Significance
The in vivo roles of plasma membrane-associated estrogen receptor (ER)α, including cross-talk with nuclear ERα, are poorly understood. We created a mouse with a point mutation of the palmitoylation site of ERα (C451A-ERα) to obtain membrane-specific loss of function. A complementary mouse lacking the ERα activation function AF-2 (ERα-AF20) provided selective loss of function of nuclear ERα actions. Physiologic studies revealed critical requirements for membrane receptors in ovarian function and thereby in fertility, and in vascular physiology. In contrast, nuclear ERα actions mediate uterine responses to estrogen and genome-wide analysis indicates that membrane-to-nuclear receptor cross-talk in vivo is quite modest in uterus. These findings demonstrate for the first time critical tissue-specific roles for membrane versus nuclear actions of a steroid hormone receptor in vivo.
Keywords: fertility, vascular effects, nongenomic effects, genomic actions
Abstract
Estrogen receptor alpha (ERα) activation functions AF-1 and AF-2 classically mediate gene transcription in response to estradiol (E2). A fraction of ERα is targeted to plasma membrane and elicits membrane-initiated steroid signaling (MISS), but the physiological roles of MISS in vivo are poorly understood. We therefore generated a mouse with a point mutation of the palmitoylation site of ERα (C451A-ERα) to obtain membrane-specific loss of function of ERα. The abrogation of membrane localization of ERα in vivo was confirmed in primary hepatocytes, and it resulted in female infertility with abnormal ovaries lacking corpora lutea and increase in luteinizing hormone levels. In contrast, E2 action in the uterus was preserved in C451A-ERα mice and endometrial epithelial proliferation was similar to wild type. However, E2 vascular actions such as rapid dilatation, acceleration of endothelial repair, and endothelial NO synthase phosphorylation were abrogated in C451A-ERα mice. A complementary mutant mouse lacking the transactivation function AF-2 of ERα (ERα-AF20) provided selective loss of function of nuclear ERα actions. In ERα-AF20, the acceleration of endothelial repair in response to estrogen–dendrimer conjugate, which is a membrane-selective ER ligand, was unaltered, demonstrating integrity of MISS actions. In genome-wide analysis of uterine gene expression, the vast majority of E2-dependent gene regulation was abrogated in ERα-AF20, whereas in C451A-ERα it was nearly fully preserved, indicating that membrane-to-nuclear receptor cross-talk in vivo is modest in the uterus. Thus, this work genetically segregated membrane versus nuclear actions of a steroid hormone receptor and demonstrated their in vivo tissue-specific roles.
Although estrogens classically serve as reproductive hormones, they induce cellular responses in almost all tissues in mammalian species. The biological effects of estrogens, and particularly of 17β-estradiol (E2), are initiated by their binding to intracellular estrogen receptors (ERs), ERα and ERβ, which classically serve as nuclear transcription factors (1, 2). The ERs regulate the transcription of hundreds of genes in a cell- and tissue-specific manner through their two activation functions (AFs), AF-1 and AF-2. The roles of the activation functions of ERα have been studied in vivo using mice deleted for ERαAF-1 or ERαAF-2 (3–5). These results, in particular the two models of ERαAF-2 inactivation (4, 6), suggested that many physiological functions strongly rely on nuclear ERα and gene transcription regulation. However, in addition to the nuclear, termed “genomic actions of ER,” the receptors stimulate rapid (from seconds to minutes), nonnuclear signal transduction, usually termed “nongenomic” or “extranuclear” effects. The rapid mobilization of intracellular calcium and the generation of cAMP by E2 were demonstrated several decades ago (7, 8). More recently, the modulation of potassium currents, phospholipase C activation, the increase in endothelial nitric oxide production, and the stimulation of protein kinase pathways (PI3K/Akt, Erk) have been described (9–13). These rapid effects have been attributed to cell membrane-initiated steroid signaling (MISS) by a subpopulation of receptors associated with the plasma membrane.
In the plasma membrane, ERα has been localized to caveolae/lipid rafts by direct binding to caveolin-1 through Ser-522 or indirectly via the scaffold protein striatin, forming complexes with G proteins (Gαi and Gβγ) (14–17). In addition, Cys-447 of human ERα is a site of palmitoylation that promotes plasma membrane association of the receptor (18). A nonpalmitoylatable Cys447Ala mutant form of ERα, or its C451A mutant mouse counterpart, expressed in cultured cells lacks interaction with caveolin-1 and downstream activation of signaling pathways and cell proliferation (18, 19). Numerous cell culture experiments further suggest potentially important kinase-mediated cross-talk between membrane and nuclear ERα that modifies genomic responses to E2 (20, 21), including recent studies revealing that MISS dependent on receptor palmitoylation influences receptor nuclear actions (22). Our current understanding of these processes has relied primarily on experimentation in cell culture. As a result, the in vivo roles of membrane-associated ERα in numerous physiologic processes are poorly understood.
Using a pharmacological approach in cell-based assays, Harrington et al. (23) synthetized estrogen–macromolecule conjugates (EDCs) to provide a gain-of-function strategy. EDC consists of estrogen attached to a large, positively charged nondegradable poly(amido)amine dendrimer via hydrolytically stable linkages. Studies in breast cancer cells clearly showed that EDC was highly effective in stimulating nonnuclear signaling but inefficient in stimulating nuclear ER target gene expression because EDC does not enter the nucleus (23). Importantly, in vivo administration of EDC fully stimulated carotid artery reendothelialization but not uterine proliferation, suggesting for the first time that activating nonnuclear ERα signaling was sufficient to promote beneficial vascular effects of estrogen (24). Membrane only ERα mice expressing the ERα E-domain under the CytoMegaloVirus promoter in an ERα−/− background were also generated, but the vascular phenotype, such as NO production or reendothelialization, was not assessed in this model (25).
To investigate the physiologic importance of membrane-initiated ERα actions in vivo, we created a knock-in mouse model with a selective loss of function of membrane ERα action by mutating the palmitoylation site at mouse ERα Cys451 to Ala (designated C451A-ERα). A complementary mutant mouse model lacking the activation function AF-2 of ERα (designated ERα-AF20) (4, 6) provided a selective loss of function of nuclear ERα actions. Physiological studies in these two models revealed critical requirements for membrane receptors in ovarian function and thereby in fertility, and in vascular physiology. In contrast, genome-wide analysis of uterine gene expression demonstrated that nuclear ERα actions mediate uterine responses to estrogen, and also that membrane-to-nuclear receptor cross-talk in vivo is modest in this tissue. These findings demonstrate critical tissue-specific roles for membrane versus genomic actions of a steroid hormone receptor in vivo.
