-dependent ERK activation (Fig. 3).
Fig. 3. Ang II induces CF migration via Nox4-dependent ERK activation.
A, Ang II induces ERK activation. The quiescent CF incubated with Ang II (10−7M) were analyzed for ERK activation by an in vitro immune complex kinase assay using Elk-1 as a substrate. A representative of three independent experiments is shown. B, Ang II stimulates Nox4-dependent H2O2 generation. CF infected with Ad.siNox4 (moi 100 for 48 h) or treated with DPI (10 µM for 30 min) prior to Ang II addition (10−7M) for 30 min were analyzed for H2O2 production using the Amplex® Red assay. *P < 0.01 vs. untreated; †P < at least 0.05 vs. Ang II ± DMSO or Ad.siGFP (n=6). C, Ang II induces ERK activation via Nox4 and ROS. CF transduced with Ad.siNox4 (moi 100 for 48 h) or pretreated with DPI (10 µM for 30 min) were incubated with Ang II (10−7M) for 30 min. ERK activation was analyzed as in A (n=3). Knockdown of Nox4 was confirmed by immunoblotting (right hand panel). D, PD98059 inhibits Ang II-induced ERK activation. The quiescent CF were treated with the ERK inhibitor PD98059 (10 µM for 1 h), p38MAPK inhibitor SB203580 (1 µM for 30 min) or JNK inhibitor SP600125 (20 µM for 30 min) prior to Ang II addition (10−7M for 30 min). ERK activation was analyzed as in A (n=3). E, Ang II stimulates CF migration via ERK. The quiescent CF were layered on Matrigel™ basement membrane matrix-coated filters, incubated with PD98059 (10 µM for 1 h) followed by Ang II (10−7M for 12 h). Cell migration was analyzed by MTT assay. *P < 0.001 vs. untreated; †P < 0.01 vs. Ang II (n=6). F, Knockdown of Nox4 attenuates Ang II-induced CF migration. CF transduced with Ad.siNox4 (moi 100 for 48 h) were incubated with Ang II (10−7M) for 12 h were analyzed for migration as in A. A–D *P < at least 0.01 vs. untreated; †P < at least 0.05 vs. Ang II (n=6).
3.4. Ang II induces MMPs 2 and 9 via NF-κB- and/or AP-1 activation, and MMP14 via Sp1
We have demonstrated that CF migration in response to Ang II is positively regulated by MMPs 2 and 9, and negatively regulated by RECK (Fig. 2). To understand the diverse effects of Ang II, we next investigated the underlying mechanisms. Ang II induced both MMP2 (Fig. 4A) and MMP9 (Fig. 4B) mRNA expression in CF, an effect that was markedly attenuated by p65 or c-Jun knockdown using lentiviral shRNA. Importantly, knockdown of p65 or c-Jun also attenuated Ang II-induced CF migration (Fig. 4C), indicating that Ang II-induced CF migration is mediated in part via NF-κB or AP-1 induced MMP2 and MMP9 expression. Further, the ERK inhibitor PD98059 inhibited Ang II induced p65 and c-Jun activation (Fig. 4D). In addition to MMPs 2 and 9, CF also express MMP14, but not MMP7 ([28, 29]), and Sp1 has been shown to positively regulate MMP14 expression [30]. Therefore, we investigated whether Ang II induces MMP14 expression via Sp1. Indeed, Ang II-induced Sp1 activation via ERK (Fig. 4E), and knockdown of Sp1 attenuated MMP14 expression (Fig. 4F).
Fig. 4. Ang II induces CF migration and collagen expression via NF-κB, AP-1 and/or Sp1-dependent MMP expression.
