Significance
Type III secretion systems are multiprotein nanomachines with the capacity to inject bacterial proteins into eukaryotic cells that are used by bacterial symbionts or pathogens to interact with their hosts. The main component of these machines is the needle complex, a multiprotein organelle composed of a bacterial envelope-associated multiring base and a filament-like structure, the needle, that projects from the bacterial surface and is linked to the base by an inner rod. Here we have studied the inner rod in detail and provided important insight into the mechanism of assembly and function of type III secretion machines. Understanding the function of type III secretion machines could lead to the development of novel antimicrobials.
Keywords: bacterial pathogenesis, organelle assembly, Salmonella pathogenesis
Abstract
Type III secretion machines are essential for the biology of many bacteria that are pathogenic or symbiotic for animals, plants, or insects. They exert their function by delivering bacterial effector proteins into target eukaryotic cells. The core component of these machines is the needle complex, a multiprotein structure that spans the bacterial envelope and serves as a conduit for proteins that transit this secretion pathway. The needle complex is composed of a multiring base embedded in the bacterial envelope and a filament-like structure, the needle, that projects from the bacterial surface and is linked to the base by the inner rod. Assembly of the needle complex proceeds in a step-wise fashion that is initiated by the assembly of the base and is followed by the export of the building subunits for the needle and inner rod substructures. Once assembled, the needle complex reprograms its specificity and becomes competent for the secretion of effector proteins. Here through genetic, biochemical, and electron microscopy analyses of the Salmonella inner rod protein subunit PrgJ we present evidence that the assembly of the inner rod dictates the timing of substrate switching and needle length. Furthermore, the identification of mutations in PrgJ that specifically alter the hierarchy of protein secretion provides additional support for a complex role of the inner rod substructure in type III secretion.
Type III secretion machines have evolved to shape the functional interface between many pathogenic or symbiotic bacteria with their respective hosts (1–3). These machines exert their function by delivering effector proteins into target cells with the capacity to modulate a variety of cellular processes for the benefit of the pathogens or symbionts that encode them (4). Type III secretion systems (TTSSs), comprising >20 proteins, are among the most complex protein secretion machines so far identified (5, 6). Salmonella Typhimurium, a cause of gastroenteritis in humans, encodes two TTSSs within its pathogenicity islands 1 (SPI-1) and 2 (SPI-2), which are essential for pathogenicity (7, 8). Through the delivery of a battery of ∼40 effector proteins, these machines allow S. Typhimurium to invade and survive within eukaryotic cells.
TTSSs consist of the envelope-associated needle complex, which mediates the passage of the secreted proteins through the bacterial envelope, and several cytoplasmic accessory proteins, which are required for the recognition and sorting of the proteins destined to travel this pathway (5, 6, 9, 10). The needle complex is composed of a multiring, envelope-associated base substructure, and a filament or needle seated on the base, projecting outward from the bacterial surface (9, 11). The needle is linked to the base by the inner rod, an incompletely characterized substructure of the needle complex (12). Assembly of the needle complex is a coordinated process, which in the S. Typhimurium SPI-1-encoded TTSS is initiated by a subset of membrane proteins (SpaP, SpaQ, SpaR, and SpaS) that are thought to form a protein channel in the inner membrane (13, 14). The inner rings of the base assemble around these membrane proteins and after addition of the independently assembled outer rings, the base substructure becomes competent for type III secretion (13, 15). However, the base substructure has very narrow substrate specificity because it can only recognize the inner rod (PrgJ) and needle (PrgI) protein subunits and a regulatory protein (InvJ). Once the full needle complex is assembled, the type III secretion machine switches specificity to become competent for the secretion of effector proteins.
The mechanisms by which the secretion machine is reprogramed are not completely understood although at least one regulatory protein, InvJ in the S. Typhimurium SPI-1 TTSS, is required for this process (16). In its absence, the needle complex is unable to switch substrates and consequently assembles abnormally long needles (16). The function of InvJ (or its homologs in other bacteria) is poorly understood and the subject of some controversy. For example, YscP, a functional homolog of InvJ in the pathogenic bacteria Yersinia enterocolitica, has been proposed to function as a molecular ruler, measuring the length of the needle and triggering substrate switching once an appropriate fixed length of the needle is achieved (17). How the measuring of the length would be carried out is not understood but it has been proposed that the fully extended form of YscP located within the lumen of the secretion channel measures the filament length by interacting with proteins at the tip and at the base of the needle complex, triggers substrate switching. An alternative model has been proposed in which InvJ is required for the assembly and/or linking of the inner rod to the base substructure, a process that leads to the firm anchoring of the needle filament to the base (12, 18). The anchoring of the needle filament leads to conformational changes on the cytoplasmic face of the needle complexes (11), which are thought to be essential for substrate switching. In this model the termination of the assembly of the inner rod is the crucial event that determines substrate switching. To better understand the role of the inner rod assembly in substrate switching we undertook biochemical, electron microscopy, and mutagenesis analyses of its building subunit, PrgJ. Collectively, our results support a role for the inner rod in determining the timing of substrate switching and needle length. Furthermore, the identification of mutations in the inner rod subunit that disrupt the hierarchy of protein secretion reveals an additional level of complexity in the function of this substructure in type III secretion.
Results
The Inner Rod Does Not Assemble in the Absence of InvJ.