Results
The C451A Mutation of ERα in Vivo Alters the Membrane Localization of ERα.
Compared with the three other cysteines present in the ligand-binding domain of ERα, C447/451 is the least reactive to iodoacetic acid, suggesting a nonexposed position of this amino acid (26), and consequently the least likely candidate for lipid modifications. Nevertheless, two independent studies have clearly shown that the palmitoylation of C447/451 is required for membrane localization of ERα (18, 19). It is likely that palmitoylation of ERα C447/451 occurs before ligand binding, when the ligand-binding domain is less tightly folded (27). Fig. S1A displays a model of palmitoylation of C447/451 showing that despite the buried nature of this residue a considerable portion of the attached palmitoyl chain extends beyond the surface of the ERα ligand-binding domain, and therefore could function as a membrane tether. To study the role of MISS actions of ERα, a knock-in strategy was used in which the palmitoylation site Cys451 was mutated to alanine using a targeting construct containing two base pair changes in exon 7 (Fig. S1B). The C451A mutation was confirmed by PCR using tail DNA (Fig. S1C).
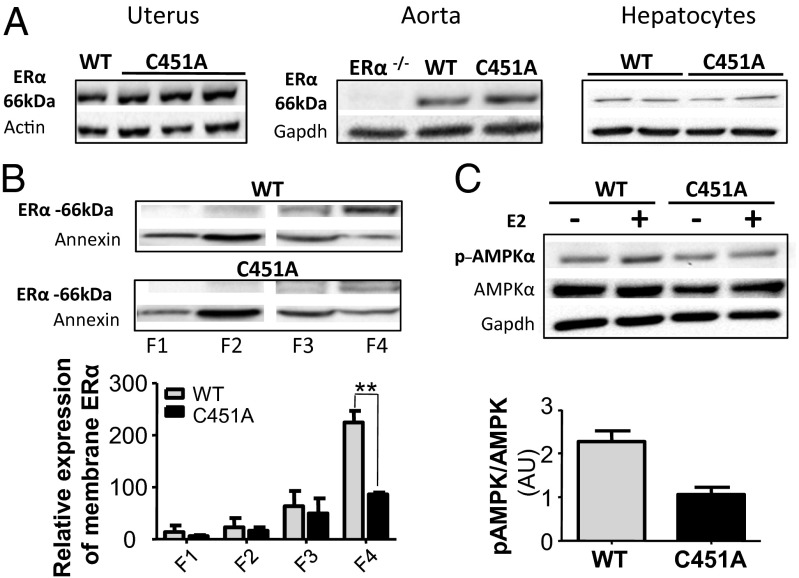
As an initial characterization of this mouse model, we evaluated the expression of ERα protein in various organs from littermate wild-type (WT-C451) and mutant C451A-ERα mice (Fig. 1 A–C). ERα protein abundance in the uterus, aorta, and hepatocytes was similar in the two groups. Plasma membranes were then prepared from primary cultures of hepatocytes that can be obtained in sufficient quantities to allow sucrose gradient isolation of plasma membranes. Whereas ERα protein was abundant in plasma membrane fractions (2–4) prepared from WT-C451 hepatocytes, a 60% decrease was observed in fraction 4 of the mutant C451A-ERα hepatocytes (Fig. 1D). Functionally, we also found that phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) was increased twofold in response to E2 in WT-C451 mice but unchanged in C451A-ERα (Fig. 1E). These data reveal that the C451A mutation effectively alters the plasma membrane localization of ERα in vivo, which further abrogates the signaling pathway.
Fig. 1.
Validation of the C451A-ERα mouse model. (A) ERα protein levels in uterus, aorta, and hepatocytes homogenates from ovariectomized WT-C451 and C451A-ERα mice. (B) Representative Western blot of plasma membrane-associated ERα isolated from WT-C451 or C451A-ERα hepatocytes by sucrose gradient. Quantification of plasma membrane-associated ERα in WT-C451 or C451A-ERα hepatocytes, n = 3. Results are normalized to annexin II expression. (C) Phospho-MAPK/MAPK abundance in hepatocytes treated or not with E2 (10−7 M, 15 min), from WT-C451 and C451A-ERα mice, n = 3. Representative Western blot is shown. AU, arbitrary units.
Membrane-Associated ERα Is Required for Ovarian Function.
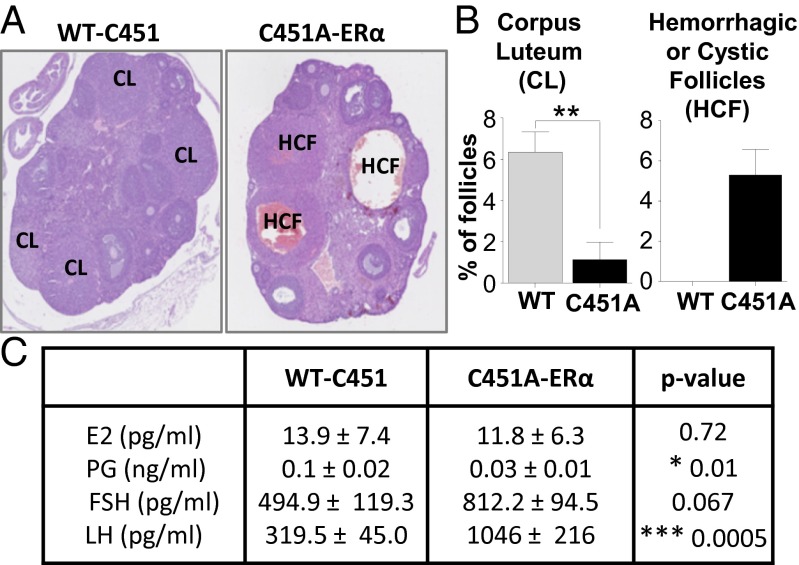
When eight C451A-ERα female mice were mated with WT-C451 males, no litters were observed despite continuous mating over a 5-mo period. To investigate the basis of this infertility, histomorphologic analysis was performed on ovaries from 10- to 12-wk-old female mice. There was an excess of large, hemorrhagic, and/or cystic follicles originating from antral follicles in C451A-ERα ovaries, along with an almost total absence of typical mature corpora lutea (Fig. 2 A and B). Counting of the number of follicles at different stages revealed that ovaries from C451A-ERα mice displayed reduced frequency of primordial follicles but normal percentages of primary, secondary, and tertiary follicles (Fig. S2A). Wall composition of the tertiary follicle was also altered, with a decrease in the percentage of the follicle area composed of granulosa cells, whereas the percentage of the thecal cell area was conserved (Fig. S2B). Additionally, there was a marked reduction in the density of glandular interstitial cells when WT-C451 were compared with C451A-ERα ovaries, which appeared loosely arranged and associated with areas of hemorrhage.
