Abstract
Objective:
To assess the cognitive effects of sertindole and olanzapine in patients diagnosed with schizophrenia. Cognition was the primary outcome of the study.
Method:
This was a 12-week double-blinded randomized clinical controlled trial. Participants were randomized to either 16–24 mg of sertindole or 10–20 mg of olanzapine.
Results:
The study had a low recruitment rate (N = 9) and was terminated before the expected number of patients was reached. No significant differences between groups were found at study end on any of the 32 cognitive subtests. A simple sign test did not show any of the comparator drugs trending towards being superior on the majority of tests. Mean change on Positive and Negative Syndrome Scale (PANSS) total and PANSS subscales from baseline to end of study were not significantly different between treatment groups. Similar results on cognition and PANSS was seen on completers and last observation carried forward analysis.
Conclusion:
In this study we did not find any significant differences between sertindole or olanzapine on PANSS subscales or neurocognitive tests in a population consisting of patients diagnosed with schizophrenia.
Keywords: cognition, olanzapine, randomized controlled trial, schizophrenia, sertindole
Introduction
Patients diagnosed with schizophrenia have cognitive deficits compared with their relatives, normal controls and patients diagnosed with other psychiatric disorders, for example, depression and bipolar disorder [Buchanan et al. 2005; Cannon et al. 1994; Caspi et al. 2003; Nielsen, 2011]. The cognitive deficits appear before first psychosis and remain stable over time [Caspi et al. 2003; Szoke et al. 2008], although some authors have proposed a more neurodegenerative hypothesis regarding the cognitive function [Levander et al. 2001; Rund, 2009].
Several central nervous system receptors are being investigated as to their effect on cognitive function in general and in patients with schizophrenia [Wallace et al. 2011]. Previous studies have shown some implication of the muscarinergic receptor system on cognitive function in patients with schizophrenia [Fagerlund et al. 2007; Freedman et al. 2008; Keefe et al. 2007; Minzenberg et al. 2004; Shekhar et al. 2008], with antagonizing drugs worsening symptoms and drugs with agonizing effects improving symptoms [Desmarais et al. 2012; McGurk et al. 2004; Minzenberg et al. 2004; Pae, 2013; Tracy et al. 1998].
The effects of the muscarinergic antagonism had not been investigated thoroughly in a clinical setting at the initiation of the study. Sertindole and olanzapine are both atypical antipsychotics [Nielsen and Nielsen, 2009], with differences in receptor affinities, especially with sertindole not showing any marked affinity for the muscarinergic receptor whereas olanzapine has a high affinity and an antagonizing effect [Correll, 2010]. In addition, sertindole does not show a tendency to induce Parkinsonism in treated patients [Seeman and Tallerico, 1998], thereby excluding the need for concomitant anticholinergic drugs.
In this study we aimed to investigate the effects of sertindole and olanzapine on cognition in patients diagnosed with schizophrenia, utilizing a computerized cognitive test battery.
Material and methods
Design
The study is a 12-week, double-blind randomized head-to-head study in which all participants are randomized to either sertindole or olanzapine. Participants were recruited in Denmark and Sweden, but due to poor recruitment in the Swedish center (one participant) only data from Denmark are reported. Primary outcome was change in cognition as measured by the CANTAB test battery (Cambridge Cognition Ltd, Bottisham, Cambridge, UK) [Lowe and Rabbitt, 1998; Sahakian and Owen, 1992].
Participants were randomized 1:1 to receive either sertindole or olanzapine. The initial dose of sertindole was 4 mg, which was increased by 4 mg every fourth day until 16 mg was reached. Treatment doses of sertindole were between 16 and 24 mg. The dose of 24 mg was only used in exceptionally cases, as the risk of QTc prolongation is dose dependent.
The initial dose of olanzapine was 10 mg and the flexible dose range was between 10 and 20 mg.
All participants were treated with a tablet of sertindole or an identical placebo tablet, and an encapsulated olanzapine tablet or a dummy tablet, so participants were either on sertindole or olanzapine throughout the study period.
Block size randomization was conducted by the pharmacy by computer and randomization block size was variable. Disclosure of blinding was done after reporting of PANSS values to the pharmacy.
Participants
Men and women, between the ages of 18 and 65 years, diagnosed with an International Classification of Diseases, 10th revision diagnosis of schizophrenia F20.0–F20.3 and F20.9 (paranoid, hebephrenic, catatonic, undifferentiated or unspecified subtypes) were eligible for participation [World Health Organization, 1992]. Patients were excluded if an electrocardiogram showed QTc prolongation or if QT prolongation over 500 ms was shown after initiation of study drugs. Before inclusion, normal levels of potassium or magnesium were required, as well as a negative pregnancy test for women. All women were required to use a safe form of birth control.
Patients with known cardiovascular disease, significant addictions, or a known partial or nonresponse to study drugs were not allowed to participate in the study. In addition, patients with a Calgary Depression Scale score of at least 7 were not allowed to participate in the study [Addington et al. 1992, 1994].
Oxazepam, zopiclone and zolpidem were allowed, but not during the last 48 h before cognitive testing. No other benzodiazepines were allowed in the study. Treatment with mood stabilizers or antidepressants was allowed if dosage had been stable during the last month before inclusion, as well as stable dosage during the study.
