Abstract
Overfishing and environmental change have triggered many severe and unexpected consequences. As existing communities have collapsed, new ones have become established, fundamentally transforming ecosystems to those that are often less productive for fisheries, more prone to cycles of booms and busts, and thus less manageable. We contend that the failure of fisheries science and management to anticipate these transformations results from a lack of appreciation for the nature, strength, complexity, and outcome of species interactions. Ecologists have come to understand that networks of interacting species exhibit nonlinear dynamics and feedback loops that can produce sudden and unexpected shifts. We argue that fisheries science and management must follow this lead by developing a sharper focus on species interactions and how disrupting these interactions can push ecosystems in which fisheries are embedded past their tipping points.
Keywords: alternative states, ecosystem flips, fisheries collapse, ocean fisheries
Overfishing and environmental change have triggered severe and unexpected consequences in diverse ecosystems supporting marine fisheries. As existing fisheries have collapsed, the ecosystems in which they were embedded have changed dramatically. These changes have brought those ecosystems to states that are often less productive and less predictable and from which recovering the fishery is more difficult. We contend that the failure of fisheries management to anticipate these transformations resulted from a lack of appreciation for the nature, strength, complexity, and outcome of species interactions. Ecologists have come to understand that networks of interacting species exhibit nonlinear dynamics and feedback loops that can produce sudden and unexpected shifts (1). We argue that fisheries science and management must follow this lead by developing a sharper focus on species interactions and how disrupting these interactions can push ecosystems in which fisheries are embedded past their tipping points.
Evolution of Fisheries Science
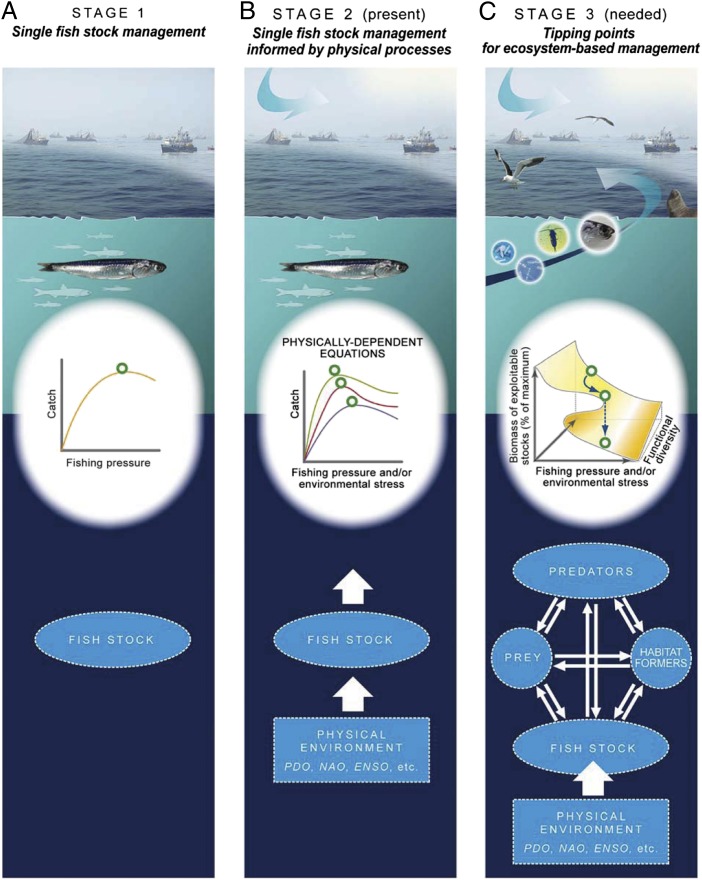
Fisheries science diverged from its parent discipline of ecology in the 1950s when quantitative models of population dynamics were developed to estimate sustainable yields (Fig. 1A). A revolution occurred in the 1980s with the recognition that variation in the physical environment affects fishery yields through its influences on larval transport, foraging success, and survival (Fig. 1B). In the meantime, ecology evolved toward experimental hypothesis testing (2) and leapt forward with an understanding of the nonlinear nature and indirect effects of species interactions (3). These characteristics of species interactions contribute to feedback loops and the resultant complex dynamics and tipping points of associated populations and ecosystems. Although fisheries science includes sophisticated ecosystem models that can aid fisheries management (4), the species interactions described in those models are primarily direct consumptive interactions that account for the flow of biomass through food webs. These models have limited capacity for accommodating the network of indirect effects among species that create complex feedback loops and tipping points; fisheries science and ecology need to reunite in a quest to understand these processes and their effects in fishery systems (Fig. 1C).