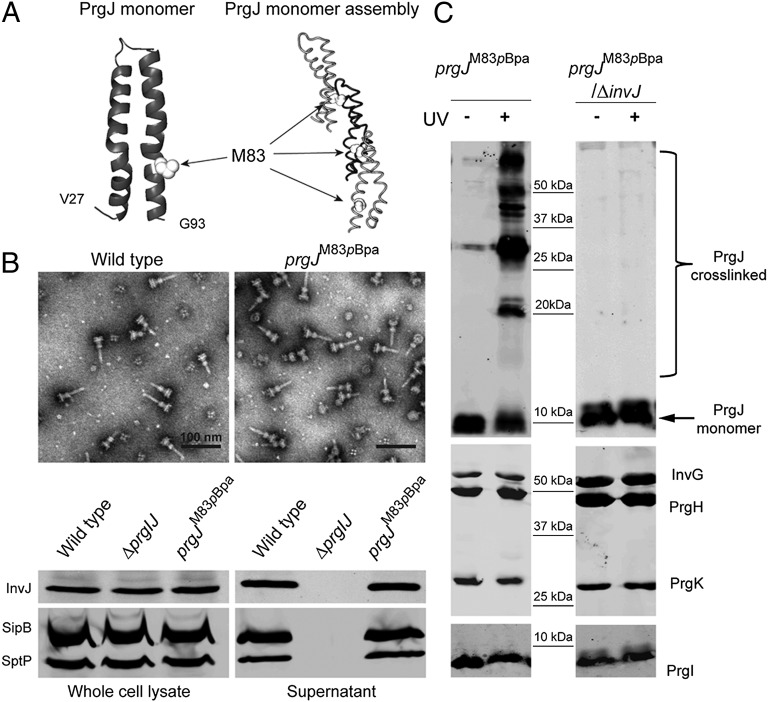
Previous studies have shown that the absence of the nonstructural regulatory protein InvJ results in the absence of the socket, a substructure within the base of the needle complex that serves as receptacle or anchoring point for the inner rod and, indirectly, for the needle substructure (12). Thus, absence of InvJ results in the very loose association of the needle to the base substructure and, consequently, an absence of substrate switching. However, these studies did not specifically determine whether InvJ was required for the assembly of the inner rod or for its anchoring to the base. The atomic structure of the inner rod subunit PrgJ is predicted to share very substantial structural similarities with PrgI, the needle subunit (19–21). Indeed, the in silico-modeled structures of PrgJ and PrgI share a similar α-helical hairpin shape flanked by flexible regions, and the two monomeric structures align very well around critical domains required for needle assembly (Fig. S1). Although the inner rod has not been unambiguously visualized by electron microscopy, the structural similarity of the building subunits indicates that the inner rod and needle structures are likely to be structurally similar. In fact, the transition between inner rod and needle substructures is not clearly demarcated in low-resolution electron microscopic images (22). Therefore, to investigate the role of InvJ in inner rod assembly we developed an assay that could report on the assembly of this substructure. We modeled the structure of the inner rod based on the solid state NMR atomic structure of the PrgI needle filament (Fig. 1) (23). Using this model we searched for residues predicted to be in the monomer–monomer interface and that, because of this location, could potentially report on inner rod assembly. We identified PrgJM83 as one of such potential residues and introduced the photo–cross-linkable amino acid p-benzoyl-l-phenylalanine (pBpa) at that position by introducing an amber codon (UAG) at the prgJM83 position and expressing an orthogonal aminoacyl tRNA synthetase-tRNA pair that incorporates this unnatural amino acid at the UAG codon (24, 25). The resulting mutant strain was shown to assemble needle complexes and carry out type III secretion in a manner indistinguishable from wild type (Fig. 1). Highly purified needle complexes from this mutant strain were exposed to UV light to promote site-specific cross-linking and examined by SDS/PAGE and Western blotting using an antibody specific for PrgJ. Exposure to UV light resulted in the appearance of a ladder of reacting bands of a molecular weight higher than that of the PrgJ monomer, which was absent from complexes not exposed to UV light or from UV light-exposed complexes obtained from wild-type bacteria (Fig. 1). Probing the different samples with antibodies directed to other components of the needle complex did not detect any changes in their SDS/PAGE migration pattern upon UV light exposure (Fig. 1) indicating that the cross-linked species represent PrgJ subunits that have been assembled into an inner rod. This conclusion is consistent with the observation that the predicted molecular weight of the shifted bands in the cross-linked sample differs among the bands via a molecular weight similar to that of the PrgJ monomer (Fig. 1). Therefore, the cross-linked species represent different multimeric forms of the inner rod subunit and can serve as a surrogate to monitor inner rod assembly. We then introduced a ∆invJ mutation into the strain expressing PrgJM83pBpa and examined the effect of the absence of InvJ in the assembly of the inner rod. We did not detect shifted PrgJ antibody reacting bands in the needle complexes from this mutant strain upon UV light exposure. These results indicate that, in the absence of InvJ, the inner rod fails to assemble. Because the ∆invJ mutant strain exhibits fully assembled needle filaments (although not firmly anchored to the base) (16), these results also demonstrate that assembly of the needle filament can occur independently from assembly of the inner rod.
Fig. 1.
The inner rod does not assemble in the absence of InvJ. (A) Structural modeling of PrgJ. The monomeric and multimeric modeled structures are shown and the position of the M83 residue selected for cross-linking experiments is indicated (white spheres). (B) S. Typhimurium-expressing PrgJM83pBpa yields intact and functional needle complex structures. Shown are electron micrographs of negatively stained needle complexes isolated from wild-type and prgJM83pBpa strains (Upper) and Western blot analysis (Lower) of whole-cell lysates and culture supernatants of the indicated strains for the presence of the type III secreted proteins SipB, SptP, and InvJ. (C) Cross-linking of PrgJM83pBpa occurs in needle complexes obtained from wild-type but not from ∆invJ S. Typhimurium mutant strains. Needle complexes were isolated from the indicated S. Typhimurium strains, exposed to UV, or left untreated as indicated, and analyzed by Western immunoblot with antibodies directed to PrgJ (Upper) or components of the needle complex (Lower).