Treatment with anticholinergic drugs was allowed for the initial 3 weeks of the study. Treatment with Electroconvulsive Therapy (ECT) within the last 3 months before inclusion or during the study was not allowed. Treatment with clozapine or depot antipsychotics as the last drug before screening resulted in exclusion, but previous treatment with the drugs was allowed.
Administration of potent cytochrome P450 enzyme CYP2D6 or CYP3A4 inhibitors was not allowed due to interaction with sertindole metabolism, as well as drugs that prolonged QTc due to pharmacodynamic interactions with sertindole.
We defined a sertindole dose of 16 mg to be equivalent to 10 mg of olanzapine, 20 mg of sertindole to be equivalent to 15 mg of olanzapine, and 24 mg of sertindole to be equivalent to 20 mg of olanzapine, as also defined by the World Health Organization in daily defined dosages.
Cognitive measures
Blinded raters performed all clinical research assessments.
The CANTAB cognitive test battery is a computerized battery [Lowe and Rabbitt, 1998; Sahakian and Owen, 1992]. The participant followed instructions to complete the subtests listed below. The approximate timescale was 60 min per test session.
Motor screening
Participants are asked to touch a series of flashing crosses on the computer touch screen. The main purpose with motor screening is to familiarize the participant with the touch screen interface and to minimize any anxiety regarding the cognitive test. Outcome measures were mean latency (defined as the time taken for the subject to touch the cross after it appeared), with a lower score being better; and mean error (the mean distance between the centre of the cross and the location the subject touched on the screen, for the 10 crosses presented to which the subject correctly responded), with a lower score being better.
Rapid visual information processing
In a white box in the center of the screen a series of pseudorandom numbers appear at a rate of 100 per minute. Participants are asked to detect a specific three-number target sequence of numbers and hit a press pad when the target sequence appears. Rapid visual information processing primarily measures attention. Outcome measures were total hits (represents the number of occasions upon which the target sequence is correctly responded to), with a higher score being better; total misses (the number of occasions the subject fails to respond to a target sequence within the response window), with a lower score being better; and mean latency (the mean time taken to respond, reported in milliseconds), with a lower score being better.
Reaction time
The participants must respond to a yellow spot appearing in the screen by moving the hand away from the press pad button. Reaction time is divided between a single point and a five-choice reaction time phase. Reaction time primarily measures processing speed. Outcome measures were reaction time (the speed with which the subject releases the press pad button in response to the onset of a stimulus in either a single location or a five-choice location); and movement time (the time taken to touch the stimulus after the press pad button has been released in trials in which stimuli appear in a single location or a five-choice location). A lower score is better for both tests. Outcome measures were also accuracy scores (the total number of trials in which the response is recorded as correct for assessment trials in which the stimuli appear in a single or a five-choice location), with a higher score being better.
Spatial working memory
A number of colored boxes are shown on the touch screen. By touching the screen and using a process of elimination, the participant searches for blue ‘tokens’ in the boxes. Participants are instructed not to return to a box where a token has been found previously. The number of boxes is gradually increased to a maximum number of eight. Spatial working memory primarily measures working memory. Outcome measures were between errors (defined as times the subject revisits a box in which a token has previously been found); within errors (defined as the number of errors made within a search, that is, the number of times a subject revisits a box already found to be empty during the same search); strategy (counting the number of times the subject begins a new search with a different box for six- and eight-box problems only); mean time to first response (the mean time between the problem being presented to the subject and the subject first touching the screen to open a box); and mean time to last response (the time between the problem being presented to the subject and the subject’s last screen touch to open a box to locate the final token for the problem). A lower score was better for all tests.
Verbal recognition/recognition memory
Participants are instructed to remember words, a total of 15, presented individually on the screen in front of them. Participants are asked to recall as many of the presented words initially after the presentation. Twenty minutes after the initial recall phase, a second retrieval phase, a recognition test is conducted. Words are presented sequentially and participants are asked to press ‘yes’ or ‘no’, according to their recognition of the words as being presented previously or not. Verbal recognition/recognition memory primarily measures verbal learning and memory. Outcome measures for immediate recall were total correct (the total number of distinct words correctly recalled from the presentation phase), with a higher score being better; total novel words (the total number of words recalled that did not appear in the presentation phase), with a lower score being better; total perseverations (the total number of times that the subject repeats the recall of a previously correctly recalled word from the presentation phase), with a lower score being better. Outcome measures for delayed recall were total correct (the total number of words that the subject correctly recognizes from the presentation phase), with a higher score being better; and total false positives (the total number of times the subject responds ‘yes’ incorrectly to a distractor word), with a lower score being better.
Pattern recognition memory
Participants are instructed to remember the patterns presented to them on the screen. Initially patterns appear sequentially on the screen, one at a time. Immediately following the encoding phase, participants carry out a recognition test, in which each pattern from the encoding phase is presented with another pattern of similar form and color, and participants must touch the pattern they saw previously. Twenty minutes following this first recognition test, a second recognition test is administered. Pattern recognition memory primarily measures visual learning and memory. Outcome measures were number correct (the number of correct responses), with a higher score being better; mean latency (details the mean time taken to respond), with a lower score being better; and percent correct (the number of correct responses, expressed as a percentage), with a higher score being better.