Fig. 1.
The paradigm shifts in fisheries management: past, present, and future. (A) Early stages of conventional fishery science, in which stocks are managed as isolated entities toward a maximum sustainable yield (small circle). (B) Current state of affairs, in which bottom up forcing from physical oceanographic variation (e.g., PDO, NAO, and El Niño/La Niña events) modifies expected maximum (three small circles) sustainable yields. (C) Our view of how fishery science should be expanded to include direct and indirect species interactions along a diversity gradient and their consequences for population and community dynamics. Strongly nonlinear responses of ecosystems to increases in environmental stress and anthropogenic forcing can force sudden phase shifts (Inset), especially in systems with low functional diversity. Illustration by Pierre Lopez, Institut de Recherche pour le Développement.
Pushing Ecosystems Past Their Tipping Points
Whereas species can be identified and counted, species interactions might be referred to as the invisible fabric of nature because the strength of their influence is detectable only after a perturbation to one or more of the interacting species. Although experimental ecology has unveiled some of this invisible fabric, fisheries science lags behind because dynamic processes are more difficult to measure and understand over the large spatial and temporal scales at which fisheries operate. Moreover, fisheries-induced perturbations are often uncontrolled, unreplicated, and unpredictable in their magnitude. Nonetheless, the influences of large-scale perturbations on the web of interacting species, which includes humans, are becoming apparent in many systems, as are the transformations in ecosystem structure and function that often result (5). This point is illustrated for fisheries-affected ecosystems by the following examples. A few others could be provided, and many others undoubtedly exist but remain undetected.
Benguela Ecosystem.
In the Northern Benguela ecosystem off Namibia, stocks of sardine (Sardinops sagax) and anchovy (Engraulis encrasicolus), which are energy-rich prey species, collapsed in the 1970s from about 10 metric tons (MT) to almost zero due to a combination of fishing and changing environmental conditions (6). Following this collapse, the bearded goby (Sufflogobius bibarbatus), a fish with lower caloric value, increased rapidly and now constitutes the major forage species for predators in the system (7). In addition, other prey with low caloric value, jellyfish (Cnidaria and Medusozoa; negligible before the early 1970s), reached a biomass estimated at more than 40 MT in the 1980s and 12.2 MT in the 2000s (8). As a consequence of the replacement of energy-rich sardines and anchovies with energy-poor gobies and jellyfish, African penguins (Spheniscus demersus) and Cape gannets (Morus capensis) have declined by 77% and 94%, respectively (9). Cape hake (Merluccius capensis) and deep water hake (Merluccius paradoxus) catches declined from >725,000 MT in 1972 to <110,000 MT since 1990, and the production of Cape fur seal (Arctocephalus pusillus) pups has fluctuated radically (10). The overharvesting of forage fish increased pelagic-benthic coupling, driving system anoxia below levels that few species other than the bearded goby can tolerate, an alternate and potentially irreversible state (11).
Western North Atlantic.
The collapse of cod (Gadus morhua) populations in the northwest Atlantic in the early 1990s resulted in large measure from overfishing. Despite widespread fishery closures, cod have not recovered, refuting predictions derived from traditional population models. On the eastern Scotian Shelf, the former prey of adult cod, released from that predation, have become hyperabundant competitors with young cod, and in some cases, likely predators on young cod (12), creating an alternative state of persistently low cod densities from which it will be difficult to escape.
The overexploitation of cod and other predatory fishes in coastal waters of the northwest Atlantic led to an increase in sea urchins (Strongylocentrotus drobachiensis), which stimulated the development of Maine’s urchin fishery in 1987 (13). This fishery pushed sea urchin stocks to collapse by the mid- to late 1990s. Released from urchin herbivory, macroalgae proliferated throughout the region, creating ideal settlement habitat for crabs (Cancer spp.) that have, in turn, become the predominant predator on sea urchins. In areas of coastal Maine where urchins were extirpated, crab predation has precluded their revival for nearly 20 y, even where urchin fishing is prohibited (13). It is not clear whether these changes could be reversed to reestablish the urchin fishery.
