Abstract
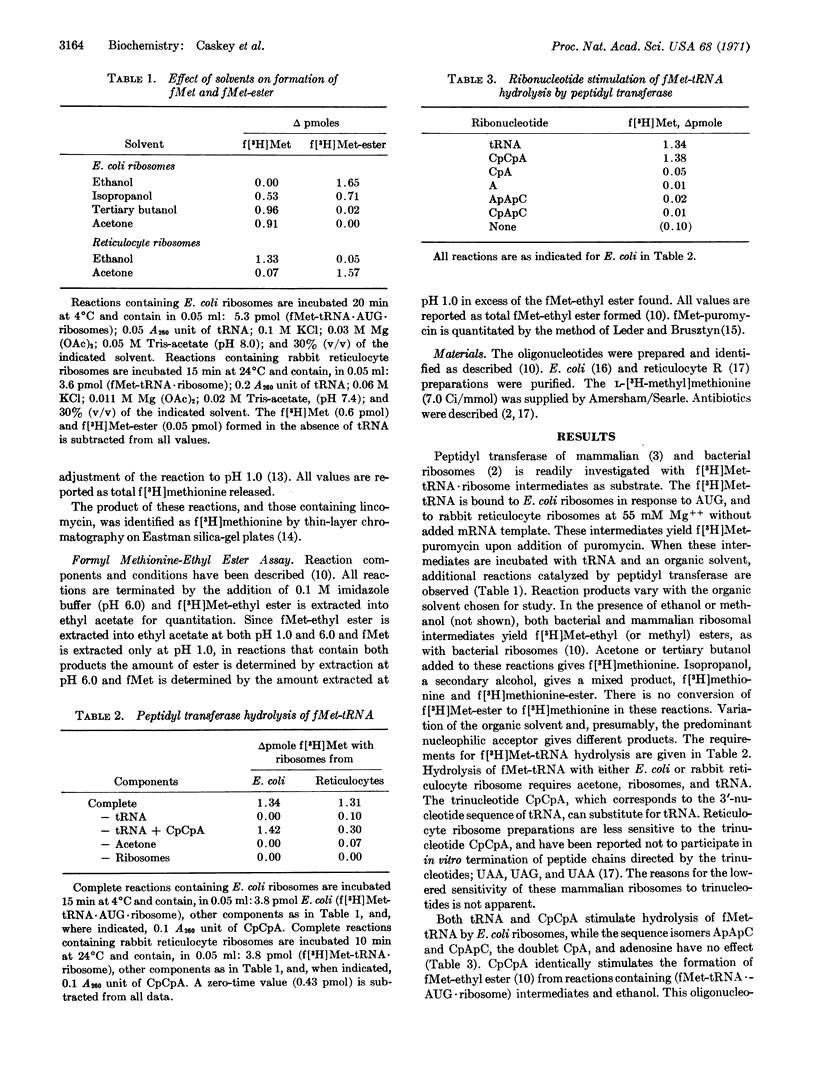
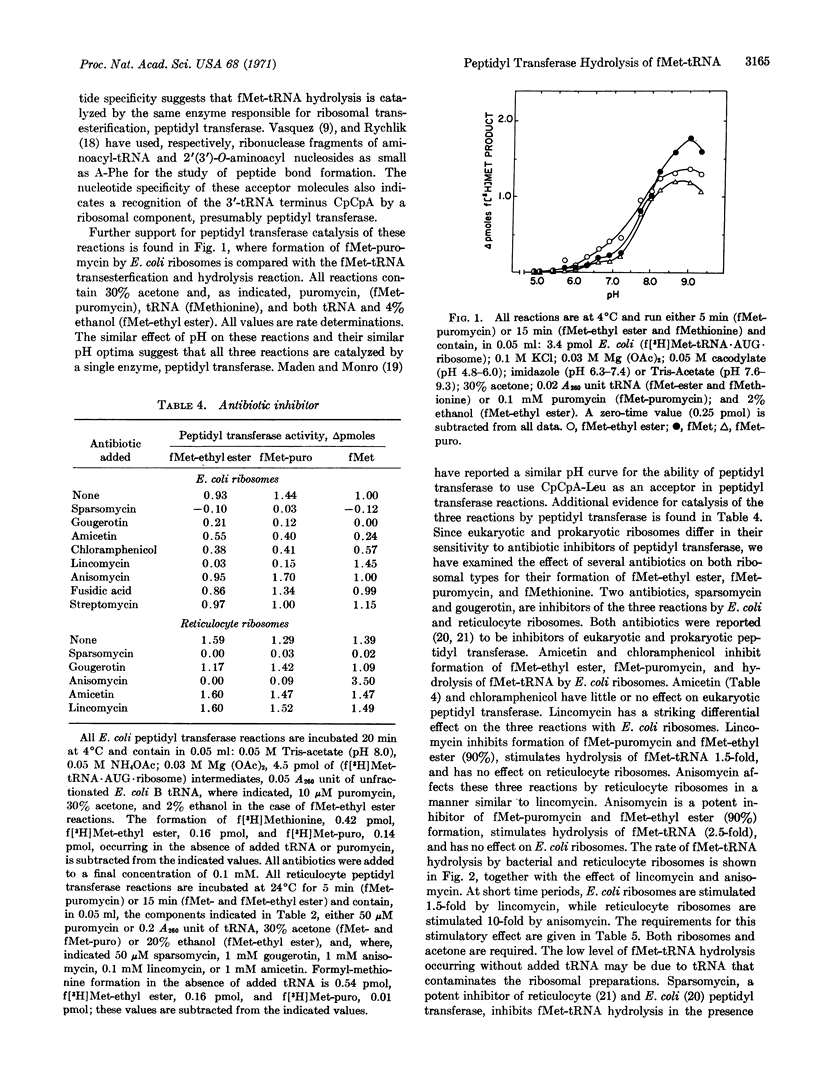
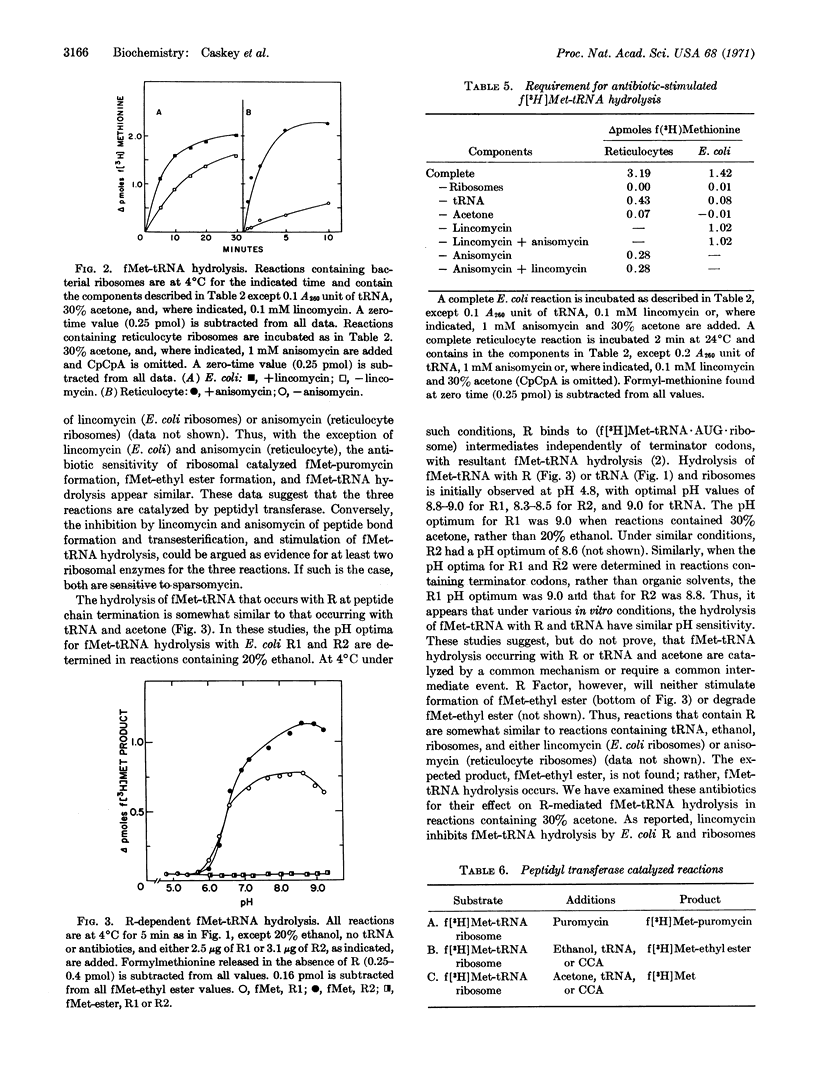
Escherichia coli and rabbit reticulocyte (f[3H]Met-tRNA·AUG·ribosome) intermediates undergo hydrolysis, with release of f[3H]methionine, upon addition of tRNA or CpCpA in the presence of acetone. This ribosomal catalyzed reaction has similar requirements, pH optimum, and antibiotic sensitivity to those of peptidyl transferase. Two antibiotics, lincomycin with E. coli ribosomes and anisomycin with reticulocyte ribosomes, inhibit peptide-bond formation and transesterification activities of peptidyl transferase, but stimulate hydrolysis of f[3H]Met-tRNA. Earlier studies have suggested peptidyl transferase activity is essential for R factor-dependent hydrolysis of f(3H)Met-tRNA. These studies indicate that peptidyl transferase has the capacity for f(3H)Met-tRNA hydrolysis and, therefore, may be responsible for peptidyl-tRNA cleavage during peptide chain termination.