Fig. 2.
Adult ovarian phenotype is disturbed in the C451A-ERα mice. (A) Representative images of WT-C451 and C451A-ERα ovaries (×25). CL, corpus luteum; HCF, hemorrhagic cystic follicles. (B) Percentages of corpora lutea and hemorrhagic/cystic follicles were determined in ovaries from 3-mo-old WT-C451 and C451A-ERα mice, n = 4. (C) Serum E2, progesterone (PG), FSH, and LH concentrations in 3-mo-old WT-C451 and C451A-ERα mice, n = 9.
We also measured steroid sex hormone levels in intact 10- to 12-wk-old female mice (Fig. 2C). C451A-ERα mice exhibited serum E2 levels similar to those of WT-C451 mice, whereas progesterone levels were markedly decreased in mutant mice. The level of FSH tended to be higher (P = 0.067) and luteinizing hormone (LH) was markedly increased (P = 0.0005) in C451A-ERα mice in comparison with WT-C451 mice. Collectively, these findings indicate that membrane ERα is required for normal ovarian morphology and function.
Membrane Actions of ERα Are Dispensable for Uterine Responses to Estrogen.
The uteri of intact WT-C451 and C451A-ERα mice were found to be normal in gross appearance (Fig. 3A). The uterotrophic response to E2 was also similar in the two genotypes when the uterine wet weights were compared in ovariectomized female mice treated or not for 28 d by E2 (8 µg⋅kg–1⋅d–1 s.c.) (Fig. 3B). To evaluate the impact of E2 on uterine epithelial proliferation, Ki-67 labeling was performed 24 h following E2 injection. A robust proliferative response was observed, similar in both WT-C451 and C451A-ERα mice (Fig. 3 C and D), and E2 treatment caused the same increase of luminal epithelial height in the two groups (Fig. 3E). The global ERα protein abundance was equivalent in the uterus homogenates from ovariectomized untreated WT-ERα and C451A-ERα and similarly decreased on both genotypes after E2 treatment (Fig. S3A). Changes in expression levels of ERβ and/or GPR30 after acute or chronic E2 treatment, as assessed by RT-quantitative PCR, were not significantly different in the two genotypes, and thereby do not support the idea of a potential compensatory roles of these receptors (Fig. S3 B and C). Thus, the C451A mutation of ERα does not alter epithelial endometrial cell proliferation or the uterotrophic response to E2, demonstrating that membrane-initiated effects of ERα are not essential for uterine actions of the hormone.
Fig. 3.
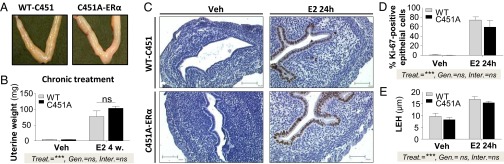
Uterine proliferative response is maintained in C451A-ERα mice. (A) Representative images of intact uterus. (B) Uterine wet weight in ovariectomized WT-C451 and C451A-ERα mice treated or not with chronic (28 d) E2 treatment (8 µg/kg per day), n = 4–6. (C) Representative Ki-67 immunodetection in uterus sections from WT-C451A and C451A-ERα mice treated or not with E2 for 24 h. (Scale bar, 100 µm.) (D) Percentages of Ki-67–positive cells in epithelium and (E) luminal epithelial height (LEH) were measured, n = 4–8. For B, D, and E, statistical results of two-way ANOVA are shown, explaining the effect of treatment (Treat.), genotype (Gen.), and the interaction (Inter.) between these two variables.
Gene Expression Profiling Analysis Reveals Differential Roles of Membrane Versus Nuclear ERα in Gene Regulation.
In contrast to the C451A mutation, previous studies in mice lacking the ERα activation function AF-2 (ERα-AF20) revealed that the uterotrophic response to E2 is entirely dependent on AF-2 (4, 6). We therefore compared the gene expression profiles in the uteri of C451A-ERα and ERα-AF20 mice (4), including littermate controls, 6 h after administration of the vehicle versus E2 (8 μg/kg) using pangenomic microarrays.
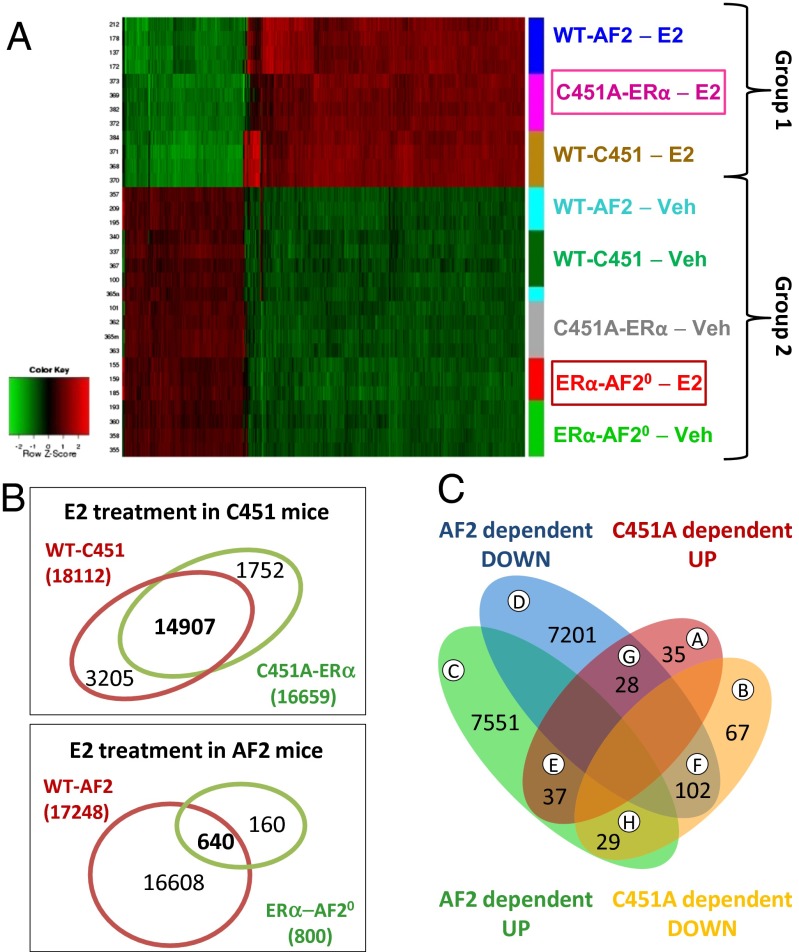
The interaction between genotype and treatment effect is shown in the heat map in Fig. 4A (P < 0.05). Cluster analysis of all of the conditions tested identified two major patterns of gene expression. The first cluster contained genes regulated by E2 in C451A-ERα mutant mice, and these genes were almost equivalent to those regulated by E2 in the control wild-type littermates of either the C451A-ERα or the ERα-AF20 mice. The patterns of genes expressed in all of the vehicle-treated animals were very similar, and they closely resembled the gene pattern observed in E2-treated ERα-AF20 mice, demonstrating an absence of transcriptional response to E2 in ERα-AF20 mice, at least in this tissue. Accordingly, E2 affected a very high number of genes in the two sets of wild-type mice: 17,248 genes in WT-AF2 and 18,112 genes in WT-C451A (Fig. S4A). The slight differences may be due to the fact that the wild-type mice are, respectively, on C57BL/6N and C57BL/6J background. More importantly, 16,659 genes were altered by E2 treatment in C451A-ERα mice, with 14,907 commonly regulated in WT-C451, which represents 82% of the genes targeted by E2 in WT-C451 mice. In contrast, only 800 genes were regulated by E2 in ERα-AF20, with 640 genes in common to those altered by E2 in WT-AF2 uterus (3.7%).