A, B, Ang II induces MMP2 and MMP9 expression via p65 and c-Jun. CF transduced with lentiviral shRNA (moi 0.5 for 48 h) were incubated with Ang II (10−7M) for 2 h. MMP2 (A) and MMP9 (B) mRNA expression was analyzed by RT-qPCR. Knockdown of p65 and c-Jun was confirmed by immunoblotting as shown on the right (n=3). *P < 0.01 vs. untreated; †P < 0.05 vs. Ang II (n=3). C, Ang II stimulates CF migration via p65 and c-Jun. CF transduced with lentiviral p65 or c-Jun shRNA (moi 0.5 for 48 h) were made quiescent, layered on Matrigel™ basement membrane matrix-coated filters, and incubated with Ang II (10−7M) for 12 h. Cell migration was analyzed by MTT assay. c-Jun and p65 served as respective off targets. *P < 0.001 vs. untreated; †P < 0.01 vs. Ang II (n=6). D, Ang II induces p65 and c-Jun activation via ERK. The quiescent CF were incubated with PD98059 (10 µM for 1 h) prior to Ang II (10−7M for 30 min) addition. Phospho-p65 and phospho-c-Jun levels were analyzed by immunoblotting using nuclear protein extracts. Lamin A/C (nuclear) and GAPDH (cytoplasmic) served as loading and purity controls (n=3). E, F, Ang II activates Sp1 via ERK. The quiescent CF were treated PD98059 (10 µM for 1 h) prior to Ang II addition (10−7M for 3 h). Sp1 activation was analyzed by ELISA using nuclear protein extracts (n=6). Lamin A/C and GAPDH served as loading and purity control (inset). *P < 0.001 vs. untreated; †P < 0.01 vs. Ang II ± DMSO (n=6). F, Ang II induces MMP14 via Sp1. CF transduced with lentiviral Sp1 shRNA (moi 0.5 for 48 h) prior to Ang II addition (10−7M for 3 h) were analyzed for MMP14 expression by immunoblotting using membrane fractions (n=3). Knockdown of Sp1 was confirmed by immunoblotting and is shown on the right. c-Jun served as an off-target. G, H, Ang II induces collagens type Ia1 (G) and IIIa1 (H) expression in part via AP-1. CF treated as A, but for 24 h were analyzed for collagen Ia1 (G) and collagen IIIa1 (H) mRNA expression by RT-qPCR and protein levels by immunoblotting (n=3). G, H*P < 0.01 vs. untreated; †P < 0.01 vs. Ang II ± GFP shRNA (n=6).
Cardiac ECM is predominantly comprised of collagens type I and III that contribute to cardiac structural integrity and function, as well as to the remodeling process. Therefore, we next investigated whether Ang II regulates collagens type Ia1 and IIIa1 in CF. Ang II enhanced the mRNA and protein expression levels of both ECM protein genes (Fig. 4G and H), and knockdown of c-Jun attenuated their expression. Together, these results demonstrate that Ang II-induces MMPs 2, 9 and 7, and collagens I and III, via ERK-dependent NF-κB, AP-1 and/or Sp1 activation (Fig. 4).
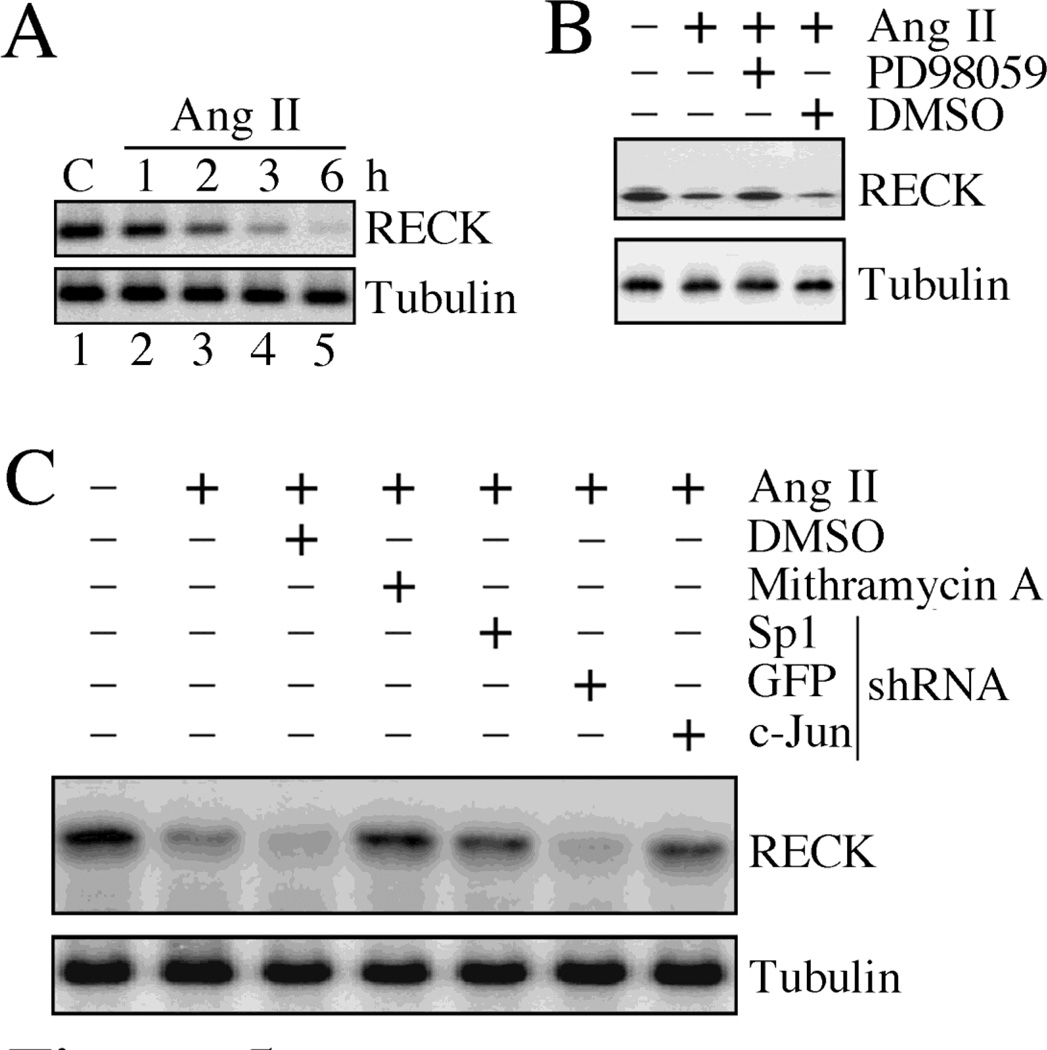
3.5. Ang II suppresses RECK expression in CF
In contrast to MMP induction (Fig. 4), Ang II suppressed RECK expression in CF in a time-dependent manner, with significantly reduced levels seen as early as 2 h (Fig. 5A), and a marked reduction at 6 h. Pre-treatment with the ERK inhibitor PD98059 reversed Ang II induced RECK suppression (Fig. 5B). Since Ang II activates Sp1 (Fig. 4E), and as Sp1 activation has been shown to negatively regulate RECK expression in cancer cells [31–33], we next investigated whether Ang II suppresses RECK via Sp1 activation. Pre-treatment of CF with the ERK inhibitor PD98059 reversed Ang II-induced RECK suppression (Fig. 5B). Similarly, pre-treatment with the Sp1 inhibitor mithramycin or Sp1 knockdown reversed Ang II-induced RECK suppression (Fig. 5C), as did c-Jun knockdown. These results indicate that Ang II suppresses RECK expression via ERK-dependent Sp1 or AP-1 activation (Fig. 5).
