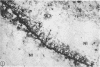
Abstract
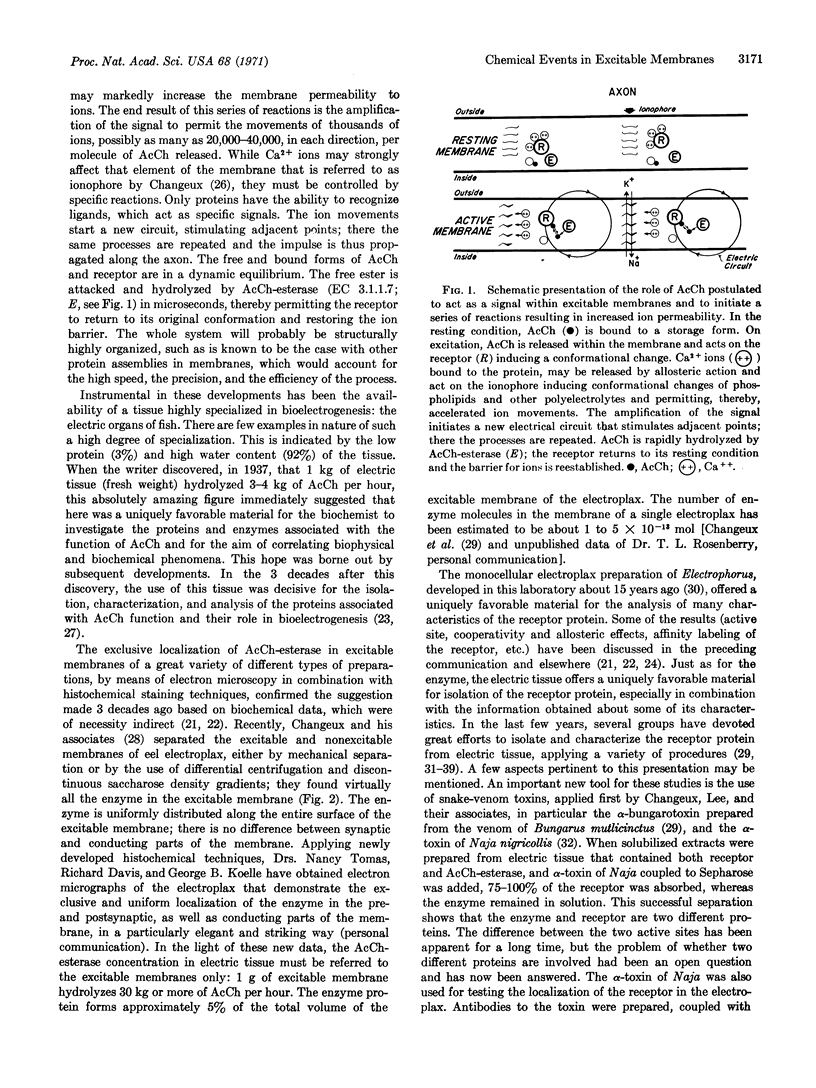
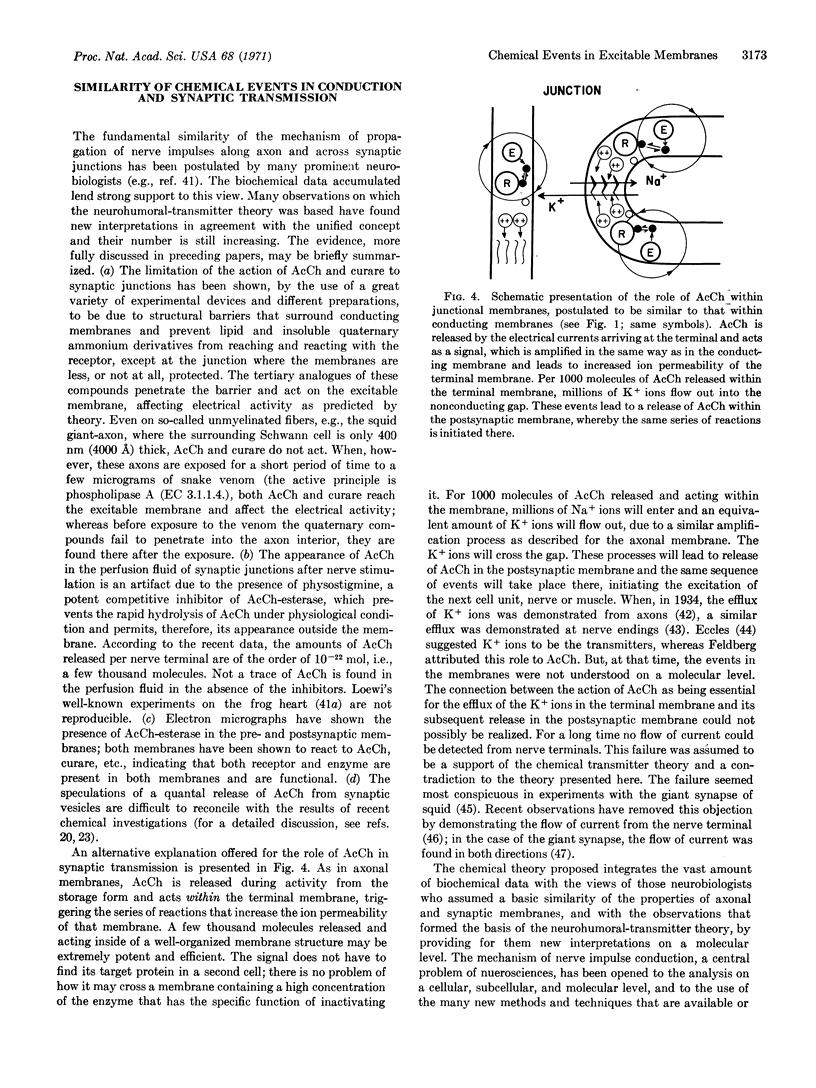
Evidence has accumulated in recent years for the central role of proteins and enzymes in the function of cell membranes. In the chemical theory proposed for the generation of bioelectricity, i.e., for the control of the ion permeability changes of excitable membranes, the protein assembly associated with the action of acetylcholine plays an essential role. Support of the theory by recent protein studies in which the excitable membranes of the highly specialized electric tissue were used will be discussed. A scheme is presented indicating the possible sequence of chemical reactions that change ion permeability after excitation. A sequence of chemical events within the excitable membranes of the synaptic junctions, i.e., within the pre- and postsynaptic membranes, similar to that proposed for the conducting membranes, is presented in a second scheme as an alternative to the hypothesis of the role of acetylcholine as a transmitter between two cells.