Mutations in the Inner Rod Protein PrgJ Affect Needle Length.
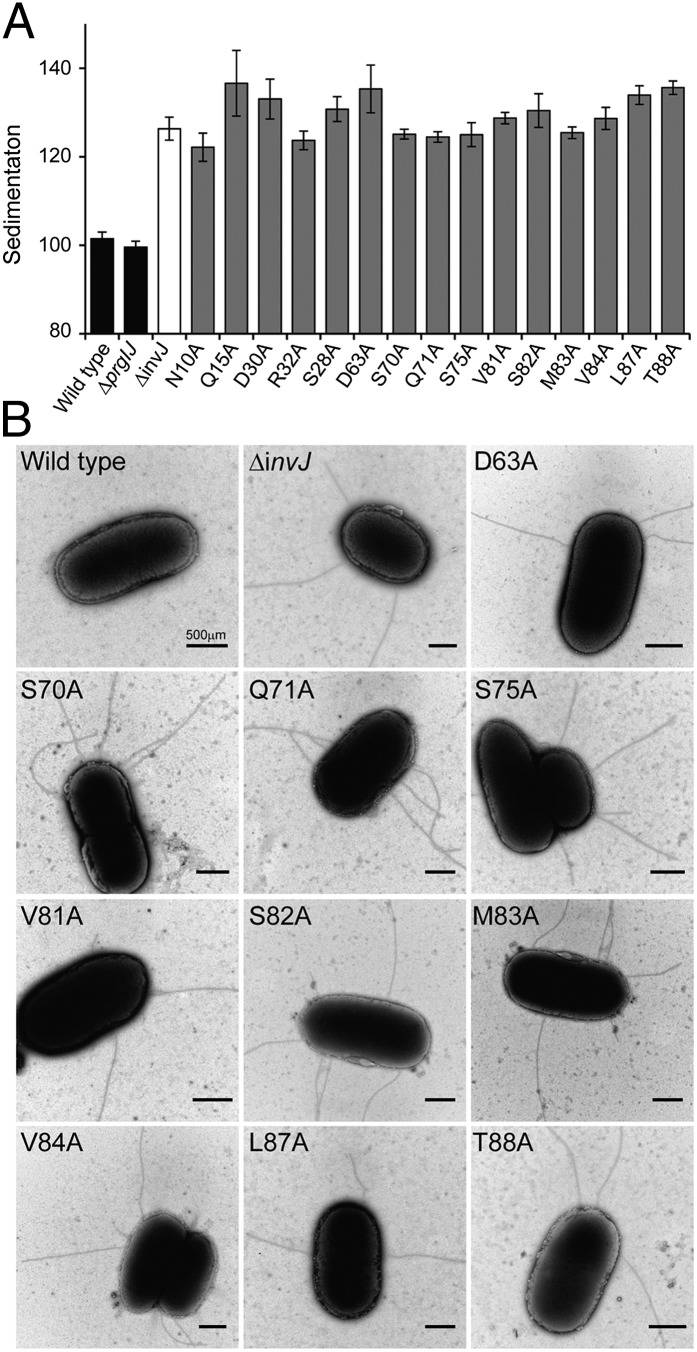
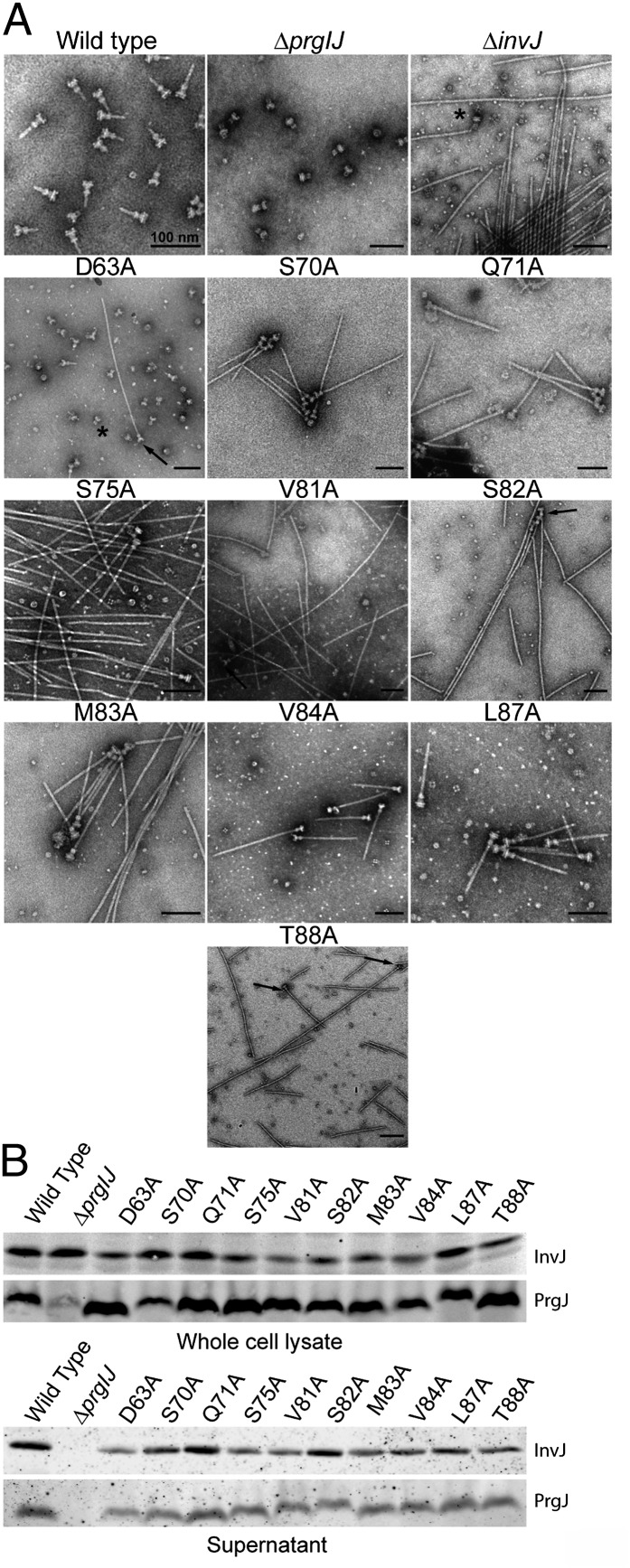
We reasoned that if completion of the assembly of the inner rod dictates the timing of substrate switching, it should be possible to obtain mutants in the inner rod subunit PrgJ with an abnormal needle length even in the presence of InvJ. We have previously observed that S. Typhimurium mutants with abnormally long needles exhibit a clumping phenotype that results in their precipitation in the culture tube (16). We therefore conducted alanine scanning mutagenesis of every residue of the inner rod protein PrgJ and screened the resulting mutants for the presence of abnormally long needles using the clumping assay. We found 15 mutants that exhibited a clumping phenotype and that therefore were likely to display long needles on their surface (Fig. 2A and Fig. S2). We examined 10 of these mutants under an electron microscope and confirmed that, as predicted by the clumping phenotype, they exhibited abnormally long needles on their surface (Fig. 2B). Absence of the regulatory protein InvJ results in abnormally long needles that are not firmly attached to the base substructure and therefore come off during the needle complex isolation procedure (12). To examine the integrity of the needle complex in the PrgJ mutants that showed long needles, we isolated needle complexes and examined them by electron microscopy. As expected, isolated needle complexes from these mutants exhibited extremely long needles (Fig. 3A). In fact, the length of the needles obtained from these mutants resembled the length of those observed in needle complexes obtained from the ∆invJ mutant strain (16). It has been previously shown that secretion of InvJ through the TTSS is required for this regulatory protein to exert its function in needle complex assembly and substrate switching (26). To ascertain whether InvJ secretion was affected in the PrgJ mutants we examined the culture supernatants of these mutants for the presence of InvJ. We found that the levels of InvJ in the culture supernatants of all the PrgJ mutants with altered needle length were indistinguishable from wild type (Fig. 3B). Furthermore, the levels of PrgJ itself were also unaltered in all the mutants (Fig. 3B). These results indicate that the defect in needle length control observed in the PrgJ mutants is not due to defects in InvJ secretion or PrgJ stability. Consistent with this observation, the needles obtained from all but one PrgJ point mutant strains were seen firmly attached to the base substructure (Fig. 