Fig. 5. Ang II suppresses RECK expression via Sp1.
A, Time-dependent suppression of RECK expression by Ang II. The quiescent CF treated with Ang II (10−7M) for the indicated time periods were analyzed for RECK expression by immunoblotting using cleared whole cell lysates (n=3). B, Ang II suppresses RECK expression via ERK. The quiescent CF were treated with PD98059 (10 µM for 1 h) prior to Ang II addition (10−7M for 3 h). RECK expression was analyzed as in A (n=3). C, Ang II suppresses RECK via Sp1. CF transduced with lentiviral Sp1, GFP and c-Jun shRNA (moi 0.5 for 48 h) or treated with mithramycin (100 nM in DMSO for 45 min) prior to Ang II addition (10−7M for 3 h) were analyzed for RECK expression by immunoblotting (n=3).
3.6. RECK suppresses MMP activation in CF
We have demonstrated that while MMP2 and 9 knockdown inhibits Ang II-induced CF migration, RECK overexpression blunts the pro-migratory effects of Ang II (Fig. 2). Since RECK is known to inhibit MMPs 2, 7, 9 and 14 [7, 10, 11] [12], we next investigated whether RECK overexpression inhibits MMP activation in CF. Since MMP7 is expressed in cardiomyocytes and inflammatory cells [28, 29], but not cardiac fibroblasts, we focused on MMPs 2, 9 and 14. We analyzed MMPs 2 and 9 activations by immunoblotting using equal amounts of culture supernatants and antibodies that detect both pro and active forms. MMP14 expression was analyzed in membrane fraction by immunoblotting. Our results show that Ang II enhanced the activities of MMPs 9 (Fig. 6A), 2 (Fig. 6B), and 14 (Fig. 6C), and RECK overexpression attenuated these effects. The Biotrak activity assays using culture supernatants (MMPs 2 and 9) and cellular extracts (MMP14) confirmed these results (right hand panels in Fig. 6A, 6B and 6C), indicating that RECK inhibits activation of both soluble (MMP2, MMP9) and membrane-bound MMP (MMP14) in CF (Fig. 6).
Fig. 6. RECK overexpression inhibits MMP activation.
A–C, Forced expression of wild type RECK (Ad.RECK) inhibits MMP9 (A), MMP2 (B) and MMP14 (C) activation. CF were transduced with Ad.RECK (moi 40 for 24 h) and then treated with Ang II (10−7M) for an additional 24 h. MMP9 (A) and MMP2 (B) enzymatic activity was analyzed by immunoblotting using equal amounts of culture supernatants and antibodies that detect both pro and active forms (left hand panels). MMP7 expression (C) was analyzed by immunoblotting using membrane fractions. Tubulin (whole cell homogenates) or GAPDH (cytoplasmic extracts) served as loading controls. The immunoreactive bands were semiquantified by densitometry and results from three independent experiments are summarized in the middle panels. Enzymatic activities were also analyzed by Biotrak activity assay kits (right hand panels) using concentrated culture supernatants containing 1 µg of total protein (MMPs 9 and 2). MMP7 enzymatic activity was also analyzed by the Biotrak activity assay kit using whole cell lysates (C, right hand panel). A–C, *P < 0.01 vs. respective untreated; †P < at least 0.05 vs. Ang II ± Ad.GFP (n=6).