Paired associates learning
Participants are instructed to observe six white squares opening one at a time on the screen, with an increasing amount of patterns being disclosed as the test progresses. After the presentation each symbol is shown in the center of the screen and the participant is requested to press the white square that contains the pattern. There are four levels of difficulty; the number of errors at each level is recorded. For participants who fail to complete all levels, an adjusted total is calculated that allows for errors predicted in the stages that were not attempted. Paired associates learning primarily measures visual learning and memory. Outcome measures were total errors (the total number of errors across all assessed problems and all stages, with an adjustment for each stage not attempted due to previous failure), with a lower score being better; mean trials to success [the total number of trials required (maximum score = 10 trials per stage) to locate all the patterns correctly in all stages attempted, and dividing the result by the number of successfully completed stages], with a lower score being better; first trial memory score (the number of patterns correctly located after the first trial, summed across the stages completed), with a higher score being better; and stages completed (key indicator of the subject’s overall success, recording how many stages were successfully completed), with a higher score being better.
Stockings of Cambridge
The participant is shown two displays containing three colored balls. There is a row of numbered boxes along the bottom of the screen. The test administrator first demonstrates to the participant how to use the balls in the lower display to copy the pattern in the upper display, and completes one demonstration problem, where the solution requires one move. The participant must then complete three further problems, one each of two moves, three moves, and four moves.
Next the participant is shown further problems and must work out in their head how many moves the solutions to these problems require, then touch the appropriate box at the bottom of the screen to indicate their response.
Stockings of Cambridge primarily measures executive function. Outcome measures were problems solved on first choice (the number of problems which were solved on the subject’s first choice), with a higher score being better; mean choices to correct (the mean number of unique box choices that the subject made on each problem to make the correct choice), with a lower score being better; latency to first choice (measured from the appearance of the balls on the screen until the box was touched), with a lower score being better; and latency to correct (measured from the appearance of the balls on the screen until the correct box was touched), with a lower score being better.
Clinical measures
The clinical symptom ratings included the PANSS consisting of 7 items for positive symptoms, 7 items for negative symptoms and 16 items for evaluation of general psychopathology, used in combination with and the ‘The Structured Clinical Interview PANSS’ (SCI-PANSS) [Kay et al. 1987, 1991].
Statistical analysis
All analyses were performed as completers analyses and intention-to-treat analyses, using the last observation carried forward (LOCF) principle. Participants were classified according to study drug, for example, sertindole versus olanzapine. All participants receiving at least one drug dose and completing at least one cognitive test were included in the intention-to-treat analysis (LOCF).
Due to a very low inclusion number in the study, only descriptive analyses with mean scores were conducted. The Mann–Whitney U test was employed.
To reduce the risk of type II errors, a correction was applied to the standard p < 0.05 significance criterion. The Bonferroni correction was judged to be too conservative in an explorative study with respect to the risk of type I errors. We selected p < 0.01 as a reasonable significance criterion.
The study was approved by the Danish Medicines Agency, Data Protection Agency and the Regional Committee on Biomedical Research Ethics of North Jutland. The study was monitored according to good clinical practice (GCP) from the International Classification of Harmonization by Aarhus University’s GCP Unit.
All participants gave informed consent and the study was conducted according to the declaration of Helsinki.
The study was registered at ClinicalTrials.gov [ClinicalTrials.gov identifier NCT00885690].
Results
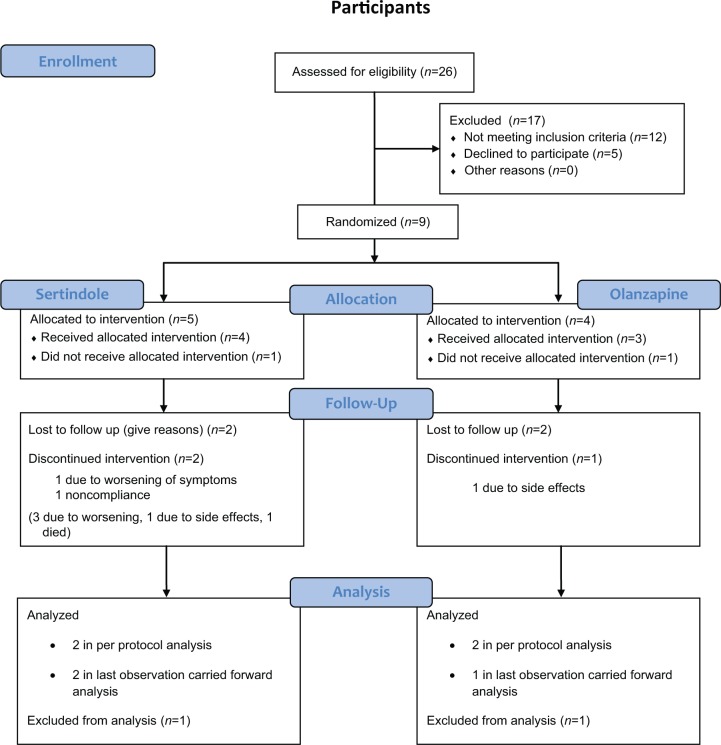
A total of 26 participants were screened for the study. Of these, 12 did not meet inclusion criteria and 5 refused to participate. Nine participants were included with five being treated with sertindole and four being treated with olanzapine. Two patients, one from each treatment arm, withdrew consent before the first dose of study medication and were excluded from further analysis. Of the nine participants included, four completed the study, resulting in a dropout rate of 56%, as shown in Figure 1. Participants were recruited in the period October 2009–July 2011 when recruitment was paused due to a low inclusion rate. The study was terminated in March 2012. Mean age at inclusion was 34.1 years [standard deviation (SD) = 11.0] for the sertindole group and 49.3 years (SD = 8.7) for the olanzapine group, p = 0.08 in the LOCF analysis. Mean age at diagnosis was 33.2 years (SD = 13.2) in the sertindole group and 36.1 (SD = 5.5) years in the olanzapine group, p = 0.56 in the LOCF analysis. In the completers analysis the mean age was 39.4 years in the sertindole group and 32.2 years in the olanzapine group, p = 1.0. A total of 75% of the participants were men in the sertindole group versus only 33% in the olanzapine group (p = 0.31), as shown in Table 1. Mean reduction in PANSS total in the sertindole group was −18.8 and −9.3 for the olanzapine group, p = 0.28 in the LOCF analysis. Similar results were found in the completers analysis with a reduction of −16.0 in the sertindole group and −9.5 in the olanzapine group, p = 1.0. There were no significant differences between treatment groups on any of the PANSS subscales investigated, as shown in Table 1.