Fig. 4.
Large-scale gene analysis on response to E2 on C451A-ERα and ERα-AF20 mice. (A) Gene expression profiles were obtained by microarray analysis (Agilent SurePrint G3 Mouse GE, 8 × 60K) of uterine samples from C451A-ERα, ERα-AF20 mice or their littermate WT controls treated with E2 (8 µg/kg, 6 h) or vehicle (Veh), n = 3–4. The heat map represents the data obtained on all samples for the differentially expressed probes exhibiting interaction between genotype and treatment (BH adjusted P < 0.001). (B) The Venn diagram illustrates the overlaps between the genes significantly (P < 0.05) up- or down-regulated in the uterus following E2 treatment in ERα-AF20 and C451A-ERα mice. (C) The Venn diagram illustrates the overlaps between the genes significantly (BH adjusted P < 0.05) up- or down-regulated in the uterus following interaction between E2 treatment and genotypes, respectively, ERα-AF20 and C451A-ERα mice.
A Venn diagram was generated to assess the overlap of up- and down-regulated genes found in the genotype and treatment groups. The vast majority of genes (7,551 and 7,201 genes in groups C and D) were respectively up-regulated or down-regulated in a specific ERα-AF2-dependent manner (Fig. 4C). In contrast, a small number of genes were regulated specifically in an ERα-C451 palmitoylation site-dependent manner (35 up-regulated and 67 down-regulated, groups A and B). Finally, a very limited number of genes was either repressed or activated by E2 via both AF2- or C451-dependent processes in the same or opposite manner (groups E–H). Therefore, there is modest, if any, cross-talk between the two receptor populations in uterus. Gene ontology analysis (Fig. S4B and Dataset S1) indicated that the limited number of palmitoylation-dependent genes (groups A and B) are involved in membrane-, transmembrane-, or extracellularly related processes or lipid transport pathways. The large number of AF2-dependent genes (groups C and D) are primarily involved in nuclear, phosphoprotein-related, or splicing events.
Altogether, the genome-wide analysis of the uterus response to E2 demonstrates that most of gene regulation by ERα is related to AF2-dependent, transcriptional nuclear processes, whereas membrane ERα signaling has a minor impact, affecting only a very limited number of genes in this tissue.
Membrane-Initiated Vascular Actions of ERα Are Abrogated in C451A-ERα Mice.
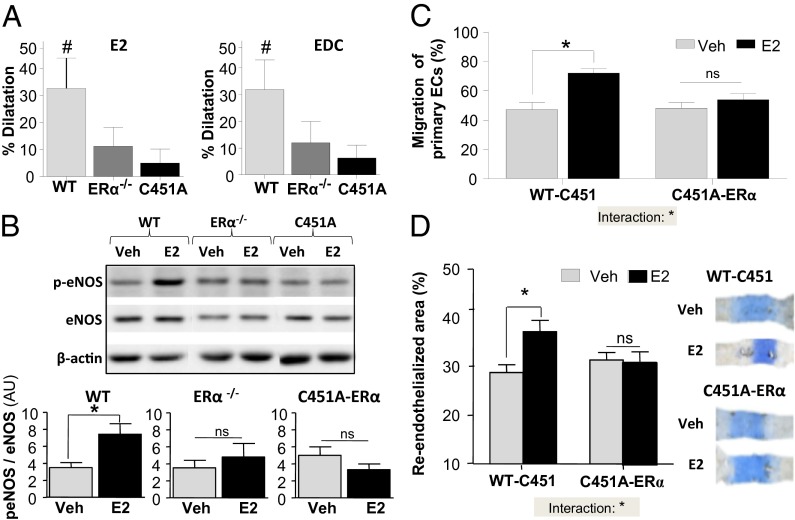
The membrane-initiated actions of E2 have been particularly explored in the vasculature, where estrogen can acutely induce vasodilation in humans (28), at least in part through the rapid stimulation of endothelial nitric oxide synthase (eNOS) activity in an ERα-dependent manner (29, 30). Studies using EDC, which selectively activates nonnuclear ER, have also previously suggested that nonnuclear ERα signaling is important for the endothelial effects of E2 in vivo (24). We then evaluated the vasodilatory effects of both E2 and EDC in WT-ERα and C451A-ERα mice by measuring dilatory responses of isolated phenylephrine-preconstricted mesenteric arteries (Fig. S5A). Within minutes, E2 (1 µM) increased the diameter of arteries from wild-type mice by 32%, whereas there was no significant vasodilatory response in arteries from ERα−/− or C451A-ERα mice (Fig. 5A, Left). EDC also induced rapid dilation of mesenteric arteries from WT-C451 with a magnitude similar to that observed with E2, and no dilation of arteries from ERα−/− or C451A-ERα mice (Fig. 5A, Right). Because rapid dilatation in response to E2 is the consequence of stimulation of eNOS through Akt-mediated phosphorylation of eNOS S1177 (31), phosphorylation of eNOS in response to E2 was evaluated in isolated carotid arteries and found to be increased in WT-C451 arteries, but not in arteries from ERα−/− or C451A-ERα mice (Fig. 5B).