3.7. RECK overexpression enhances collagen expression and secretion in CF
Since RECK is an MMP inhibitor, and as MMPs exert collagenolytic activity, we next examined whether RECK overexpression enhances Ang II-induced collagen expression and secretion. Our results show that Ang II induced collagens types Ia1 (Fig. 7A) and IIIa1 (Fig. 7B) protein levels, and RECK overexpression further enhanced their expression. Moreover, RECK overexpression enhanced Ang II-induced increases in soluble collagens secretion (Fig. 7C). These results indicate that RECK potentiates Ang II-induced collagens expression and secretion (Fig. 7).
Fig. 7. RECK overexpression enhances Ang II-induced collagen expression.
A, B, Forced expression of wild type RECK enhances Ang II-induced collagens type Ia1 (A) and type IIIa1 (B) levels. CF transduced with Ad.RECK (moi 40 for 24 h) were treated with Ang II (10−7M) for 12 h, and then analyzed for collagen expression by immunoblotting using cleared whole cell lysates. The immunoreactive bands from three independent experiments were semiquantified by densitometry and are shown in the respective lower panels. A, B*P < 0.05 vs. respective untreated; †P < 0.05 vs. Ang II ± Ad.GFP (n=3). C, RECK overexpression enhances total collagen release. CF were transduced with Ad.RECK as in A, and then treated with Ang II for 48 h. Equal amounts of culture supernatants were analyzed for recently synthesized collagens by Sircol collagen assay and normalized to cell numbers. In addition to cell numbers, Tubulin in whole cell lysates also served as a control (inset). *P < at least 0.01 vs. untreated; †P < 0.05 vs. Ang II ± Ad.GFP (n=6).
4. Discussion
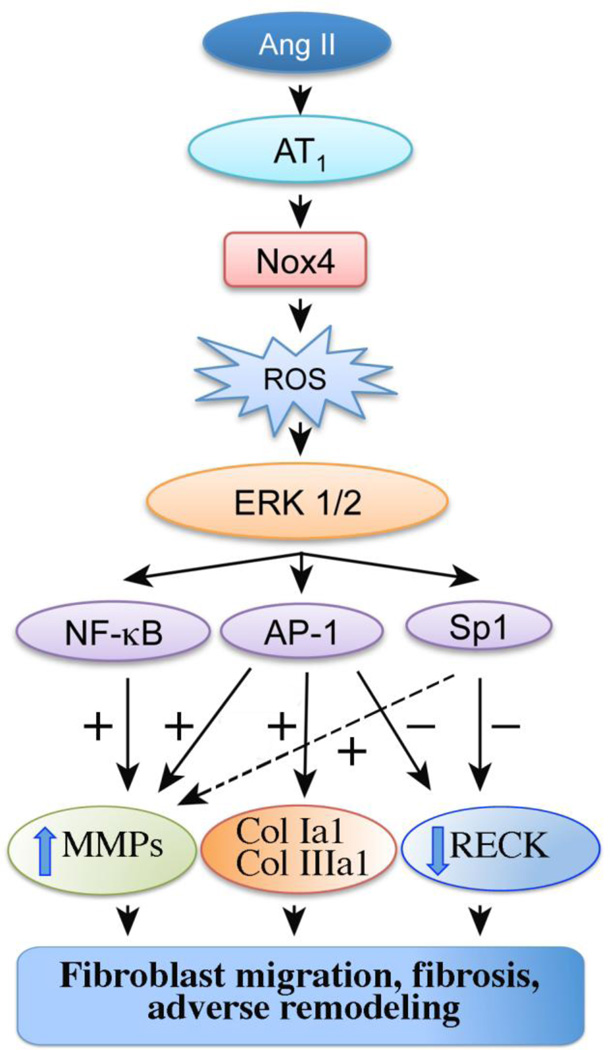
RECK is a unique membrane-anchored MMP regulator [7], that has been shown to inhibit the activity of MMPs 2, 7, 9 and 14 [7, 10–12]. Enhanced expression of the MMPs contributes causally to the myocardial fibrosis and adverse remodeling that can occur in the heart after injury. Though reduced or lack of RECK expression in the progression of certain cancers is well described [8, 9], its expression and role in CVD, particularly in hypertensive heart disease, have not previously been reported. Here we show for the first time that Ang II-induced cardiac fibrosis is characterized by increased expression of MMPs 2, 7, 9 and 14, but suppressed RECK expression, suggesting differential regulation of MMPs and their endogenous inhibitor RECK by Ang II. Supporting these in vivo observations, Ang II induced MMPs 2, 9 and 14 expression in cardiac fibroblasts in vitro via ERK-dependent NF-κB, AP-1, and/or Sp1 activation, but suppressed RECK via Sp1 activation. Further, Ang II induced the synthesis and secretion of collagens type Ia1 and IIIa1 via AP-1 activation, and RECK overexpression enhanced this effect. Notably, while forced expression of RECK inhibits, its knockdown potentiates Ang II-induced CF migration (Fig. 8).