3A), which is in sharp contrast to the needles from the ∆invJ mutant, which easily detached during needle complex purification (12). Needle complexes isolated from the PrgJD63A mutant showed the presence of large numbers of bases without needles and many detached long filaments (Fig. 3A and Fig. S3). The location of this mutation in the modeled inner rod predicts that this residue may be important in the interaction of PrgJ with the needle protein PrgI (Fig. S4), thus its mutation may lead to a weaker link between the inner rod and the needle filament. However, more experiments will be required to test this hypothesis. Most PrgJ mutations showing firmly attached but long needles mapped to sites predicted to be involved in PrgJ/PrgJ monomer interactions (Fig. S4). Although not affecting the stability of the resulting needle complexes and thus allowing for the firm anchoring of the needle filaments, we hypothesize that these mutations may lead to relatively slower inner rod assembly rates. Because termination of the assembly of the inner rod ultimately leads to substrate switching and needle length determination, slower assembly rates would result in longer needles as observed in these mutants. Taken together, these results indicate that mutations in PrgJ can lead to aberrant needle length control even in the presence of wild-type levels of the regulatory protein InvJ.
Fig. 2.
Mutations in the inner rod protein PrgJ affect TTSS needle length. (A) Culture sedimentation analysis of the indicated PrgJ alanine mutant strains during growth under TTSS inducing conditions. Bacteria that express long needles sediment in the culture tubes due to needle-mediated clumping. Sedimentation was calculated by comparing the OD600 values pre- and postvortexing of standing bacterial cultures and are expressed as arbitrary units after standardization relative to wild type, which is given the arbitrary value of 100. Error bars represent the standard deviation of three independent samples. (B) Electron micrographs of negatively stained bacterial cells reveal long TTSS needle filaments. Indicated are the different PrgJ mutants examined as well as wild type and the ∆invJ mutant control.
Fig. 3.
Analysis of PrgJ mutants exhibiting abnormal TTSS needle length. (A) Electron micrographs of isolated needle complexes from the indicated S. Typhimurium strains. (B) Western blot analysis of whole-cell lysates and culture supernatants of the indicated S. Typhimurium mutant strains.
PrgJ Mutant Strains with Aberrant Needle Length Are Able to Assemble Functional Needle Complexes That Can Undergo Substrate Switching.