Figure 1.
Patient flow throughout the study.
Table 1.
Demographics.
| LOCF |
Completers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sertindole (n = 4) | Olanzapine ( = 3) | p | Sertindole (n = 2) | Olanzapine (n = 2) | p | |||||
| Men | 3 (75%) | 1 (33%) | 0.31 | 2 (100%) | 1 (50%) | 0.32 | ||||
| Age at inclusion (years) | 34.1 | SD = 11.0 | 49.3 | SD = 8.7 | 0.08 | 40.5 | SD = 8.9 | 54.0 | SD = 3.3 | 0.12 |
| Age at diagnosis | 33.2 | SD = 13.2 | 36.1 | SD = 5.5 | 0.56 | 39.4 | SD = 11.1 | 32.2 | – | 1.0 |
| Inclusion | 95% CI | 95% CI | 95% CI | 95% CI | ||||||
| PANSS total | 71 | 52.3–89.7 | 54.3 | 42.5–66.2 | 0.16 | 64.5 | 40.6–88.4 | 56.5 | 32.6–80.4 | 0.44 |
| PANSS positive | 20 | 10.3–29.7 | 15.7 | 8.7–22.7 | 0.47 | 15.5 | 4.4–26.6 | 14.5 | 0.18–28.8 | 0.68 |
| PANSS negative | 17 | 13.1–20.9 | 15 | 8.5–21.5 | 0.72 | 16.5 | 11.7–21.3 | 17.5 | 12.7–22.3 | 0.44 |
| PANSS general | 34 | 28.4–39.6 | 23.7 | 20.6–26.6 | 0.03 | 32.5 | 24.5–40.5 | 24.5 | 24.5–40.5 | 0.12 |
| End of study | 95% CI | 95% CI | 95% CI | 95% CI | ||||||
| PANSS total | 45 | 39.3–50.7 | 52.3 | 38.9–65.6 | 0.37 | 48.5 | 31.0–66.0 | 47 | 40.6–53.4 | 1 |
| PANSS positive | 12.3 | 7.6–16.9 | 8.3 | 6.7–10.0 | 0.12 | 12 | 2.5–21.5 | 8 | 4.8–11.2 | 0.22 |
| PANSS negative | 13.5 | 8.5–18.5 | 13.7 | 8.0–19.4 | 0.86 | 11 | 1.5–20.5 | 15.5 | 7.5–23.5 | 0.44 |
| PANSS general | 26.5 | 20.8–32.2 | 23 | 21.6–24.4 | 0.21 | 23.5 | 23.9 -27.1 | 23.5 | 21.9–25.1 | 0.12 |
| Mean change | 95% CI | 95% CI | 95% CI | 95% CI | ||||||
| PANSS total | −18.8 | −24.3 to −13.2 | −9.3 | −22.8 to 4.1 | 0.28 | −16.0 | −22.4 to −9.6 | −9.5 | −39.7 to 20.7 | 1 |
| PANSS positive | −7.8 | −14.5 to −1.0 | −7.3 | −12.7 to −2.0 | 1 | −3.5 | −5.1 to −1.9 | −6.5 | −17.6 to 4.6 | 0.68 |
| PANSS negative | −3.5 | −7.0 to 0.0 | −1.3 | −7.2 to 4.5 | 0.37 | −5.5 | −10.3 to −0.7 | −2.0 | −14.7 to 10.7 | 0.44 |
| PANSS general | −7.5 | −10.7 to −4.3 | −0.7 | −3.6 to 2.3 | 0.03 | −7.0 | −16.5 to 2.5 | −1.0 | −7.4 to 5.4 | 0.12 |
CI, confidence interval; LOCF, last observation carried forward; PANSS, Positive and Negative Syndrome Scale.