Fig. 8.
Schema showing possible signal transduction pathways involved in the differential regulation of MMPs and RECK in Ang II-induced collagen synthesis, fibroblast migration, fibrosis, and adverse remodeling. +: positive regulation, −: negative regulation, broken arrow: though we have not investigated it here, Sp1 has also been shown to transcriptionally upregulate MMP9.
Although both RECK and TIMPs inhibit MMP activity, marked differences exist between these two classes of MMP inhibitors. For example, while TIMPs are secreted proteins [34], RECK is expressed on the plasma membrane as a GPI-anchored protein [7]. As soluble proteins, TIMPs act both locally and distally, whereas as a membrane-anchored protein, the inhibitory effect of RECK may be localized. Further, TIMPs inhibit all MMPs, whereas RECK to date has only been shown to inhibit MMPs 2, 7, 9 and 14 [7, 10–12]. However, based on structural similarities between various subclasses of MMPs, it’s plausible that RECK may inhibit other MMPs as well. In addition, while the expression of TIMPs is mostly regulated at the transcriptional level, RECK expression is regulated both transcriptionally and post-transcriptionally. Interestingly TIMPs also exert anti-apoptotic and prosurvival effects, independent of their MMP regulation. Whether RECK exerts similar or opposing effects remains to be determined.
In contrast to studies in cancer [8, 9], there are virtually no reports describing the role of RECK in either human or animal models of cardiovascular disease. In the one study we were able to identify [35], the investigators reported the increased expression of RECK in the fibrotic atria of patients with atrial fibrillation (AF). In those subjects, immunohistochemistry revealed RECK positive staining in cells present in the ECM [35]. RECK expression was also detected as diffuse positive spots or cell accumulations in the interstitium. Immunoblotting confirmed increased RECK expression in right atrial free walls [35]. Based on these observations, the authors speculated that increased RECK expression may be a compensatory mechanism in this late stage of the disease, since those subjects also showed increased levels of MMP2 and TIMPs, and fibrosis [35]. These important association studies however did not provide mechanistic insights into RECK expression, role, and regulation in fibrosis.
Here we show that Ang II infusion in mice induces cardiac fibrosis with increased expression of MMPs, but suppressed expression of RECK. Similar to its in vivo effects, Ang II induced MMPs 2, 9 and 14 in cardiac fibroblasts in vitro. Ang II stimulated Nox4-dependent H2O2 production, the activation of the redox-sensitive transcription factors NF-κB and AP-1, and the induction of MMPs. While both NF-κB and AP-1 have been shown to positively regulate MMP9 transcription, various cis-regulatory elements have been shown to play a role MMP2 regulation. In addition to two AP-2 and at least four Sp1 binding sites in its proximal promoter region [36], the MMP2 promoter also contains several AP-1 binding sites, and FosB, JunB, c-Jun, and Fra1 contributed to its increased transcription [37]. In addition, p53, nm-23β, and RE-1 have also been shown to regulate MMP2 transcription [38]. We analyzed a 4.1 kb mouse MMP2 promoter (GenBank: AB125668.1) and the first exon by both www.cbrc.jp/research/db/TFSEARCH.html and MatInspector Professional® software and identified several putative NF-κB binding sites; five in the promoter region and one in the 5’UTR (data not shown), suggesting a potential role for NF-κB in MMP2 regulation. Also, a role for NF-κB in MMP2 expression has been previously reported [38–40], suggesting that MMP2 regulation is complex. Here we show that Ang II-induced MMP2 expression is attenuated by p65 or c-Jun knockdown.
In contrast to the induction of the MMPs, Ang II suppressed RECK expression in part via Sp1 activation. The RECK proximal promoter region contains two Sp1-binding sites [33], and ERK-dependent Sp1 activation has been shown to repress RECK expression and promote HER-2/neu oncogene-induced tumor cell invasion [32]. Consistent with those results in tumor cells, we show here that Ang II suppresses RECK expression in cardiac fibroblasts via ERK/Sp1 activation. Interestingly, Sp1 activation induces MMP14 expression [30], suggesting that Ang II-mediated Sp1 activation differentially regulates MMPs and their inhibitor RECK in CF, most likely resulting in a net increase in MMP production/activation, ECM destruction, and fibroblast migration.
