Significance
Coastal mangrove forests support a diverse array of associated species and provide ecosystem services to human communities. Mangroves cannot tolerate extreme freezing temperatures and so are generally limited to tropical environments. However, climate change in the form of increasing temperatures has the potential to facilitate increases in mangrove abundance near tropical–temperate transition zones. Here, we use 28 y of satellite imagery to demonstrate that increases in mangrove area have already occurred along the northeast coast of Florida. These increases correspond to decreases in the frequency of extreme cold events in this region. We also identify a temperature-related ecological threshold of −4°C. These results suggest that landscape-scale increases in mangrove area may occur in other regions where this threshold is crossed.
Keywords: ecological threshold, frost tolerance, Landsat, coastal wetlands
Abstract
Regional warming associated with climate change is linked with altered range and abundance of species and ecosystems worldwide. However, the ecological impacts of changes in the frequency of extreme events have not been as well documented, especially for coastal and marine environments. We used 28 y of satellite imagery to demonstrate that the area of mangrove forests has doubled at the northern end of their historic range on the east coast of Florida. This expansion is associated with a reduction in the frequency of “extreme” cold events (days colder than −4 °C), but uncorrelated with changes in mean annual temperature, mean annual precipitation, and land use. Our analyses provide evidence for a threshold response, with declining frequency of severe cold winter events allowing for poleward expansion of mangroves. Future warming may result in increases in mangrove cover beyond current latitudinal limits of mangrove forests, thereby altering the structure and function of these important coastal ecosystems.
The biological impacts of climate change—temperature increases in particular—have been documented across a large number of terrestrial ecosystems (1). For example, increasing annual mean temperatures are associated with shifts in the abundance and distributions of hundreds of plant species toward higher latitudes and elevations (2). Although most investigations into the response of ecosystems to climate change have focused on the impacts of gradual increases in mean temperatures, there is growing recognition that changes in the frequency of extreme weather events can also have profound impacts (3, 4). Thresholds in the mechanisms by which individuals respond to environmental conditions can lead to ecosystem-level nonlinear responses, particularly when foundation species are already close to environmental tipping points (5, 6). Recent evidence suggests that several important ecosystems may soon cross such thresholds (7). For example, CO2 concentrations of 450 ppm and a temperature increase of +2 °C have been identified as tipping points for mass coral bleaching and mortality (8). In rocky intertidal systems, temperature-related thresholds are linked to increased mortality and reproductive failure in barnacles and mussels (9, 10).
Although an increasing number of such ecological regime shifts are predicted in future years (11), there are still surprisingly few empirical examples of climate-related thresholds in natural systems (12, 13). In practice, identifying threshold responses on regional scales is challenging because of the difficulty involved with observing ecosystems over long enough time periods and large spatial scales (13). In addition, covarying environmental variables and biotic and abiotic interactions can make it difficult to isolate the environmental factors driving threshold responses.
The “velocity” of climate-driven change appears greatest in the coastal zone (14), a region that includes more than 70% of the world’s population and some of our most biologically productive ecosystems (15). Mangrove forests are among the most ecologically and economically important of these coastal ecosystems, providing food and habitat to a diverse array of terrestrial and marine species. Ecosystem services provided by mangroves include food, nesting, and nursery grounds for commercially important fish and invertebrates, wood production, waste processing, coastal protection, and recreation (16, 17). The value of these services has been estimated at more than $1.6 trillion per year (18). The serious threats that mangrove forests face as a result of coastal development, aquaculture, and timber production have been well documented (19, 20). However, our understanding of how climate-induced changes in environmental conditions are impacting these systems is limited relative to that of terrestrial systems (21). This is in part because long-term observations of changes in coastal and marine systems are rare by comparison with those on land (1).
The historical northern limit of mangroves in eastern North America, believed to be set by cold temperatures, is located near 30°N, just north of St. Augustine, FL (22). Salt marshes dominate the more temperate climates to the north, whereas mangroves and salt marsh coexist in an ecotone to the south (28°N to 30°N in Florida). Distribution modeling suggests the hypothesis that mangrove–salt marsh ecotones around the world persist near climate-related thresholds (23, 24), whereby small increases in temperature could lead to large increases in the relative abundance of mangroves and therefore in the structure and function of these important coastal ecosystems. Local observations of mangroves encroaching into salt marshes are accumulating (25–30), but regional assessments of mangrove expansion are lacking. Hypothesized mechanisms for these local mangrove encroachments include increased temperature, increased precipitation, revegetation of areas cleared for agriculture, altered tidal regimes, and/or increases in nutrient levels and sedimentation (29, 30). However, to date, we are aware of no study that has linked landscape-scale changes in mangrove abundance or spatial extent to changes in climate. We used 28 y of Landsat Thematic Mapper satellite imagery to demonstrate that a large poleward expansion of mangroves has occurred along the east coast of Florida. To test the hypothesis that temperature thresholds are responsible for transitions from salt marsh to mangroves, we analyzed changes in mangrove area over a period of 28 y against historical temperature records from a series of coastal weather stations spanning the mangrove–salt marsh transition zone. Moreover, we used other data to test alternative hypotheses that these changes were driven by changes in mean temperature or precipitation, revegetation of abandoned agricultural lands, eutrophication from agricultural or urban runoff, or altered tidal regimes.