The eight neurocognitive tests are described with LOCF analysis in Table 2 and completers analysis in Table 3. A total of 32 neurocognitive results were available for each analysis type (LOCF and completers analysis) and no single significant difference was found in either analysis type, as shown in Tables 2 and 3. A simple sign test did not show any trend towards either treatment group being superior, with sertindole-treated patients showing better test results in 15 subtests, olanzapine-treated patients showing better results in 16 subtests, and both drugs being equally good in the last subtest in the LOCF analysis. In the completers analysis sertindole-treated patients showed better results in 12 subtests, olanzapine-treated patients in 17 subtests, and both drugs did equally well in 3 subtests.
Table 2.
Last observation carried forward analysis of CANTAB.
| Mean change |
LOCF |
||||
|---|---|---|---|---|---|
| Sertindole (n = 4) | 95% CI | Olanzapine (n = 3) | 95% CI | p | |
| MOT | |||||
| Mean latency | 7.7 | −175.9 to 191.2 | −49.1 | −424.4 to 326.2 | 0.72 |
| Mean error | −2.2 | −7.0 to 2.7 | −0.6 | −5.1 to 3.9 | 0.48 |
| RVP | |||||
| Total hits | 4.5 | −3.1 to 12.1 | −0.7 | −12.4 to 11.1 | 0.29 |
| Total misses | −4.5 | −12.1 to 3.1 | 5.3 | −3.7 to 14.4 | 0.08 |
| Mean latency | 2.7 | −188.5 to 193.9 | −9.3 | −255.0 to 236.4 | 0.72 |
| RTI | |||||
| Simple reaction time | 15.1 | −29.6 to 59.8 | 187.2 | −75.6 to 450.0 | 0.16 |
| Five-choice reaction time | −4.7 | −43.9 to 34.5 | 108.2 | −3.3 to 219.7 | 0.08 |
| Simple movement time | −3.7 | −100.3 to 92.9 | 38.0 | −164.1 to 240.1 | 0.72 |
| Five-choice movement time | 25.7 | −86.3 to 137.6 | 80.6 | −60.9 to 222.1 | 0.48 |
| Simple accuracy score | −0.3 | −1.4 to 0.9 | 0.7 | −0.1 to 1.5 | 0.19 |
| Five-choice accuracy score | 0.0 | −1.0 to 1.0 | −0.7 | −2.3 to 1.0 | 0.44 |
| SWM | |||||
| Between errors | 6 | −0.6 to 12.6 | −8.0 | −33.1 to 17.1 | 0.29 |
| Within errors | −0.3 | −0.9 to 0.4 | −2.7 | −4.8 to −0.5 | 0.04 |
| Strategy | 0.3 | −3.1 to 3.6 | 0.0 | −2.4 to 3.6 | 0.72 |
| Mean time to first response (four boxes) | −635.1 | −1705.0 to 434.9 | 350.4 | −142.5 to 843.3 | 0.08 |
| Mean time to last response (four boxes) | −3023.8 | −6084.9 to 37.3 | −2639.3 | −6711.3 to 1432.8 | 0.72 |
| VRM (immediate) | |||||
| Total correct | −1.8 | −6.4 to 2.9 | −1.0 | −2.4 to 0.4 | 0.59 |
| Total novel words | −0.5 | −1.7 to 0.7 | −0.3 | −1.1 to 0.5 | 1.0 |
| Total perseverations | −0.8 | −1.4 to −0.1 | −1.0 | −3.4 to 1.4 | 0.70 |
| VRM (delayed) | |||||
| Total correct | −2.0 | −4.2 to 0.2 | 0.3 | −4.2 to 4.9 | 0.37 |
| Total false positives | −0.3 | −0.9 to 0.4 | −0.3 | −1.1 to 0.5 | 0.82 |
| PRM (immediate) | |||||
| Number correct | −1.0 | −2.0 to 0.0 | −0.7 | −3.6 to 5.0 | 0.47 |
| Mean latency | −289.5 | −484.8 to −94.2 | 411.7 | −426.5 to 1249.9 | 0.16 |
| PRM (delayed) | |||||
| Percent correct | −8.3 | −16.7 to 0.0 | 5.6 | −30.4 to 41.5 | 0.37 |
| PAL | |||||
| Total errors (adjusted) | 0.5 | −17.3 to 18.3 | −24.7 | −69.2 to 19.8 | 0.48 |
| Mean trials to success | 0.0 | −0.9 to 1.0 | −0.7 | −1.5 to 0.2 | 0.15 |
| First trial memory score | 0.0 | −3.2 to 3.2 | 3.0 | −1.2 to 7.2 | 0.21 |
| Stages completed | 0.0 | − | 0.7 | −0.1 to 1.5 | 0.07 |
| OTS | |||||
| Problems solved on first choice | 3.0 | −0.5 to 6.5 | 2.0 | −3.1 to 7.1 | 0.86 |
| Mean choices to correct | −0.2 | −0.5 to 0.1 | −0.4 | −1.1 to 0.2 | 0.48 |
| Latency to first choice | −4114.0 | −8847.7 to 619.7 | −706.2 | −5744.2 to 4331.9 | 0.16 |
| Latency to correct | −5477.2 | −10,524.6 to −429.7 | 642.4 | −16,131.8 to 17,416.6 | 0.72 |
CI, confidence interval; LOCF, last observation carried forward; MOT, motor screening task; OTS, one touch Stockings of Cambridge; PAL, paired associates learning; PRM, pattern recognition memory; RTI, reaction time; RVP, rapid visual information processing; SWM, spatial working memory; VRM, verbal recognition memory.