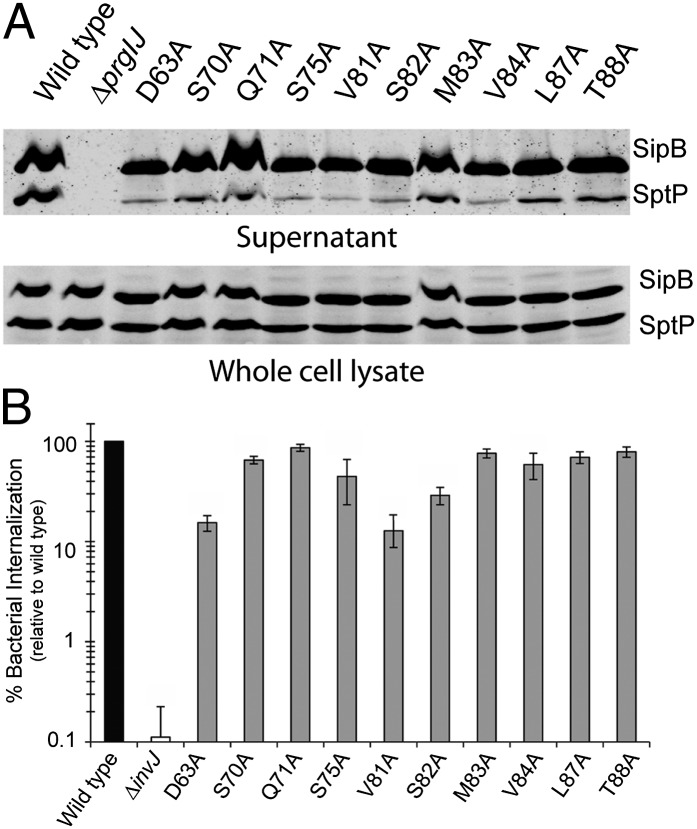
Absence of the regulatory protein InvJ results in type III secretion machines that are locked in a mode only competent for the secretion of the needle and inner rod proteins (16, 27, 28). The TTSS in the ∆invJ mutant is therefore nonfunctional (29). We examined the PrgJ mutants that exhibited abnormally long needles for their ability to undergo substrate switching by examining their culture supernatants for the presence of type III secreted proteins. Unlike the ∆invJ mutant, we found that all of the PrgJ mutants exhibiting long needles were competent for substrate switching demonstrated by their ability to secrete middle (translocase SipB) and late (effector SptP) substrate proteins (Fig. 4A). Consistent with this observation, these mutants showed a functional needle complex because they were competent for mammalian cell invasion (Fig. 4B), a measure of the functionality of the SPI-1 TTSS (30). Introduction of a ∆invJ mutation to a strain expressing PrgJM83A abolished secretion indicating that the ΔinvJ phenotype is dominant over the PrgJM83A phenotype (Fig. S5). Taken together, these results indicate that the phenotype of the PrgJ mutants is distinct from the phenotype of the ∆invJ mutant and supports a model in which InvJ would exert its function before inner rod assembly. Therefore, the aberrant needle length observed in the PrgJ mutants is unlikely to be due to abnormal InvJ function and/or secretion.
Fig. 4.
PrgJ mutant strains with aberrant needle length assemble a functional TTSS that undergoes substrate switching. (A). Western blot analysis of whole-cell lysates and culture supernatants of the indicated strains examining the presence of the protein translocase SipB and the effector protein SptP. (B) Bacterial invasion of cultured Henle-407 cells by the indicated PrgJ mutant and control strains was determined using the gentamicin protection assay. Values represent the percentage of bacteria that survive gentamicin treatment, are normalized relative to the values of wild-type bacteria, and are the average of three determinations. Error bars represent the standard deviation of three independent experiments.
Mutations in PrgJ Lead to an Altered Hierarchy in Type III Secretion.
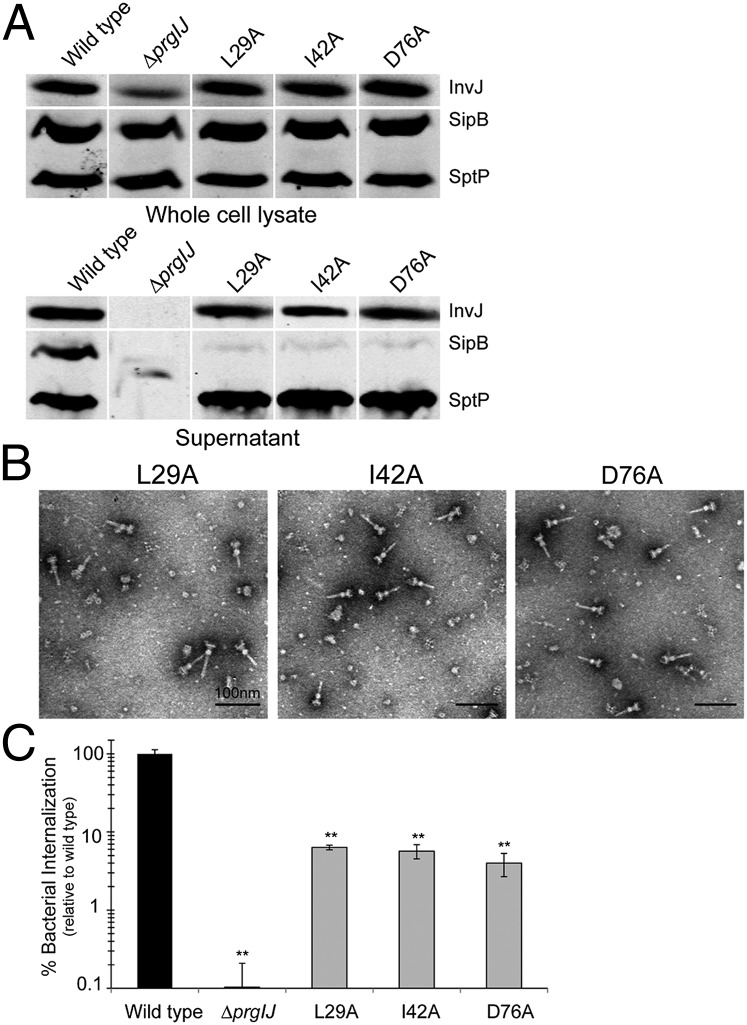
It has been previously shown that there is a hierarchy in the type III secretion process so that different proteins are engaged at different times (10, 29). Proteins necessary for the assembly of the needle and inner rod substructures of the needle complex are engaged first. However, the hierarchy in the secretion process extends beyond needle complex assembly because the protein translocases, which mediate the passage of effectors through the target host cell membrane, must be secreted before the effector proteins themselves. Indeed, a cytoplasmic sorting platform has been shown to be critical to the establishment of the secretion hierarchy (10). We examined the PrgJ mutants for their ability to secrete proteins within the different hierarchical categories of type III secretion. We examined the secretion of the regulatory protein InvJ, the translocase SipB, and the effector SptP, which are representatives of early, middle, and late type III secretion substrates, respectively (29, 31, 32). We found that most of the mutants exhibited a normal or near normal secretion profile (Fig. S6). However, PrgJD31A and PrgJY77A mutants exhibited a complete absence of type III secretion suggesting that these mutations lead to a complete loss of PrgJ function (Fig. S5). Consistent with this observation, these mutant strains showed abundant needle complex bases with no needles (Fig. S7). Most interestingly, however, the PrgJL29A, PrgJI42A, and PrgJD76A mutants exhibited a severely reduced secretion of the protein translocase SipB but wild-type levels of secretion of the early and late substrates InvJ and SptP (Fig. 5A), as well as wild-type needle complexes (Fig. 5B). Consistent with the lack of secretion of the protein translocases, these mutants showed a significantly reduced ability to enter cultured mammalian cells (Fig. 5C). These results indicate that the inner rod is also involved in the establishment of the hierarchy of the secretion process, thus suggesting a more complex role for this substructure in type III secretion.
Fig. 5.