RECK inhibits MMPs 2, 7, 9 and 14 [7, 10–12]. These MMPs contribute causally to myocardial fibrosis and adverse remodeling [41–44]. RECK inhibits MMP2 and MMP9 secretion and enzymatic activity [7]. Recently, RECK has also been shown to inhibit MMP9 transcription [45]. In those studies, RECK overexpression was shown to prevent Fra-1 and c-Jun binding to the TRE-1 site within the MMP9 promoter region. RECK however failed to affect MMP2 transcription or mRNA expression [45]. RECK forms a complex with MMP14 and inhibits its maturation and proteolytic activity [12]. RECK also promotes the rapid internalization and decay of MMP14 [12]. RECK competitively inhibits MMP7 activity [11]. Surprisingly, RECK is also a target of MMP proteolytic activity. Using purified proteins, both MMPs 2 and 7 have been shown to cleave RECK in vitro [11]. It is thus possible that decreased transcription via Sp1 activation and increased degradation by MMPs might have contributed to reduced RECK expression in vivo in hearts of Ang II infused animals. Of note, RECK has also been shown to inhibit ADAMs 10 and 17 (TACE) [46, 47], whose enhanced expression showed a strong positive correlation with cardiac remodeling and failure [48, 49]. Further, knockdown of ADAM17 has been shown to inhibit angiotensin II-induced myocardial hypertrophy and fibrosis [50].
The matrix metalloproteinase system is the major proteolytic system involved in the degradation of ECM components in the heart, and cardiac fibroblasts via MMPs regulate ECM degradation and deposition, and thus cardiac remodeling. Initially at least, ligand-dependent rapid activation of preformed MMPs present in ECM degrade various components of ECM, followed later by the sustained induction and activation of MMPs and excessive ECM degradation. This results in the breakdown of myocyte–matrix interface, myocyte misalignment and slippage, LV dilation, and dysfunction, suggesting that sustained MMP induction is deleterious to the heart. In fact, gene deletion of various MMPs has been shown to be protective in various models of myocardial injury [41–44]. Here we show increased levels of MMPs 2, 7, 9 and 14 in the heart even after 14 days of Ang II administration. The initial breakdown in ECM components by MMPs is followed by CF migration and proliferation, and enhanced ECM deposition, and fibrosis. Our results show increased interstitial and perivascular fibrosis following Ang II infusion. In contrast to MMP expression, Ang II infusion is associated with inhibition of RECK both in vivo and in vitro in CF. Thus the combination of MMP induction and RECK downregulation may result in sustained activation of MMPs, increased collagenolytic activity, CF migration and proliferation, fibrosis, and adverse remodeling. These results suggest that strategies that upregulate RECK expression in vivo might attenuate the deleterious effects of the sustained activation of various MMPs and ADAMs in CVD, specifically in hypertensive heart disease.
Supplementary Material
Highlights.
-
►
RECK is a unique membrane-anchored MMP inhibitor
-
►
Angiotensin II induces MMP expression in vivo and in vitro
-
►
Ang II suppresses RECK expression in vivo and in vitro
-
►
RECK overexpression inhibits Ang II-induced MMP expression and fibroblast migration
-
►
Strategies that upregulate RECK may attenuate fibrosis and adverse remodeling
Acknowledgements
BC is a recipient of the Department of Veterans Affairs Research Career Scientist award, and is supported by VA Office of Research and Development Biomedical Laboratory Research and Development Service Award 1IO1BX000246 and the NIH/NHLBI grant HL-86787. The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- ADAM
a disintegrin and metalloproteinase domain
- AP-1
activator protein-1
- ARB
angiotensin receptor blockers
- ACE
angiotensin converting enzyme
- AT1
angiotensin II type 1 receptor
- AT2
angiotensin II type II receptor
- CF
cardiac fibroblasts
- CMV
cytomegalovirus
- CVD
cardiovascular disease
- DPI
diphenylene iodonium
- ECM
extracellular matrix
- EGFP
enhanced green fluorescent protein
- Elk
Elk, Est-like protein
- ERK
Extracellular Signal-Regulated Kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GPI
glycophosphatidylinositol
- JNK
c-Jun amino-terminal kinase
- LV
left ventricle
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type 1-MMP
- MTT
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NF-κB
nuclear factor kappa B
- Nox
NADPH oxidase
- NADPH
nicotinamide adenine dinucleotide phosphate
- RECK
reversion-inducing-cysteine-rich protein with Kazal motifs
- ROS
reactive oxygen species
- SBP
systolic blood pressure
- shRNA
short hairpin RNA
- siMMP
small inhibitory RNA against MMP
- Sp1
Specific protein 1
- TACE
tumor necrosis factor, alpha, converting enzyme
- TIMP
Tissue Inhibitor of Metalloproteinase
- TPA
12-O-Tetradecanoylphorbol-13-acetate
- TRE
TPA DNA response element
- UTR
untranslated region
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000 Sep;52(3):415–472. [PubMed] [Google Scholar]