Table 3.
Completers analysis of CANTAB.
| Mean change |
Completers |
||||
|---|---|---|---|---|---|
| Sertindole (n = 2) | 95% CI | Olanzapine (n = 2) | 95% CI | p | |
| MOT | |||||
| Mean latency | 84.2 | 75.9–92.5 | −191.6 | −504.5 to 121.4 | 0.12 |
| Mean error | −3.5 | −8.3 to 1.4 | 0.8 | −6.2 to 7.7 | 0.12 |
| RVP | |||||
| Total hits | 7.0 | −2.5 to 16.5 | −4.0 | −23.1 to 15.1 | 0.12 |
| Total misses | −7.0 | −16.5 to 2.5 | 4.0 | −15.1 to 23.1 | 0.12 |
| Latency | 14.4 | −570.3 to 599.1 | −25.8 | −571.7 to 520.2 | 0.44 |
| RTI | |||||
| Simple reaction time | 25.7 | −47.2 to 98.6 | 211.5 | −365.2 to 788.1 | 0.44 |
| Five-choice reaction time | 14.5 | −57.4 to 86.4 | 89.3 | −139.4 to 318.0 | 0.44 |
| Simple movement time | −0.6 | −63.5 to 62.4 | 98.6 | −210.6 to 407.9 | 0.44 |
| Five-choice movement time | 30.1 | −107.5 to 167.8 | 104.0 | −187.5 to 395.5 | 0.44 |
| Simple accuracy score | 0.5 | −1.1 to 2.1 | 0.5 | −1.1 to 2.1 | 1.0 |
| Five-choice accuracy score | 0.0 | −3.2 to 3.2 | 0.0 | − | 1.0 |
| SWM | |||||
| Between errors | 3.0 | −6.5 to 12.5 | −18.0 | −30.7 to −5.3 | 0.12 |
| Within errors | −0.5 | −2.1 to 1.1 | −3.5 | −5.1 to −1.9 | 0.12 |
| Strategy | 1.0 | −5.4 to 7.4 | −0.5 | −5.3 to 4.3 | 0.44 |
| Mean time to first response (four boxes) | −966.4 | −3414.1 to 1481.3 | 386.9 | −705.1 to 1478.9 | 0.12 |
| Mean time to last response (four boxes) | −3522.4 | −11,170.2 to 4125.4 | −3730.9 | −10,654.7 to 3192.9 | 1.0 |
| VRM (immediate) | |||||
| Total correct | −4.5 | −9.3 to 0.3 | −1.0 | −4.2 to 2.2 | 0.12 |
| Total novel words | 0 | − | −0.5 | −2.1 to 1.1 | 0.32 |
| Total perseverations | −1.0 | − | −1.5 | −6.3 to 3.3 | 1.0 |
| VRM (delayed) | |||||
| Total correct | −2.0 | −5.2 to 1.2 | 1.0 | −8.5 to 10.5 | 0.44 |
| Total false positives | −0.5 | −2.1 to 1.1 | −0.5 | −2.1 to 1.1 | 1.0 |
| PRM (immediate) | |||||
| Number correct | −0.5 | −2.1 to 1.1 | 2.0 | −4.4 to 8.4 | 0.22 |
| Mean latency | −293.1 | −550.6 to −35.6 | 180.1 | −1214.0 to 1575.5 | 0.44 |
| PRM (delayed) | |||||
| Percent correct | −4.2 | −17.4 to 9.1 | 16.7 | −36.4 to 69.7 | 0.22 |
| PAL | |||||
| Total errors (adjusted) | −2.0 | —24.3 to 20.3 | −33.0 | −122.1 to 56.1 | 0.44 |
| Mean trials to success | −0.2 | −1.5 to 1.2 | −1.0 | −1.9 to −0.1) | 0.12 |
| First trial memory score | 0.0 | −3.2 to 3.2 | 4.5 | −0.3 to 9.3 | 0.12 |
| Stages completed | 0.0 | − | 1.0 | − | 0.08 |
| OTS | |||||
| Problems solved on first choice | 5.0 | −1.4 to 11.4 | 4.0 | 0.8 to 7.2 | 0.68 |
| Mean choices to correct | −0.3 | −0.9 to 0.2 | −0.7 | −1.1 to −0.3 | 0.12 |
| Latency to first choice | −2894.8 | −8905.7 to 3116.0 | 1100.4 | −4343.8 to 6544.7 | 0.12 |
| Latency to correct | −4038.0 | −10,590.7 to 2514–7 | 3630.3 | −30,378.8 to 37,639.4 | 1.0 |
CI, confidence interval; LOCF, last observation carried forward; MOT, motor screening task; OTS, one touch Stockings of Cambridge; PAL, paired associates learning; PRM, pattern recognition memory; RTI, reaction time; RVP, rapid visual information processing; SWM, spatial working memory; VRM, verbal recognition memory.