Keywords: protein assembly, acetylcholine, Ca++, α-toxin, electroplax Na+ efflux
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., HILL A. V., HOWARTH J. V. The positive and negative heat production associated with a nerve impulse. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):149–187. doi: 10.1098/rspb.1958.0012. [DOI] [PubMed] [Google Scholar]
- BULLOCK T. H., HAGIWARA S. Intracellular recording from the giant synapse of the squid. J Gen Physiol. 1957 Mar 20;40(4):565–577. doi: 10.1085/jgp.40.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E., Wassermann N. H., Erlanger B. F. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasie J. K., Worthington C. R. Planar liquid-like arrangement of photopigment molecules in frog retinal receptor disk membranes. J Mol Biol. 1969 Feb 14;39(3):417–439. doi: 10.1016/0022-2836(69)90136-3. [DOI] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J. P., Tsuji S., Boquet P., Pillot J., Ryter A., Changeux J. -P. Localization of the cholinergic receptor protein by immunofluoroscence in eel electroplax. FEBS Lett. 1971 Aug 1;16(2):92–94. doi: 10.1016/0014-5793(71)80340-x. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Gautron J., Israël M., Podleski T. Séparation de membranes excitables à partir de l'organe électrique d'Electrophorus electricus. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 3;269(18):1788–1791. [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Huchet M., Meunier J. C. Extraction à partir du tissu électrique de gymnote d'une protéine présentant plusieurs propriétés caractéristiques du récepteur physiologique de l'acétylcholine. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jun 8;270(23):2864–2867. [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., O'Brien R. D. Binding sites for cholinergic ligands in a particulate fraction of Electrophorus electroplax. Proc Natl Acad Sci U S A. 1971 May;68(5):1047–1050. doi: 10.1073/pnas.68.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Vartiainen A. Further observations on the physiology and pharmacology of a sympathetic ganglion. J Physiol. 1934 Dec 14;83(1):103–128. doi: 10.1113/jphysiol.1934.sp003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Moore J. W. Synaptic current at the squid giant synapse. Science. 1969 Oct 24;166(3904):510–512. doi: 10.1126/science.166.3904.510. [DOI] [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Counting acetylcholine receptors in the electroplax. In: Molecular properties of drug receptors. Ciba Found Symp. 1970:247–261. doi: 10.1002/9780470719763.ch12. [DOI] [PubMed] [Google Scholar]
- Kasai M., Changeux J. P. Démonstration de l'excitation par des agonistes cholinergiques à partir des fractions de membranes purifiées, in vitro. C R Acad Sci Hebd Seances Acad Sci D. 1970 Mar 9;270(10):1400–1403. [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J. C., Huchet M., Boquet P., Changeux J. P. Séparation de la protéine réceptrice de l'acétylcholine et de l'acétylcholinestérase. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jan 4;272(1):117–120. [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- Nachmansohn D. Proteins in bioelectricity: the control of ion movements across excitable membranes. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1034–1041. doi: 10.1073/pnas.61.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. D., Gilmour L. P. A muscarone-binding material in electroplax and its relation to the acetylcholine receptor. I. Centrifugal assay. Proc Natl Acad Sci U S A. 1969 Jun;63(2):496–503. doi: 10.1073/pnas.63.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. D., Gilmour L. P., Eldefrawi M. E. A muscarone-binding material in electroplax and its relation to the acetylcholine receptor. II. Dialysis assay. Proc Natl Acad Sci U S A. 1970 Feb;65(2):438–445. doi: 10.1073/pnas.65.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- SCHOFFENIELS E., NACHMANSOHN D. An isolated single electroplax preparation. I. New data on the effect of acetylcholine and related compounds. Biochim Biophys Acta. 1957 Oct;26(1):1–15. doi: 10.1016/0006-3002(57)90047-1. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand F. S., Barajas L. Effect of modifications in conformation of protein molecules on structure of mitochondrial membranes. J Ultrastruct Res. 1968 Oct;25(1):121–155. doi: 10.1016/s0022-5320(68)80065-6. [DOI] [PubMed] [Google Scholar]