Mutations in PrgJ lead to altered hierarchy in type III secretion. (A) Western blot analysis of whole-cell lysates and culture supernatants of the indicated strains examining the presence of early (InvJ), middle (SipB), and late (SptP) substrates. (B) Electron micrographs of isolated needle complexes from the indicated S. Typhimurium strains. (C) Bacterial invasion of cultured Henle-407 cells by the indicated PrgJ mutant and control strains was determined using the gentamicin protection assay. Values represent the percentage of bacteria that survive gentamicin treatment, are normalized relative to the values of wild-type bacteria, and are the average of three determinations. **P < 0.001 relative to the values of wild type. Error bars represent standard deviation of three independent experiments.
Discussion
Assembly of the type III secretion needle complex is highly ordered and occurs in sequential steps (13, 14). A key and unique step in this process is the assembly of the needle and connecting inner rod substructures, which require a functional TTSS (16). Once the needle complex is fully assembled, the TTSS switches substrate specificity becoming competent for the secretion of translocases and effector proteins. The mechanisms by which the TTSS changes substrate specificity are incompletely understood and are the subject of some controversy. A regulatory protein, which in the S. Typhimurium SPI-1 TTSS is known as InvJ, plays a central role in this process. In its absence, substrate switching does not occur, resulting in nonfunctional needle complexes with abnormally long and fragile needles (16). At least two models have been proposed to account for the function of this regulatory protein or its homologs in other systems. One model proposes that this regulator exerts its function as a molecular ruler by measuring the length of the needle in an extended configuration within the secretion channel (17). An alternative model for the function of InvJ has been proposed based on the observation that needle complexes obtained from the ∆invJ mutant lack the socket (the substructure within the needle complex) that dockswith the inner rod thus anchoring the needle (12). This model proposes that InvJ aids in the formation of the socket thus facilitating the attachment and/or assembly of the inner rod, a process essential for substrate switching. Therefore, in this model the function of InvJ would be similar to that proposed for scaffolding proteins involved in flagellar assembly, which, like InvJ, do not form part of the final flagellar structure and are secreted into the culture medium (33).
Here we have provided evidence to support the model that the completion of the assembly of the inner rod dictates the timing of substrate switching of the TTSS needle complex and thus determines needle length (Fig. S8). This conclusion is supported by several pieces of evidence. First, the direct evidence we provided that, in the absence of the regulatory protein InvJ the inner rod fails to assemble, thus explaining the phenotype of the ∆invJ mutant (i.e., absence of substrate switching and longer, nonattached needles). Second, through an extensive genetic screen, we identified specific mutations in the inner rod protein PrgJ that lead to abnormally long needles. However, unlike the needle complexes assembled in the ∆invJ mutant, the identified PrgJ mutants undergo substrate switching and assemble functional needle complexes that have very long but firmly attached needle substructures. Most of the identified PrgJ mutants mapped to residues that our modeling of the inner rod are predicted to be involved in monomer–monomer interactions. Therefore, we propose that the PrgJ mutations exhibiting longer needles may lead to inner rods that assemble at a slower rate than wild type, delaying but not preventing substrate switching, and thus resulting in longer needles. Expression and secretion levels of InvJ in the different PrgJ mutants were indistinguishable from wild type. This observation coupled with the fact that the resulting needles were firmly attached to the base indicates that the function of InvJ is unaffected in these mutants. These data are consistent with a model in which termination of the assembly of the inner rod is the triggered for the substrate switching event (12, 18). It is also consistent with the previous observation indicating that overexpression of the inner rod protein PrgJ or the needle protein PrgI leads to shorter and longer needles, respectively. Finally, our findings and the proposed model are compatible with the observations that the filament length in wild-type needle complexes follows a normal distribution with a significant range in size (12).