- 2.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009 Aug;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Patterson NL, Iyer RP, de Castro Bras LE, Li Y, Andrews TG, Aune GJ, et al. Using proteomics to uncover extracellular matrix interactions during cardiac remodeling. Proteomics Clin Appl. 2013 Aug;7(7–8):516–527. doi: 10.1002/prca.201200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010 Sep;15(5):415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-alphainduced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002 Dec 13;91(12):1119–1126. doi: 10.1161/01.res.0000047090.08299.d5. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci U.S.A. 1998 Oct 27;95(22):13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003 Jun-Sep;22(2–3):167–175. doi: 10.1023/a:1023043315031. [DOI] [PubMed] [Google Scholar]
- 9.Nagini S. RECKing MMP: relevance of reversion-inducing cysteine-rich protein with kazal motifs as a prognostic marker and therapeutic target for cancer (a review) Anticancer Agents Med Chem. 2012 Sep;12(7):718–725. doi: 10.2174/187152012802650237. [DOI] [PubMed] [Google Scholar]
- 10.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001 Dec 14;107(6):789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 11.Omura A, Matsuzaki T, Mio K, Ogura T, Yamamoto M, Fujita A, et al. RECK forms cowbell-shaped dimers and inhibits matrix metalloproteinase-catalyzed cleavage of fibronectin. J Biol Chem. 2009 Feb 6;284(6):3461–3469. doi: 10.1074/jbc.M806212200. [DOI] [PubMed] [Google Scholar]
- 12.Miki T, Takegami Y, Okawa K, Muraguchi T, Noda M, Takahashi C. The reversioninducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J Biol Chem. 2007 Apr 20;282(16):12341–12352. doi: 10.1074/jbc.M610948200. [DOI] [PubMed] [Google Scholar]
- 13.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, et al. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011 Jun;50(6):928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valente AJ, Clark RA, Siddesha JM, Siebenlist U, Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates Angiotensin-II-induced Interleukin-18 expression, and Nox2- dependent cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2012 Jul;53(1):113–124. doi: 10.1016/j.yjmcc.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, et al. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin IIinfused mice. Am J Physiol Renal Physiol. 2008 Sep;295(3):F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masson S, Latini R, Bevilacqua M, Vago T, Sessa F, Torri M, et al. Within-patient variability of hormone and cytokine concentrations in heart failure. Pharmacol Res. 1998 Mar;37(3):213–217. doi: 10.1006/phrs.1998.0288. [DOI] [PubMed] [Google Scholar]
- 17.Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000 Jan;21(1):53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 18.Graziani G, Badalamenti S, Del Bo A, Marabini M, Gazzano G, Como G, et al. Abnormal hemodynamics and elevated angiotensin II plasma levels in polydipsic patients on regular hemodialysis treatment. Kidney Int. 1993 Jul;44(1):107–114. doi: 10.1038/ki.1993.219. [DOI] [PubMed] [Google Scholar]
- 19.Simoes e Silva AC, Diniz JS, Pereira RM, Pinheiro SV, Santos RA. Circulating renin Angiotensin system in childhood chronic renal failure: marked increase of Angiotensin-(1–7) in end-stage renal disease. Pediatr Res. 2006 Dec;60(6):734–739. doi: 10.1203/01.pdr.0000246100.14061.bc. [DOI] [PubMed] [Google Scholar]
- 20.Valente AJ, Yoshida T, Gardner JD, Somanna N, Delafontaine P, Chandrasekar B. Interleukin-17A stimulates cardiac fibroblast proliferation and migration via negative regulation of the dual-specificity phosphatase MKP-1/DUSP-1. Cell Signal. 2012 Feb;24(2):560–568. doi: 10.1016/j.cellsig.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNFalpha)- stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem. 2009 May 22;284(21):14414–14427. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valente AJ, Sakamuri SS, Siddesha JM, Yoshida T, Gardner JD, Prabhu R, et al. TRAF3IP2 mediates interleukin-18-induced cardiac fibroblast migration and differentiation. Cell Signal. 2013 Nov;25(11):2176–2184. doi: 10.1016/j.cellsig.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesan B, Valente AJ, Das NA, Carpenter AJ, Yoshida T, Delafontaine JL, et al. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cell Signal. 2013 Jan;25(1):359–371. doi: 10.1016/j.cellsig.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colston JT, de la Rosa SD, Freeman GL. Impact of brief oxidant stress on primary adult cardiac fibroblasts. Biochem Biophys Res Commun. 2004 Mar 26;316(1):256–262. doi: 10.1016/j.bbrc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Schorb W, Conrad KM, Singer HA, Dostal DE, Baker KM. Angiotensin II is a potent stimulator of MAP-kinase activity in neonatal rat cardiac fibroblasts. J Mol Cell Cardiol. 1995 May;27(5):1151–1160. doi: 10.1016/0022-2828(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 26.Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase Cdelta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension. 2008 Mar;51(3):704–711. doi: 10.1161/HYPERTENSIONAHA.107.098459. [DOI] [PubMed] [Google Scholar]