Keywords: reticulocytes, E. coli, anisomycin, R factors, lincomycin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Caskey C. T. Mammalian peptide chain termination. II. Codon specificity and GTPase activity of release factor. Proc Natl Acad Sci U S A. 1971 Mar;68(3):619–624. doi: 10.1073/pnas.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Characterization of three proteins involved in polypeptide chain termination. Cold Spring Harb Symp Quant Biol. 1969;34:469–477. doi: 10.1101/sqb.1969.034.01.053. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Fahnestock S., Neumann H., Shashoua V., Rich A. Ribosome-catalyzed ester formation. Biochemistry. 1970 Jun 9;9(12):2477–2483. doi: 10.1021/bi00814a013. [DOI] [PubMed] [Google Scholar]
- Fahnestock S., Rich A. Ribosome-catalyzed polyester formation. Science. 1971 Jul 23;173(3994):340–343. doi: 10.1126/science.173.3994.340. [DOI] [PubMed] [Google Scholar]
- Fahnestock S., Rich A. Synthesis by ribosomes of viral coat protein containing ester linkages. Nat New Biol. 1971 Jan 6;229(1):8–10. doi: 10.1038/newbio229008a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Beaudet A. L., Caskey C. T. Peptide chain termination with mammalian release factor. Proc Natl Acad Sci U S A. 1970 Sep;67(1):99–106. doi: 10.1073/pnas.67.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Monro R. E. Ribosome-catalyzed peptidyl transfer. Effects of cations and pH value. Eur J Biochem. 1968 Nov;6(2):309–316. doi: 10.1111/j.1432-1033.1968.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Milman G., Goldstein J., Scolnick E., Caskey T. Peptide chain termination. 3. Stimulation of in vitro termination. Proc Natl Acad Sci U S A. 1969 May;63(1):183–190. doi: 10.1073/pnas.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Cabrer B., Parmeggiani A., Vazquez D. Inhibition by siomycin and thiostrepton of both aminoacyl-tRNA and factor G binding to ribosomes. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1796–1800. doi: 10.1073/pnas.68.8.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro R. E., Celma M. L., Vazquez D. Action of sparsomycin on ribosome-catalysed peptidyl transfer. Nature. 1969 Apr 26;222(5191):356–358. doi: 10.1038/222356a0. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Staehelin T., Celma M. L., Vazquez D. The peptidyl transferase activity of ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:357–368. doi: 10.1101/sqb.1969.034.01.042. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Vazquez D. Ribosome-catalysed peptidyl transfer: effects of some inhibitors of protein synthesis. J Mol Biol. 1967 Aug 28;28(1):161–165. doi: 10.1016/s0022-2836(67)80085-8. [DOI] [PubMed] [Google Scholar]
- Rychlík I., Cerná J., Chládek S., Zemlicka J., Haladová Z. Substrate specificity of ribosomal peptidyl transferase: 2'(3')-O-aminoacyl nucleosides as acceptors of the peptide chain on the amino acid site. J Mol Biol. 1969 Jul 14;43(1):13–24. doi: 10.1016/0022-2836(69)90075-8. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Caskey C. T. Peptide chain termination. V. The role of release factors in mRNA terminator codon recognition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1235–1241. doi: 10.1073/pnas.64.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Milman G., Rosman M., Caskey T. Transesterification by peptidyl transferase. Nature. 1970 Jan 10;225(5228):152–154. doi: 10.1038/225152a0. [DOI] [PubMed] [Google Scholar]
- Tompkins R. K., Scolnick E. M., Caskey C. T. Peptide chain termination. VII. The ribosomal and release factor requirements for peptide release. Proc Natl Acad Sci U S A. 1970 Mar;65(3):702–708. doi: 10.1073/pnas.65.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez D., Battaner E., Neth R., Heller G., Monro R. E. The function of 80 S ribosomal subunits and effects of some antibiotics. Cold Spring Harb Symp Quant Biol. 1969;34:369–375. doi: 10.1101/sqb.1969.034.01.043. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Zamir A., Elson D. The possible involvement of peptidyl transferase in the termination step of protein biosynthesis. Biochemistry. 1969 Dec;8(12):5161–5168. doi: 10.1021/bi00840a070. [DOI] [PubMed] [Google Scholar]