Once assembled, the needle complex becomes competent for the secretion of translocases and effector proteins. However, there is a hierarchy in this secretion process because the protein translocases must be deployed on the target host cell membrane before the secretion of the effectors (29, 31). The establishment of this hierarchy is incompletely understood although it is known to require a cytoplasmic sorting platform that is sequentially loaded with the proteins destined to be secreted (10). Interestingly, we found a set of PrgJ mutants that exhibited an altered hierarchy in the secretion process. These mutants assembled an apparently normal needle complex, exhibiting needles with a length indistinguishable from wild type. However, these mutants were specifically defective for the secretion of protein translocases and showed apparently normal secretion of both early (InvJ) and late (SptP) substrates. Although the understanding of the mechanisms by which the inner rod influences the hierarchy of protein secretion would require further studies, this finding suggests a complex role for the inner rod in type III secretion. In addition, these findings suggest the presence of mechanisms by which the inner rod and needle structures can transduce signals to the cytoplasmic elements of the type III secretion machine that are essential for its function. Consistent with this hypothesis, previous studies have reported a functional interplay between the inner rod subunit and cytoplasmic components of the TTSS (34).
In summary, these studies place the inner rod substructure as a central element in the process of needle length determination and substrate switching, thus providing major insight into the mechanism of reprogramming of the type III secretion machine.
Materials and Methods
Bacterial Strains, Plasmids, and Culture Conditions.
All strains were derived from S. Typhimurium strain SJW2941 (9). Mutant strains were constructed as previously described (35). Alanine-scanning mutagenesis of prgJ was carried out using standard molecular genetic techniques. All prgJ mutants were cloned into a low-copy expression vector pWSK29 (36) derivative that also coexpressed wild-type prgI (to maintain wild-type PrgI:PrgJ expression ratios), and introduced into a ∆prgI prgJ S. Typhimurium mutant strain for phenotype screening. All S. Typhimurium strains were grown at 37 °C in LB broth supplemented with 0.3 M NaCl with low aeration to stimulate the expression of components and substrates of the SPI-1 TTSS. When appropriate, the following compounds were added to the medium: ampicillin (100 μg/mL), spectinomycin (50 μg/mL), chloramphenicol (15 μg/mL), tetracycline (15 μg/mL), arabinose (0.0125%), or pBpa (0.1 mM) (Bachem).
PrgJ Structure Modeling.
In silico modeling of PrgJ was carried out using the Protein Homology/analogy Recognition Engine V 2.0 (Phyre2) (37). Following secondary structure prediction and multiple sequence alignment a model for the PrgJ monomer was obtained, showing the most similarity to the PrgI structure (2JOW, RCSB protein databank). To generate a multimonomer assembly model for PrgJ, individual PrgJ monomers were aligned to monomer subunits in the solid state NMR structure generated for the PrgI filament (2LPZ, RCSB protein databank) using PyMol (38). The C-terminal helical domain of PrgJ (PrgJD57–PrgJG93), which shows the highest level of conservation between PrgJ and PrgI homologs, has an rmsd value of 1.113 when aligned with the PrgI monomer structure.
Culture Sedimentation Analysis.
Culture sedimentation was determined for cells grown under TTSS-inducing conditions. Optical density measurements were collected both pre- and postagitation. Sedimentation potential was calculated by dividing the postagitation value by the preagitation value (OD600 post vortex − OD600 pre vortex) × 100. Values over 120 were considered to be positive for cell sedimentation.
Needle Complex Isolation and Analysis.
Needle complexes were isolated from cultures grown under TTSS-inducing conditions as previously described (9, 12). Samples were processed through 27.5% (wt/vol) CsCl gradients and the collected fractions were assayed for the distribution of the structural components of the needle complex (PrgH, PrgK, InvG, PrgI, and PrgJ) by immunoblot and examined by negative stain and transmission electron microscopy as previously described (9, 12).
Evaluation of TTSS Function.
The functionality of the S. Typhimurium SPI-1 TTSS was evaluated by examining the secretion of substrate proteins to culture supernatants (39), and by evaluating the ability of the different S. Typhimurium mutant strains to invade culture epithelial cells (31), as previously described.
Site-Specific Cross-Linking.
For site-specific cross-linking experiments, the photoreactive amino acid pBpa (24, 25) was incorporated into PrgJ by replacing the codon for methionine 83 (M83) with a TAG amber codon. Incorporation of the unnatural amino acid was accomplished by amber codon suppression in the presence of the pSUP plasmid encoding an Escherichia coli nonsense suppressor tRNA–tRNA synthetase system that can accommodate the atypical residue (24, 25). Cultures were grown in LB broth supplemented with 0.3 M NaCL and 1 mM pBpa. For cross-linking experiments, purified needle complex samples were exposed to UV irradiation for 30 min at 4 °C. Cross-linking was assessed by immunoblot analysis using antibodies directed toward PrgJ and the other structural components of the needle complex (PrgH, PrgK, PrgI, and InvG).
Transmission Electron Microscopy.
Whole bacterial cell and purified NC samples (5 μL volume) were applied to glow discharged carbon–formvar-coated copper grids. Bacterial cells and purified NC samples were negatively stained with 1% and 2% PTA, pH 7.0 respectively. Images were collected using an FEI Tecnai Biotwin microscope at 80 kV and using the Morada Soft Imaging system and a 6-M pixel CCD camera (Olympus).
Supplementary Material
Acknowledgments
We thank members of the J.E.G. laboratory for careful review of the manuscript. This work was supported by National Institutes of Health Grant AI30492 (to J.E.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319698111/-/DCSupplemental.