- 27.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005 Apr 25;579(11):2533–2540. doi: 10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 28.Furman C, Copin C, Kandoussi M, Davidson R, Moreau M, McTaggiart F, et al. Rosuvastatin reduces MMP-7 secretion by human monocyte-derived macrophages: potential relevance to atherosclerotic plaque stability. Atherosclerosis. 2004 May;174(1):93–98. doi: 10.1016/j.atherosclerosis.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Boixel C, Fontaine V, Rucker-Martin C, Milliez P, Louedec L, Michel JB, et al. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol. 2003 Jul 16;42(2):336–344. doi: 10.1016/s0735-1097(03)00578-3. [DOI] [PubMed] [Google Scholar]
- 30.Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000 Jan 25;242(1–2):75–86. doi: 10.1016/s0378-1119(99)00549-1. [DOI] [PubMed] [Google Scholar]
- 31.Chang HC, Liu LT, Hung WC. Involvement of histone deacetylation in ras-induced down-regulation of the metastasis suppressor RECK. Cell Signal. 2004 Jun;16(6):675–679. doi: 10.1016/j.cellsig.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Hsu MC, Chang HC, Hung WC. HER-2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion. J Biol Chem. 2006 Feb 24;281(8):4718–4725. doi: 10.1074/jbc.M510937200. [DOI] [PubMed] [Google Scholar]
- 33.Sasahara RM, Takahashi C, Noda M. Involvement of the Sp1 site in ras-mediated downregulation of the RECK metastasis suppressor gene. Biochem Biophys Res Commun. 1999 Nov 2;264(3):668–675. doi: 10.1006/bbrc.1999.1552. [DOI] [PubMed] [Google Scholar]
- 34.Moore L, Fan D, Basu R, Kandalam V, Kassiri Z. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev. 2012 Sep;17(4–5):693–706. doi: 10.1007/s10741-011-9266-y. [DOI] [PubMed] [Google Scholar]
- 35.Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008 Jan-Feb;12(1):189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999 Oct 8;274(41):29130–29137. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 37.Bergman MR, Cheng S, Honbo N, Piacentini L, Karliner JS, Lovett DH. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem J. 2003 Feb 1;369(Pt 3):485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JG, Dahi S, Mahimkar R, Tulloch NL, Alfonso-Jaume MA, Lovett DH, et al. Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia. Proc Natl Acad Sci U.S.A. 2005 Nov 8;102(45):16345–16350. doi: 10.1073/pnas.0508085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Koh G. Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2000 Mar 16;269(2):401–405. doi: 10.1006/bbrc.2000.2308. [DOI] [PubMed] [Google Scholar]
- 40.Lan YQ, Zhang C, Xiao JH, Zhuo YH, Guo H, Peng W, et al. Suppression of IkappaBalpha increases the expression of matrix metalloproteinase-2 in human ciliary muscle cells. Mol Vis. 2009;15:1977–1987. [PMC free article] [PubMed] [Google Scholar]
- 41.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006 Apr;47(4):711–717. doi: 10.1161/01.HYP.0000208840.30778.00. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006 Jun 27;113(25):2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 43.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000 Jul;106(1):55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zile MR, Baicu CF, Stroud RE, Van Laer A, Arroyo J, Mukherjee R, et al. Pressure overload-dependent membrane type 1-matrix metalloproteinase induction: relationship to LV remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2012 Apr 1;302(7):H1429–H1437. doi: 10.1152/ajpheart.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi S, Simizu S, Osada H. RECK negatively regulates matrix metalloproteinase-9 transcription. Cancer Res. 2009 Feb 15;69(4):1502–1508. doi: 10.1158/0008-5472.CAN-08-2635. [DOI] [PubMed] [Google Scholar]
- 46.Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EP, Omura A, et al. RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat Neurosci. 2007 Jul;10(7):838–845. doi: 10.1038/nn1922. [DOI] [PubMed] [Google Scholar]
- 47.Hong KJ, Wu DC, Cheng KH, Chen LT, Hung WC. RECK inhibits stemness gene expression and tumorigenicity of gastric cancer cells by suppressing ADAM-mediated Notch1 activation. J Cell Physiol. 2013 Jul 23; doi: 10.1002/jcp.24434. [DOI] [PubMed] [Google Scholar]
- 48.Satoh M, Nakamura M, Saitoh H, Satoh H, Maesawa C, Segawa I, et al. Tumor necrosis factor-alpha-converting enzyme and tumor necrosis factor-alpha in human dilated cardiomyopathy. Circulation. 1999 Jun 29;99(25):3260–3265. doi: 10.1161/01.cir.99.25.3260. [DOI] [PubMed] [Google Scholar]
- 49.Fedak PW, Moravec CS, McCarthy PM, Altamentova SM, Wong AP, Skrtic M, et al. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation. 2006 Jan 17;113(2):238–245. doi: 10.1161/CIRCULATIONAHA.105.571414. [DOI] [PubMed] [Google Scholar]
- 50.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, et al. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011 Jan;57(1):123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.