Caribbean Coral Reef Systems.
Most Caribbean reefs underwent a rapid shift from coral to algal dominance during the 1980s (14). Although disease was the proximate cause, an additional factor was a failure by the scientists to understand the role of herbivory in the system. Increased fish trapping caused a decline in the abundance of herbivorous fish. This decline did not have an obvious effect on the abundance of macroalgae, whereas the dominant urchin, Diadema antillarum, was abundant. When disease caused the mass mortality of Diadema, macroalgae proliferated over the reefs, thereby reducing reef coral recruitment (15). The coral decline likely reduced recruitment habitats for herbivorous fishes, and the reduction in fish numbers and diversity allowed macroalgae to continue to dominate the system. These complex interactions among four major tropical taxa—coral, macroalgae, fish, and urchins—have created a self-perpetuating process that may have locked reef ecosystems into an alternative, nearly coral-free state (16, 17).
River-Ocean Linkages.
Physical transport and migratory animals can also connect sets of interacting species across ecosystems, as occurs between rivers and coastal ocean habitats. Rivers deliver fresh water, solutes, sediments, detritus, and organisms to coastal oceans, and changes in the linkage between river and ocean can reverberate in both habitats through species interactions. For example, fish landings off the Nile delta, which collapsed during the first few decades following the construction of the Aswan Dam in 1965, have more than recovered since the 1990s due to increased nitrogen and phosphorus from land-based fertilizer and sewage (18). However, because ecosystem impacts caused by nutrient loading are strongly nonlinear, changes in nutrient delivery can unexpectedly tip estuaries from impoverished to productive to collapsed hypoxic states. These alterations in state are driven by interactions among bacteria, heterotrophic and mixotrophic protists, and algae, which can all more than double their biomass daily under copious nutrient loading (19). Pacific salmon offer one of the more notorious examples of the disruption of species interactions involving an interecosystem migrant. Although the direct impacts of dams and watershed degradation on anadromous salmon populations have long been of major concern, there is growing evidence that these changes alter the interactions between salmonids and their predators, prey, and pathogens, creating ecosystems in which salmon no longer thrive (20).
Securing Ecology at the Heart of Fisheries Science
These examples demonstrate the need for understanding how direct species interactions are altered by fishing and environmental change and which indirect effects will become prominent as a result. Their unifying theme is how the synergy between species interactions and changing environmental conditions, including exploitation, pushed systems past their tipping points. This synergy emerges from two sources: (i) alterations in the nature and strength of direct species interactions by environmental variation (21), and (ii) propagations of these direct interactions along multiple indirect pathways (22).
Understanding species interactions in large, complex ecosystems is a daunting challenge (Fig. 1C). However, it is becoming clear that the dynamics of such systems are now often driven by humans (23, 24), a few other key species, and their interactions with the rest of the system (25). We believe that a primary empirical direction for fisheries science must be toward identifying those key interactions; a primary theoretical direction is to use those interactions as the foundation for “minimally realistic models” (4) that can be deployed to explore which perturbations might drive ecosystems toward tipping points (26). Although controlled experiments, which have served basic ecology so well, are usually infeasible in fisheries systems, there are other promising approaches for identifying the key interactions; multispecies time series can be used for inverse modeling (27), and certain chance events or management interventions constitute perturbations from which we can learn about key interactions when they are accompanied by well-designed protocols for careful data collection.
Managing the Invisible Fabric of Nature
As we seek to build understanding of species interaction webs into the criteria that guide fisheries management, it is critical to understand how humans are embedded in these webs (28–30). It may even be possible to use that knowledge to recover a system after its collapse. For example, overharvesting the carnivorous snail, Concholepas concholepas, along the coast of Chile caused a cascading effect that led to a mussel monoculture in the rocky intertidal zone. Experimental work on the intertidal food web suggested that protecting the remaining snails from harvest could, in time, turn this system around; 12 y later, the mussel monoculture had reverted to a diverse community of barnacles and macroalgae (30).