References
- 1.Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444(7119):567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 2.Lindeberg M, Cunnac S, Collmer A. The evolution of Pseudomonas syringae host specificity and type III effector repertoires. Mol Plant Pathol. 2009;10(6):767–775. doi: 10.1111/j.1364-3703.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston GM. Metropolitan microbes: Type III secretion in multihost symbionts. Cell Host Microbe. 2007;2(5):291–294. doi: 10.1016/j.chom.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Galán JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5(6):571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Büttner D. Protein export according to schedule: Architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol Mol Biol Rev. 2012;76(2):262–310. doi: 10.1128/MMBR.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S, Chaudhury S, McShan AC, Kaur K, De Guzman RN. Structure and biophysics of type III secretion in bacteria. Biochemistry. 2013;52(15):2508–2517. doi: 10.1021/bi400160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galán JE. Salmonella interactions with host cells: Type III secretion at work. Annu Rev Cell Dev Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 2003;5(8):501–511. doi: 10.1046/j.1462-5822.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 9.Kubori T, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280(5363):602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 10.Lara-Tejero M, Kato J, Wagner S, Liu X, Galán JE. A sorting platform determines the order of protein secretion in bacterial type III systems. Science. 2011;331(6021):1188–1191. doi: 10.1126/science.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marlovits TC, et al. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306(5698):1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marlovits TC, et al. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature. 2006;441(7093):637–640. doi: 10.1038/nature04822. [DOI] [PubMed] [Google Scholar]
- 13.Wagner S, et al. Organization and coordinated assembly of the type III secretion export apparatus. Proc Natl Acad Sci USA. 2010;107(41):17745–17750. doi: 10.1073/pnas.1008053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukhan A, Kubori T, Wilson J, Galán JE. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J Bacteriol. 2001;183(4):1159–1167. doi: 10.1128/JB.183.4.1159-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diepold A, Wiesand U, Cornelis GR. The assembly of the export apparatus (YscR,S,T,U,V) of the Yersinia type III secretion apparatus occurs independently of other structural components and involves the formation of an YscV oligomer. Mol Microbiol. 2011;82(2):502–514. doi: 10.1111/j.1365-2958.2011.07830.x. [DOI] [PubMed] [Google Scholar]
- 16.Kubori T, Sukhan A, Aizawa SI, Galán JE. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc Natl Acad Sci USA. 2000;97(18):10225–10230. doi: 10.1073/pnas.170128997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Journet L, Agrain C, Broz P, Cornelis GR. The needle length of bacterial injectisomes is determined by a molecular ruler. Science. 2003;302(5651):1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- 18.Wood SE, Jin J, Lloyd SA. YscP and YscU switch the substrate specificity of the Yersinia type III secretion system by regulating export of the inner rod protein YscI. J Bacteriol. 2008;190(12):4252–4262. doi: 10.1128/JB.00328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong D, et al. The Salmonella type III secretion system inner rod protein PrgJ is partially folded. J Biol Chem. 2012;287(30):25303–25311. doi: 10.1074/jbc.M112.381574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyraz O, et al. Protein refolding is required for assembly of the type three secretion needle. Nat Struct Mol Biol. 2010;17(7):788–792. doi: 10.1038/nsmb.1822. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, et al. Differences in the electrostatic surfaces of the type III secretion needle proteins PrgI, BsaL, and MxiH. J Mol Biol. 2007;371(5):1304–1314. doi: 10.1016/j.jmb.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schraidt O, et al. Topology and organization of the Salmonella typhimurium type III secretion needle complex components. PLoS Pathog. 2010;6(4):e1000824. doi: 10.1371/journal.ppat.1000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loquet A, et al. Atomic model of the type III secretion system needle. Nature. 2012;486(7402):276–279. doi: 10.1038/nature11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin JW, Martin AB, King DS, Wang L, Schultz PG. Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proc Natl Acad Sci USA. 2002;99(17):11020–11024. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin JW, Schultz PG. In vivo photocrosslinking with unnatural amino Acid mutagenesis. ChemBioChem. 2002;3(11):1135–1137. doi: 10.1002/1439-7633(20021104)3:11<1135::AID-CBIC1135>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Rüssmann H, Kubori T, Sauer J, Galán JE. Molecular and functional analysis of the type III secretion signal of the Salmonella enterica InvJ protein. Mol Microbiol. 2002;46(3):769–779. doi: 10.1046/j.1365-2958.2002.03196.x. [DOI] [PubMed] [Google Scholar]
- 27.Sukhan A, Kubori T, Galán JE. Synthesis and localization of the Salmonella SPI-1 type III secretion needle complex proteins PrgI and PrgJ. J Bacteriol. 2003;185(11):3480–3483. doi: 10.1128/JB.185.11.3480-3483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collazo CM, Zierler MK, Galán JE. Functional analysis of the Salmonella typhimurium invasion genes invl and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15(1):25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 29.Collazo CM, Galán JE. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64(9):3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galán JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collazo CM, Galán JE. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24(4):747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaniga K, Uralil J, Bliska JB, Galán JE. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol Microbiol. 1996;21(3):633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 33.Macnab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 34.Cherradi Y, et al. Interplay between predicted inner-rod and gatekeeper in controlling substrate specificity of the type III secretion system. Mol Microbiol. 2013;87(6):1183–1199. doi: 10.1111/mmi.12158. [DOI] [PubMed] [Google Scholar]
- 35.Kaniga K, Bossio JC, Galán JE. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13(4):555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 36.Stocker NG, Fairweather NL, Spratt BG. Versatile low-copy-number vectors for cloning in E. coli. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- 37.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: A case study using the Phyre server. Nat Protoc. 2009;4(3):363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 38. DeLano WL (2002) The PyMOL Molecular Graphics System. Available at www.pymol.org.
- 39.Kaniga K, Tucker S, Trollinger D, Galán JE. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177(14):3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.