Fig. 5.
Membrane-initiated endothelial effects of E2 are abrogated in C451A-ERα mice. (A) Percentage of relaxation of mesenteric arteries from WT-C451, ERα −/−, and C451A-ERα mice following addition of E2 (1 µM) or EDC (ethinyl-estradiol dendrimer conjugate, 1 µM), n = 8–9. (B) Phospho-eNOS/eNOS abundance in carotid arteries from WT-C451, ERα −/−, and C451A-ERα mice treated or not with E2 (10−8 M, 30 min), n = 6. Representative Western blot is shown. AU, arbitrary units. (C) Percentage of migration of primary endothelial cells treated or not with E2 (10−9 M, 16 h), n = 3–4. (D) Carotid artery reendothelialization in ovariectomized WT-C451 and C451A-ERα mice treated or not with E2 (80 µg⋅kg–1⋅d–1, 2 wk), n = 8–10. For C and D, statistical results of two-way ANOVA are shown, reporting the statistical interaction between treatment and genotype.
To directly evaluate how ERα C451 palmitoylation and the resulting plasma membrane targeting of the receptor does affect a functional response of endothelial cells to E2, endothelial cell migration was tested in scratch assays using primary cells isolated from the aorta of WT-C451 and C451A-ERα mice. Indeed, activation of endothelial cell migration by E2 is mediated by plasma membrane-associated ERα coupled to eNOS via GαI (24). E2 stimulated migration of WT-C451 endothelial cells, but not of C451A-ERα endothelial cells (Fig. 5C). The role of membrane-associated ERα in the vascular actions of E2 was then investigated in vivo by evaluating reendothelialization of the carotid artery (Fig. 5D). E2 treatment caused an increase in endothelial repair in control WT-C451mice, whereas it had no effect in C451A-ERα mice (Fig. 5D).
Previous cell culture studies indicated that disrupting the palmitoylation of ERα resulted in a receptor that is more sensitive to E2-dependent degradation (22). We therefore evaluated possible alterations in ER abundance, in presence of E2 treatment, in the arteries of C451A-ERα mice. First, ERα and ERβ mRNA expression was assessed in aorta from ovariectomized C451A-ERα and WT-C451 mice treated with vehicle versus E2 (8 μg/kg) for 6 h or 4 wk (Fig. S5 B and C). Aortas from both vehicle and E2-treated C451A-ERα mice displayed ERα and ERβ mRNA levels similar to those of WT mice. ERα protein abundance was also equivalent in aorta from untreated ovariectomized C451A-ERα and WT-C451 mice, and a similar decrease in the amount of the receptor was observed after E2 treatment (80 µg⋅kg–1⋅d–1 for 2 wk) in the two genotypes (Fig. S5D). Thus, although previous in vitro experiments suggested that ERα palmitoylation influences the abundance of the receptor protein (22), this was not detected in vivo in arteries and uterus.
Altogether, these findings indicate that preventing membrane-initiated ERα signaling by targeting the palmitoylation site of ERα abrogates major vascular actions of E2, including rapid artery dilation and the promotion of endothelial repair, despite the presence of a global similar ERα arterial abundance.
Membrane-Initiated Vascular Actions of ERα Are Conserved in ERα-AF20 Mice.
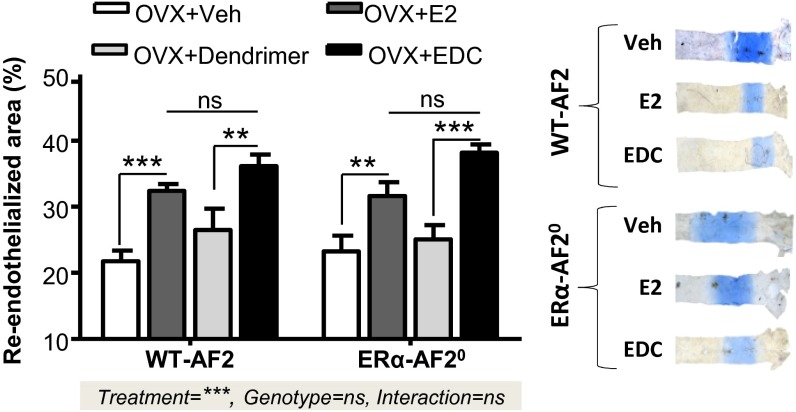
The marked contrast between ERα function in the uterus of C451-ERα and ERα-AF20 mice suggests that parallel studies in the two models may reveal potential tissue-specific roles for nuclear versus nonnuclear receptors. We have previously shown that the activation function AF2 is dispensable for the accelerating effect of E2 on endothelial healing (4). Here, we first confirmed a similar E2-induced acceleration of reendothelialization after carotid injury in WT-AF2 and ERα-AF20 mice (Fig. 6). We further directly tested whether membrane-initiated ERα function is retained in ERα-AF20 mice and found that indeed EDC caused similar promotion of endothelial repair in WT-AF2 and ERα-AF20 mice (Fig. 6). Therefore, whereas nuclear ERα actions mediated by AF-2 are abrogated, nonnuclear ERα function is intact in the ERα-AF20 mice, indicating that the conformation of the membrane ERα-AF20 allows its activation by E2 as well as by EDC.
Fig. 6.
Membrane effects of ERα are preserved in ERα-AF20 mutant mice. Carotid artery reendothelialization in ovariectomized WT-AF2 and AF2-ERα mice treated with E2 (80 µg⋅kg–1⋅d–1, 2 wk) or vehicle (Veh) and EDC (240 µg⋅kg–1⋅d–1, 2 wk) or control dendrimer, n = 8–10. Statistical results of two-way ANOVA are shown, explaining the effect of treatment, genotype, and interaction between these two variables.
Discussion
In the present study we investigated the physiological roles of a pool of ERα localized at the plasma membrane by mutating in mice the ERα C451 recognized as a key palmitoylation site in vitro. This genetic loss-of-function model revealed that ERα C451 is absolutely required for plasma membrane location and signals that are critical for arterial effects of E2, including the stimulation of vasodilation and the promotion of endothelial repair, but also female fertility. In contrast, the uterine proliferation in response to E2 occurs independently of membrane ERα. Complementary studies in ERα-AF20 mice further demonstrated a preservation of membrane ERα action and vascular function in this model. Moreover, genome-wide analysis of uterine gene expression revealed that membrane ERα signaling has only a minor impact on gene expression in this sex target tissue. These observations reveal tissue-specific roles for membrane versus nuclear ERα in vivo. In that broader context, this work genetically segregated nonnuclear and nuclear actions of a steroid hormone receptor and demonstrated their tissue specificity.