This success story may not be an isolated one. In analyzing 20 marine protected areas in tropical and temperate coastal oceans as replicated “human exclusion experiments,” Micheli et al. (31) found that the abundance of species targeted by fisheries, which are often at high trophic positions, increased inside the no-take zones. However, up to a third and, on average, 19% of the nontarget species, decreased in abundance inside these zones, which is strong evidence that impacts of human fishers ramify through networks of species interactions. Indeed, an increasing number of studies document some degree of recovery and sustainability in certain fisheries (24) and ancillary benefits of wise management for biodiversity (32). These cases generally involve comanagement by local fishers, managers, and scientists (29), combining local or traditional knowledge, leadership, and peer pressure by fishers with rigorous scientific methods and innovative technologies.
Of course, it may be impossible to recover some ecosystems that have moved past their tipping points, which is why it is crucial to cultivate a greater appreciation for the fact that ecosystems have tipping points. In the management arena, it may be possible to estimate what a phase shift might look like by using models of instantaneous biomass fluxes through an ecosystem (33) in expanded sensitivity analyses that explore what a large perturbation might do to a system (34). It may also be possible to use time series data to discern when a system is approaching a tipping point (35, 36). However, because phase shifts and tipping points are functions of indirect interactions and feedback loops, there will be no substitute for a clearer understanding of the direct and indirect interactions among human and nonhuman species. Understanding these linkages and their consequences is not only crucial for making policy decisions (which areas, stocks, or species to protect; when to fish and when to conserve), but also for anticipating, diagnosing, and reacting to the trends and surprises that will continue to emerge as these systems confront global change.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Scheffer M. Critical Transitions in Nature and Society. Princeton: Princeton Univ Press; 2009. [Google Scholar]
- 2.Peterson CH. 1990. Behavioural Mechanisms of Food Selection, ed Hughes RN (Springer, Berlin), pp 821–846.
- 3.Wootton JT. The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst. 1994;25:443–466. [Google Scholar]
- 4.Plaganyi EE. 2007. Models for an ecosystem approach to fisheries. FAO Fisheries Technical Paper No. 477 (FAO, Rome)
- 5.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 6.Cury P, Shannon LJ. Regime shifts in upwelling ecosystems: Observed changes and possible mechanisms in the northern and southern Benguela. Prog Oceanogr. 2004;60(2-4):223–243. [Google Scholar]
- 7.Utne-Palm AC, et al. Trophic structure and community stability in an overfished ecosystem. Science. 2010;329(5989):333–336. doi: 10.1126/science.1190708. [DOI] [PubMed] [Google Scholar]
- 8.Lynam CP, et al. Jellyfish overtake fish in a heavily fished ecosystem. Curr Biol. 2006;16(13):R492–R493. doi: 10.1016/j.cub.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Ludynia K, Roux J-P, Jones R, Kemper J, Underhill LG. Surviving off junk: Low-energy prey dominates the diet of African penguins Spheniscus demersus at Mercury Island, Namibia, between 1996 and 2009. Afr J Mar Sci. 2010;32(3):562–572. [Google Scholar]
- 10.Hutchings L, et al. The Benguela current: An ecosystem of four components. Prog Oceanogr. 2009;83(Special Issue):15–32. [Google Scholar]
- 11.Roux J-P, et al. Jellyfication of marine ecosystems as a likely consequence of overfishing and small pelagic fishes: Lessons from the Benguela. Bull Mar Sci. 2013;89(1):249–284. [Google Scholar]
- 12.Bundy A, Fanning LP. Can Atlantic cod (Gadus morhua) recover? Exploring trophic explanations for the non-recovery of the cod stock on the eastern Scotian Shelf, Canada. Can J Fish Aquat Sci. 2005;62(7):1474–1489. [Google Scholar]