Discussion
At the end of the study we did not find any significant differences on PANSS scales or neurocognitive tests between treatment groups on either LOCF or completers analyses.
When the protocol was written, a hospital chart review to estimate possible participants for the study was conducted. The initial chart review suggested that approximately 20% of the clinical patient population were possible participants. In this chart review, we were unable to estimate patients’ attitude towards study drugs. When inclusion was initiated, the main barrier to inclusion was either patients’ or clinicians’ concern for metabolic side effects of olanzapine [Citrome et al. 2011]. Many of the screened patients had been offered treatment with olanzapine previously, but had refused due to concerns about side effects. Sertindole had been withdrawn due to concerns about increased cardiac mortality and had been reintroduced shortly before initiation of the current study [Peuskens et al. 2008]. Despite the early concern of cardiac mortality, most patients and clinicians were positive towards potential treatment with sertindole.
Gallhofer and colleagues compared sertindole with haloperidol in a 12-week study with reaction time decomposition and Wisconsin Card Sorting Test (WCST) as outcome measures. Participants treated with sertindole did significantly better in all subtests of the reaction time decomposition test than participants treated with haloperidol. A similar pattern was seen in the WCST, except for the non perseverative errors, for which sertindole and haloperidol did not differ at study end point in this study of 34 participants [Gallhofer et al. 2007]. Nielsen and colleagues compared adjunctive treatment with sertindole or placebo with an original treatment with clozapine and did not show any difference in cognitive outcomes between the groups [Nielsen et al. 2012]. More than 25 randomized trials on patients diagnosed with schizophrenia have been conducted comparing olanzapine with other antipsychotic drugs with most studies being against atypical antipsychotic drugs only. Some studies investigated the effects of olanzapine compared with typical antipsychotic drugs only, and other studies had both typical and atypical drugs as comparator drugs. No uniform patterns of change in cognitive function in specific domains were found in patients treated with olanzapine.
Due to a low inclusion rate, the number of participants proposed by power calculation was not reached (N = 100). Our study did not show any of the comparator drugs being superior on cognitive outcomes or PANSS. The low inclusion rate increased the risk of type II error, but a simple sign test did not shown any of the comparator drugs trending towards being superior on the majority of tests. Our study did not evaluate previous educational level or job training, which could have influenced the participants’ ability to perform the cognitive tests. We did not specify an antipsychotic drug washout period to minimize the effects of previous treatment, but did not allow treatment with depot antipsychotics immediately before inclusion in the study. No history of previous drug treatment for the individual patient was recorded, but participants could not have been previously treated with olanzapine or sertindole.
Conclusion
In this study we did not find any significant differences between sertindole or olanzapine on PANSS subscales or neurocognitive tests in a population consisting of patients diagnosed with schizophrenia. The study lacks power due to a low inclusion rate, but a simple sign test does not show trends towards any drug being more effective on cognitive function.
Acknowledgments
The authors thanks the GCP Unit from Aarhus University for monitoring the study and the Hospital Pharmacy North Denmark Region and APL Pharmacy, Stockholm for their assistance with study drugs and randomization.
Footnotes
Funding: H. Lundbeck supported the study with a study grant and study medication. They had no influence on conduct of the study or preparation of the manuscript.
Conflict of interest statement: The Unit for Psychiatric Research provided support with researchers’ salaries and overheads. R. Nielsen has received research grants from H. Lundbeck for clinical trials, received speaking fees from Bristol-Myers Squibb, Astra Zeneca, Janssen & Cilag, Lundbeck, Servier, Otsuka Pharmaceuticals and has acted as advisor to Astra Zeneca and Otsuka Pharmaceuticals. J. Nielsen has received research support from Pfizer and Lundbeck and has received speakers’ honoraria from AstraZeneca, Bristol-Myers Squibb, HemoCue and Lundbeck.
Contributor Information
René Ernst Nielsen, Unit for Psychiatric Research, Aalborg Psychiatric Hospital, Aalborg University Hospital, Mølleparkvej 10, 9000 Aalborg, Denmark.
Florence Odur, Psychiatric Clinic, Malmo University Hospital, Malmo, Sweden.
Torben Østergaard, Clinic for Young People with Schizophrenia (OPUS), Aalborg University Hospital, Aalborg Psychiatric Hospital, Aalborg, Denmark.
Povl Munk-Jørgensen, Aarhus University Hospital, Risskov, Denmark.
Jimmi Nielsen, Center for Schizophrenia, Aalborg University Hospital, Aalborg Psychiatric Hospital, Aalborg, Denmark.