We first determined whether mutation of the palmitoylation site of mouse ERα alters the expression level of the protein in vivo, because in vitro studies (22) had shown that such a mutated human ERα (C447A) was more sensitive to E2-dependent degradation. The mutated C451A-ERα protein was normal in abundance in the different murine tissues analyzed. With respect to the abrogation of vascular E2 effects in the C451A-ERα mouse, it was also important to demonstrate that the abundance of the C451A-ERα protein in the vasculature in vivo is normal under basal and E2-stimulated conditions. Furthermore, the anticipated decrease in plasma membrane association of the mutant protein in vivo mirrors in vitro observations (18, 19), and it validates the C451A-ERα mouse as a selective loss-of-function model attenuating membrane receptor localization.
Our study on the reproduction of the C451A-ERα mice demonstrates the crucial role of membrane ERα in female fertility. This is at least in part due to alterations in ovarian function, with the ovaries of adult C451A-ERα mice being almost completely devoid of corpora lutea and displaying a high number of hemorrhagic and/or cystic follicles, indicating that the maturation of follicles into corpora lutea (luteinization, which allows the transition to the next estrous cycle) is abnormal. The capacity for ovulation per se has not yet been directly evaluated in C451A-ERα mice. The absence of corpora lutea, which produce progesterone, likely underlies the low serum progesterone found in C451-ERα mice. Furthermore, the level of LH in intact mice was increased, suggesting that the control of LH production by the hypothalamic–pituitary axis is adversely affected in C451-ERα mice. Female mice deficient in either ERαAF-1 or -2 (named ERα-AF10 and ERα-AF20, respectively) are also sterile, primarily owing to absence of uterine hypertrophy (3, 4, 6). Thus, both plasma membrane and nuclear actions of ERα are absolutely required for female fertility.
We then explored to what extent membrane ERα is important for estrogen vascular effects. We found that both E2 and EDC elicit a rapid vasorelaxant effect in isolated mesenteric arteries from wild-type mice, whereas these effects are lost in ERα−/− and C451A-ERα mice. eNOS1177 phosphorylation in response to E2 was also abolished in arteries from C451A-ERα mice, as was the accelerative action of E2 on endothelial healing both in vivo and in vitro. In the past, the selective membrane ER activator EDC was shown to be sufficient to stimulate endothelial cell migration and the acceleration of reendothelialization (24). Furthermore, the acceleration of endothelial repair by E2 or EDC in ERα-AF20 mice was preserved, demonstrating that the MISS effects are fully activable in this model. Altogether, the current findings indicate that the activation of membrane ERα is both necessary and sufficient to promote endothelial repair and contribute to the integrity of the endothelial monolayer.
In contrast, C451A-ERα mice also revealed that receptor palmitoylation and membrane targeting are dispensable for uterine growth responses to E2. Previous work showed that the selective activation of membrane ERα by EDC does not induce uterine hypertrophy (24), suggesting that MISS activation alone is not sufficient to promote a proliferative response in uterine epithelium. Using a loss-of-function strategy, we now show that MISS effects involving ERα palmitoylation are also not necessary. In contrast, previous studies have demonstrated the importance of genomic (i.e., transcriptional) activity of ERα in this process, because E2 does not induce a uterine proliferative response in ERα-AF10 or in ERα-AF20 mice (5, 6).
We further explored the gene expression profiles of ERα-AF20 and C451A-ERα mice in the uterus, a tissue that is highly responsive to E2. Genome-wide gene array studies revealed that, in contrast to nuclear ERα-mediated processes, membrane ERα-mediated signaling has only a minor impact on gene expression. A small set of genes was found to be regulated in the uterus by both membrane and nuclear ERα. Altogether, we can propose that C451A-ERα and ERα-AF20 mice can be considered as mouse models of membrane ERα and nuclear ERα loss of function, respectively, but with preservation of nuclear ERα and membrane ERα, respectively.
Cell culture studies (18, 19, 22, 32) previously suggested that rapid E2-dependent signaling may modify ERα transcriptional activity and thereby modulate final receptor-dependent nuclear actions, representing cross-talk between nonnuclear and nuclear receptor subpopulations. The function of many transcription factors is regulated through protein kinase-mediated phosphorylation, and these transcription factors may be targets for extranuclear actions of estrogen (20, 33). An important role for ERα MISS was reported in cancer cell proliferative responses to E2 (34–36), and ERK 2, a downstream effector of the MAPK pathway, cooperates in regulating gene transcription (21). Similarly, a cross-talk between membrane-initiated signaling of steroid receptors, such as progesterone or androgen receptor, and gene regulation and cell proliferation was also reported in various culture models of cancer cells (37). However, in contrast to the conclusions raised from these models of cultured cancer cells, our large-scale gene analysis reveals that although MISS via ERα can influence the regulation of a small subset of genes in the uterus, the impact of nonnuclear ERα on gene regulation is quite modest in this organ, paradigmatic of the proliferative action of E2.
This context-specific role for membrane-initiated signals fits along the complexity of the palmitoylation process (38), emphasizing the regulation and the spatiotemporal dynamics of protein palmitoylation. These levels of complexity should be addressed in future studies, even if such in vivo studies seem particularly difficult.
The discoveries that were rendered possible via the generation of the C451A-ERα mouse model indicate that membrane and nuclear actions of ERα in vivo are highly tissue-specific in some key targets and functions. The C451A-ERα mouse now provides a valuable loss-of-function model for the study of the role of plasma membrane-associated ERα in numerous additional physiological and pathophysiological processes affected by the receptor, as well as a means to conceive new selective ERα modulators with an optimized medical profile.
Materials and Methods
Mice.
All procedures involving experimental animals were performed in accordance with the principles established by the Institut National de la Santé et de la Recherche Médicale and were approved by the local Ethical Committee of Animal Care. The C451A-ERα knock-in mouse line was generated on a C57BL6/N background through the strategy outlined in Fig. S1B at the Mouse Clinical Institute (Illkirch, France). ERα −/−, ERα-AF20 mice have been previously described (4, 39). C451A-ERα and their corresponding wild-type littermates (WT-C451) were ovariectomized at 4 wk of age. For acute E2 treatment, they were injected s.c. with vehicle (castor oil) or 17β-estradiol (E2, 8 µg/kg) 3 wk after ovariectomy. For chronic E2 treatment, ovariectomized mice were implanted with s.c. pellets releasing either vehicle or E2 (8 or 80 µg⋅kg–1⋅d–1 as indicated, 60-d release; Innovative Research of America).