- 13.Steneck RS, McNaught D, Leland A, Vavrinec J. Ecosystem flips, locks and feedbacks: The lasting effects of fisheries on Maine’s kelp forest ecosystem. Bull Mar Sci. 2013;89(1):31–56. [Google Scholar]
- 14.Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25(11):633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Arnold SN, Mumby PJ, Steneck RS. Running the gauntlet to coral recruitment through a sequence of local multiscale processes. Mar Ecol Prog Ser. 2010;414:91–105. [Google Scholar]
- 16.Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol. 2008;23(10):555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci USA. 2008;105(42):16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oczkowski AJ, Nixon SW, Granger SL, El-Sayed AF, McKinney RA. Anthropogenic enhancement of Egypt’s Mediterranean fishery. Proc Natl Acad Sci USA. 2009;106(5):1364–1367. doi: 10.1073/pnas.0812568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries. 2002;25(4B):704–726. [Google Scholar]
- 20.Kareiva P, Marvier M, McClure M. Recovery and management options for spring/summer chinook salmon in the Columbia River basin. Science. 2000;290(5493):977–979. doi: 10.1126/science.290.5493.977. [DOI] [PubMed] [Google Scholar]
- 21.Travis J. The significance of geographic variation in species interactions. Am Nat. 1996;148(Suppl):S1–S8. [Google Scholar]
- 22.Dahlgren J, et al. Plant defenses to no avail? Responses of plants of varying edibility to food web manipulations in a low arctic scrubland. Evol Ecol Res. 2009;11(8):1189–11203. [Google Scholar]
- 23.Castilla JC. Coastal marine communities: Trends and perspectives from human-exclusion experiments. Trends Ecol Evol. 1999;14(7):280–283. doi: 10.1016/s0169-5347(99)01602-x. [DOI] [PubMed] [Google Scholar]
- 24.Castilla JC. Conservation and social-ecological systems in the 21st century of the Anthropocene era. Contrib Sci. 2012;8(1):11–21. [Google Scholar]
- 25.Paine RT. Food-web analysis through field measurement of per-capita interaction strength. Nature. 1992;355(6355):73–75. [Google Scholar]
- 26.Blamey LK, Plaganyi EE, Branch GM. Was overfishing of predatory fish responsible for a lobster-induced regime shift in the Benguela? Ecol Modell. 2014;273(1):140–150. [Google Scholar]
- 27.Essington TE. Getting the right answer from the wrong model: Evaluating the sensitivity of multispecies fisheries advice to uncertain species interactions. Bull Mar Sci. 2004;74(3):563–582. [Google Scholar]
- 28.Botsford LW, Castilla JC, Peterson CH. The management of fisheries and marine ecosystems. Science. 1997;277(5325):509–515. [Google Scholar]
- 29.Gutiérrez NL, Hilborn R, Defeo O. Leadership, social capital and incentives promote successful fisheries. Nature. 2011;470(7334):386–389. doi: 10.1038/nature09689. [DOI] [PubMed] [Google Scholar]
- 30.Castilla JC, Branch GM, Barkai A. 1994. Exploitation of two critical predators: The gastropod Concholepas concholepas and the rock lobster Jasus lalandii. Rocky Shores: Exploitation in Chile and South Africa, ed, Siegfried WR (Springer, Berlin), Vol 103, pp. 101–130.
- 31.Micheli F, Halpern BS, Botsford LW. Trajectories and correlates of community change in no-take marine reserves. Ecol Appl. 2004;14(6):1709–1723. [Google Scholar]
- 32.Gelcich S, et al. Territorial user rights for fisheries as ancillary instruments for marine coastal conservation in Chile. Conserv Biol. 2012;26(6):1005–1015. doi: 10.1111/j.1523-1739.2012.01928.x. [DOI] [PubMed] [Google Scholar]
- 33.Christensen V, Walters CJ. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol Modell. 2004;172(2-4):109–139. [Google Scholar]
- 34.Link J, et al. Response of balanced network models to large-scale perturbation: Implications for evaluating the role of small pelagics in the Gulf of Maine. Ecol Modell. 2009;220(3):351–369. [Google Scholar]
- 35.Scheffer M, et al. Early-warning signals for critical transitions. Nature. 2009;461(7260):53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, et al. Flickering gives early warning signals of a critical transition to a eutrophic lake state. Nature. 2012;492(7429):419–422. doi: 10.1038/nature11655. [DOI] [PubMed] [Google Scholar]