References
- Addington D., Addington J., Maticka-Tyndale E. (1994) Specificity of the Calgary Depression Scale for Schizophrenics. Schizophr Res 11: 239–244 [DOI] [PubMed] [Google Scholar]
- Addington D., Addington J., Maticka-Tyndale E., Joyce J. (1992) Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res 6: 201–208 [DOI] [PubMed] [Google Scholar]
- Buchanan R., Davis M., Goff D., Green M., Keefe R., Leon A., et al. (2005) A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull 31: 5–19 [DOI] [PubMed] [Google Scholar]
- Cannon T., Zorrilla L., Shtasel D., Gur R., Gur R., Marco E., et al. (1994) Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 51: 651–661 [DOI] [PubMed] [Google Scholar]
- Caspi A., Reichenberg A., Weiser M., Rabinowitz J., Kaplan Z., Knobler H., et al. (2003) Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res 65: 87–94 [DOI] [PubMed] [Google Scholar]
- Citrome L., Holt R., Walker D., Hoffmann V.(2011) Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig 31: 455–482 [DOI] [PubMed] [Google Scholar]
- Correll C. (2010) From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry 25(Suppl. 2): S12–S21 [DOI] [PubMed] [Google Scholar]
- Desmarais J., Beauclair L., Margolese H. (2012) Anticholinergics in the era of atypical antipsychotics: Short-term or long-term treatment? J Psychopharmacol 26: 1167–1174 [DOI] [PubMed] [Google Scholar]
- Fagerlund B., Soholm B., Fink-Jensen A., Lublin H., Glenthoj B. (2007) Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol 30: 3–12 [DOI] [PubMed] [Google Scholar]
- Freedman R., Olincy A., Buchanan R., Harris J., Gold J., Johnson L., et al. (2008) Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 165: 1040–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallhofer B., Jaanson P., Mittoux A., Tanghoj P., Lis S., Krieger S. (2007) Course of recovery of cognitive impairment in patients with schizophrenia: a randomised double-blind study comparing sertindole and haloperidol. Pharmacopsychiatry 40: 275–286 [DOI] [PubMed] [Google Scholar]
- Kay S., Fiszbein A., Opler L. (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276 [DOI] [PubMed] [Google Scholar]
- Kay S., Opler L., Spitzer R., Williams J., Fiszbein A., Gorelick A. (1991) SCID-PANSS: two-tier diagnostic system for psychotic disorders. Compr Psychiatry 32: 355–361 [DOI] [PubMed] [Google Scholar]
- Keefe R., Bilder R., Davis S., Harvey P., Palmer B., Gold J., et al. (2007) Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry 64: 633–647 [DOI] [PubMed] [Google Scholar]
- Levander S., Jensen J., Grawe R., Tuninger E. (2001) Schizophrenia – progressive and massive decline in response readiness by episodes. Acta Psychiatr Scand (Suppl. 408): 65–74 [DOI] [PubMed] [Google Scholar]
- Lowe C., Rabbitt P. (1998) Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Cambridge Neuropsychological Test Automated Battery. International Study of Post-operative Cognitive Dysfunction. Neuropsychologia 36:915–923 [DOI] [PubMed] [Google Scholar]
- McGurk S., Green M., Wirshing W., Wirshing D., Marder S., Mintz J., et al. (2004) Antipsychotic and anticholinergic effects on two types of spatial memory in schizophrenia. Schizophr Res 68: 225–233 [DOI] [PubMed] [Google Scholar]
- Minzenberg M., Poole J., Benton C., Vinogradov S. (2004) Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry 161: 116–124 [DOI] [PubMed] [Google Scholar]
- Nielsen R. (2011) Cognition in schizophrenia – a systematic review. Drug Discov Today: Ther Strategies 8: 43–48 [Google Scholar]
- Nielsen R., Levander S., Thode D., Nielsen J. (2012) Effects of sertindole on cognition in clozapine-treated schizophrenia patients. Acta Psychiatr Scand 126: 31–39 [DOI] [PubMed] [Google Scholar]
- Nielsen R., Nielsen J. (2009) Antipsychotic drug treatment for patients with schizophrenia. Clin Med Ther 1: 1053–1068 [Google Scholar]
- Pae C. (2013) Role of the cholinesterase inhibitors in the treatment of schizophrenia. Expert Opin Investig Drugs 22: 293–298 [DOI] [PubMed] [Google Scholar]
- Peuskens J., Tanghoj P., Mittoux A. (2008) The sertindole cohort prospective (SCoP) study: rationale, design and methodology. Pharmacoepidemiol Drug Saf 17: 425–433 [DOI] [PubMed] [Google Scholar]
- Rund B. (2009) Is schizophrenia a neurodegenerative disorder? Nord J Psychiatry 63: 196–201 [DOI] [PubMed] [Google Scholar]
- Sahakian B., Owen A. (1992) Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 85: 399–402 [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Tallerico T. (1998) Antipsychotic drugs which elicit little or no Parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry 3: 123–134 [DOI] [PubMed] [Google Scholar]
- Shekhar A., Potter W., Lightfoot J., Lienemann J., Dube S., Mallinckrodt C., et al. (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 165: 1033–1039 [DOI] [PubMed] [Google Scholar]
- Szoke A., Trandafir A., Dupont M., Meary A., Schurhoff F., Leboyer M. (2008) Longitudinal studies of cognition in schizophrenia: meta-analysis. Br J Psychiatry 192: 248–257 [DOI] [PubMed] [Google Scholar]
- Tracy J., Monaco C., Abraham G., Josiassen R., Pollock B. (1998) Relation of serum anticholinergicity to cognitive status in schizophrenia patients taking clozapine or risperidone. J Clin Psychiatry 59: 184–188 [PubMed] [Google Scholar]
- Wallace T., Ballard T., Pouzet B., Riedel W., Wettstein J. (2011) Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav 99: 130–145 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992) ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization [Google Scholar]