Isolation of Plasma Membranes.
Primary hepatocyte cultures were isolated from the livers of 8- to10-wk-old mice by a modification of the collagenase method (40). Briefly, livers from WT-C451 and C451A-ERα mice were perfused using the portal vein with HBSS before collagenase perfusion (C5138; Sigma). Hepatocytes were then put in cultures in DMEM. Plasma membranes were isolated by a discontinuous sucrose step gradient, from which 12 fractions were recovered (41). The first fractions (1–4) contained the plasma membrane proteins, which were identified after protein precipitation by Western blot using polyclonal antibodies against either ERα or annexin II.
Mouse Carotid Artery Injury.
Perivascular carotid artery electric injury was performed as previously described (3) in ovariectomized mice treated or not for 2 wk before surgery with E2 (80 µg⋅kg–1⋅d–1 in 60-d-release pellets) or EDC (ethinyl-estradiol dendrimer conjugated, 240 µg⋅kg–1⋅d–1). Briefly, the left common carotid artery was exposed via an anterior cervicotomy. The electric injury was applied to the distal part (3 mm precisely) of the common carotid artery with a bipolar microregulator. The percentage of reendothelialization was calculated relative to the initial deendothelialized area by assessing Evans blue dye uptake 3 d after injury. Images were acquired under a DMR 300 Leica microscope, using Leica Application Suite V3.8 and ImageJ softwares.
Vascular Reactivity of Isolated Mesenteric Arteries.
As previously described (42), 5-mm-long segments of second-order mesenteric arteries were dissected and mounted between two glass cannulae and bathed in a physiological salt solution (pH 7.4, PO2 160 mm Hg, and PCO2 37 mm Hg). Pressure was controlled by a servo-perfusion system, and diameter changes were measured continuously using a video-monitored system (Living Systems Instruments). Artery viability was assessed using KCl (80 mM) and endothelium integrity with acetylcholine (1 µM). EDC- (1 µM) and E2- (0.01–1 µM) dependent dilation was then assessed after precontraction with phenylephrine to 70% of the maximal contractile response obtained with KCl (80 mM).
Mouse Endothelial Cell Migration Assay.
Endothelial cells were isolated from the aortae of wild-type and C451A-ERα mice, and cell migration was assessed using methods modified from those previously described (43). At first passage, the cells were transferred into six-well plates, and following growth to confluence in EGM-2 medium (Lonza) containing 10% FBS, a defined linear region of cells was removed using a pipette tip. The initial area devoid of cells was marked and quantified on images obtained at baseline, and treatments with vehicle or E2 (10−9 M) were initiated in phenol red-free DMEM with 5% charcoal-stripped FBS. Sixteen hours later the cells were fixed and stained with Coomassie blue, and repeat images were obtained to quantify the remaining area devoid of cells. Cells were imaged using an inverted phase-contrast microscope (Nikon ECLIPSE TS100; Nikon Corporation) and a digital camera (Infinity 1). The area devoid of cells was quantified using Adobe ImageReady CS2 software. The degree of migration was calculated as the percentage of the initial area devoid of cells that was occupied by cells following 16-h treatment.
Hormone Assays.
Serum levels of LH and FSH were determined using the Multiplex Immunoassay Technology Xmap (MILLIPLEX; Millipore). Progesterone and 17β-estradiol were measured by gas chromatography–mass spectrometry (44), with minor modifications. These assays were performed on intact 10- to12-wk-old female mice.
Immunohistochemistry.
Paraffin-embedded transverse sections (4 µm) from formalin-fixed uterine or ovary specimens were stained as previously described (5) with anti–Ki-67 antigen (RM-9106; Thermo-scientific). Sections were examined under a Nikon Eclipse E400 microscope using image analysis software Morpho Expert. For uterus, the luminal epithelial height and stromal height of endometrium is the mean of 10 measurements, whereas the percentage of Ki-67–positive epithelial cells is the mean of three measurements in each transverse uterus section. Ovarian function was evaluated in 3-mo-old adult mice by counting the number of follicles in each class (45). The number of follicles in each category was expressed as the percentage of total number of follicles. Analysis of the area of the granulosa and theca was performed on the largest tertiary follicle of each section, as previously described (46), and expressed as a percentage of the total area of follicle.
Microarray.
Total RNA was extracted using TriPure reagent (Roche) and reverse-transcribed (High-Capacity cDNA Reverse Transcription Kit; Applied Biosystems). Microarray data were obtained from 200 ng of uterine total RNA labeled with Cy3 using Low Input Quickamp Labeling Kit (Agilent) and hybridized to Agilent SurePrint G3 Mouse GE (8 × 60K) microarrays following the manufacturer’s instructions. All experimental details are available in the Gene Expression Omnibus (GEO) database under accession no. 53237. Data were analyzed under R (R 2.13; www.R-project.org). Hierarchical clustering was applied to the samples and the probes using 1-Pearson correlation coefficient as distance and Ward’s criterion for agglomeration. Functional analysis was carried out using DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov), and comparisons are realized with the Venn Diagrams plug-in based upon the VENNY tool developed by J. C. Oliveros.
Western Blotting.
Total proteins were separated on a 10% SDS/PAGE gel and transferred to a nitrocellulose membrane. The primary antibodies used are as follows: MC20 (sc-542; Santa Cruz Biotechnology), Gapdh (sc-32233; Santa Cruz Biotechnology), pSer1177-eNOS (612392; BD Bioscience), eNOS (610297; BD Bioscience), pThr172-AMPK (2531; Cell Signaling), AMPK (2532; Cell Signaling), actin (A2066; Sigma), and annexin II (sc-9061; Santa Cruz Biotechnology). Revelation was performed using an HRP-conjugated secondary antibody and visualized by ECL detection according to the manufacturer’s instructions (Amersham Biosciences/GE Healthcare), using ChemiDoc Imaging System (Bio-Rad). Bands were quantified using ImageJ densitometry.
Statistical Analyses.
Results are expressed as the mean ± SEM. To test the effect of treatments or genotypes, t test or Mann–Whitney test was performed. To test the interaction between treatments and genotypes, a two-way ANOVA was carried out. When an interaction was observed between two variables, the effect of treatment was studied in each genotype using the Bonferroni post hoc test. A value of P < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Supplementary Material
Acknowledgments
The C451A-ERα mouse was generated at the Mouse Clinical Institute by M. C. Birling and coworkers. We thank Dr. Christopher Mayne for creating the model of ERα-C451A palmitate (Fig. S1A) and Rosa Sirianni, who performed the endothelial cell migration experiments. We thank Dr. Benita Katzenellenbogen and Dr. Henrik Laurell for fruitful discussions and suggestions for the writing of the manuscript. We also thank the staff of the animal facility (J.-C. Albouys, M. J. Fouque, and G. Carcasses); C. Bleuart for anatomopathology at the École Nationale Vétérinaire de Toulouse, the staff of the Anexplo platform (A. Desquennes and S. Legonidec); and M. Buscato [Institut National de la Santé et de la Recherche Médicale (INSERM) 1048] for skillful technical assistance. We also thank Y. Lippi and P. Martin for the excellent contribution to microarray analysis carried out at GeT-TRIX Genopole Toulouse facility. The work at I2MC-INSERM U1048 is supported by Institut National de la Santé et de la Recherche Médicale, Université et CHU de Toulouse, Faculté de Médecine Toulouse-Rangueil, Fondation pour la Recherche Médicale, Fondation de France, Fondation de l'Avenir, Conseil Régional Midi-Pyrénées, and AVIESAN-Astra-Zeneca. The work at INSERM U1083-Centre National de la Recherche Scientifique (CNRS) Unité Mixte de Recherche 6214 is supported by INSERM, CNRS, Centre Hospitalier Universitaire and Université d'Angers, Fondation de France, Fondation de l'Avenir and Conseil Régional Pays de la Loire, Conseil Régional Midi-Pyrénées, and Fondation pour la Recherche Médicale. M.A. and A.A. were supported by grants from the Ministère de la Recherche and Groupe de Réflexion sur la Recherche Cardiovasculaire, respectively. This work was supported by National Institutes of Health Grants DK015556 and HL087564 (to J.A.K. and P.W.S., respectively).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nih.gov/geo (accession no. 53237).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322057111/-/DCSupplemental.
References
- 1.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov. 2011;10(10):778–792. doi: 10.1038/nrd3551. [DOI] [PubMed] [Google Scholar]
- 3.Billon-Galés A, et al. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci USA. 2009;106(6):2053–2058. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billon-Galés A, et al. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA. 2011;108(32):13311–13316. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abot A, et al. The AF-1 activation function of estrogen receptor α is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154(6):2222–2233. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- 6.Arao Y, et al. Estrogen receptor α AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA. 2011;108(36):14986–14991. doi: 10.1073/pnas.1109180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265(5589):69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 8.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152(12):4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoncini T. Mechanisms of action of estrogen receptors in vascular cells: relevance for menopause and aging. Climacteric. 2009;12(Suppl 1):6–11. doi: 10.1080/13697130902986385. [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol. 2009;308(1-2):3–8. doi: 10.1016/j.mce.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem. 2011;286(17):14737–14743. doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda K, Karas RH. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids. 2013;78(6):589–596. doi: 10.1016/j.steroids.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, et al. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci USA. 2004;101(49):17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoncini T, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambliss KL, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87(11):E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 18.Acconcia F, et al. Palmitoylation-dependent estrogen receptor alpha membrane localization: Regulation by 17beta-estradiol. Mol Biol Cell. 2005;16(1):231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedram A, et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 20.Lonard DM, O’Malley BW. Nuclear receptor coregulators: Modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8(10):598–604. doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. Genomic collaboration of estrogen receptor alpha and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol. 2011;31(1):226–236. doi: 10.1128/MCB.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F. Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity. Mol Endocrinol. 2012;26(5):762–774. doi: 10.1210/me.2011-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington WR, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 24.Chambliss KL, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120(7):2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedram A, et al. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284(6):3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegy GB, et al. Carboxymethylation of the human estrogen receptor ligand-binding domain-estradiol complex: HPLC/ESMS peptide mapping shows that cysteine 447 does not react with iodoacetic acid. Steroids. 1996;61(6):367–373. doi: 10.1016/0039-128x(96)00042-6. [DOI] [PubMed] [Google Scholar]
- 27.Gee AC, Katzenellenbogen JA. Probing conformational changes in the estrogen receptor: Evidence for a partially unfolded intermediate facilitating ligand binding and release. Mol Endocrinol. 2001;15(3):421–428. doi: 10.1210/mend.15.3.0602. [DOI] [PubMed] [Google Scholar]
- 28.Gilligan DM, Quyyumi AA, Cannon RO., 3rd Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89(6):2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- 29.Lantin-Hermoso RL, et al. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273(1 Pt 1):L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 30.Caulin-Glaser T, García-Cardeña G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81(5):885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: Mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280(20):19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 32.Madak-Erdogan Z, et al. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22(9):2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Björnström L, Sjöberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16(10):2202–2214. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- 34.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238(1):1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Poulard C, et al. Activation of rapid oestrogen signalling in aggressive human breast cancers. EMBO Mol Med. 2012;4(11):1200–1213. doi: 10.1002/emmm.201201615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razandi M, Pedram A, Levin ER. Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol Endocrinol. 2000;14(9):1434–1447. doi: 10.1210/mend.14.9.0526. [DOI] [PubMed] [Google Scholar]
- 37.Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol. 2007;69(1):171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- 38.Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191(7):1229–1238. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 40.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J Cell Biol. 1969;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terrillon S, et al. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol. 2003;17(4):677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 42.Tarhouni K, et al. Key role of estrogens and endothelial estrogen receptor α in blood flow-mediated remodeling of resistance arteries. Arterioscler Thromb Vasc Biol. 2013;33(3):605–611. doi: 10.1161/ATVBAHA.112.300334. [DOI] [PubMed] [Google Scholar]
- 43.Sundgren NC, et al. Coupling of Fcγ receptor I to Fcγ receptor IIb by SRC kinase mediates C-reactive protein impairment of endothelial function. Circ Res. 2011;109(10):1132–1140. doi: 10.1161/CIRCRESAHA.111.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liere P, et al. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;739(2):301–312. doi: 10.1016/s0378-4347(99)00563-0. [DOI] [PubMed] [Google Scholar]
- 45.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 46.Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. doi: 10.1210/en.2012-1954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.