Results
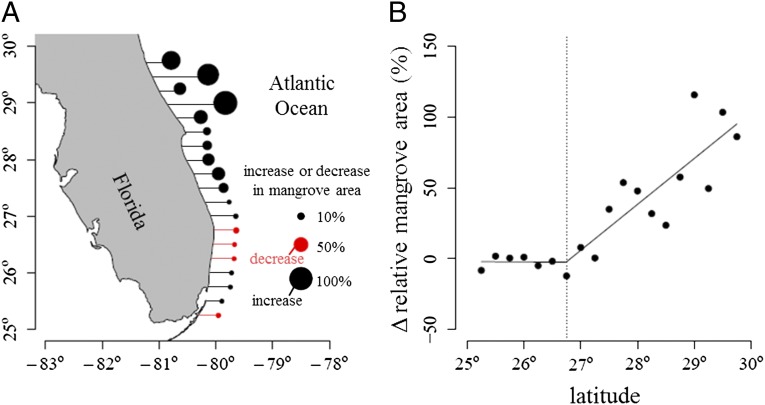
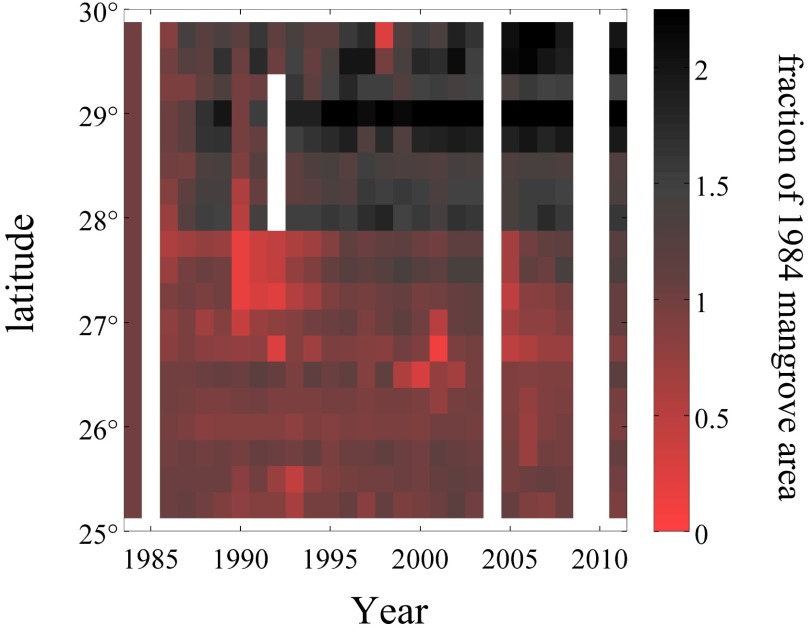
The area of mangrove forests increased dramatically between 1984 and 2011 near the northern range limit of mangroves in Florida (Fig. 1). Over this time period, the spatial extent of mangroves between 29° and 29.75°N doubled (Table S1). A distinct division in the dynamics of mangroves was evident near 26.75°N (Fig. 1B); coastal zones to the north of this latitude showed increases in mangrove area whereas zones to the south showed little change or small decreases in area. Piecewise regression analysis identified 26.75°N as a breakpoint in the relationship between latitude and change in mangrove area (Fig. 1B): north of 26.75°N, the magnitude of the change in mangrove area along the east coast of Florida was positively correlated with latitude (r2 = 0.69; P < 0.001), but there was no relationship between latitude and change in mangrove area south of 26.75°N (r2 = 0.03; P = 0.74). In absolute terms, mangrove area increased by 1,240 ± 93 ha along the east coast of Florida between 1984 and 2011 (Table S1). Regions to the north of 26.75°N increased by a total of 1,700 ± 130 ha whereas regions to the south decreased by 464 ± 35 ha. Coastal areas across Florida experienced a decrease in mangrove area after a hard freeze during the winter of 1989 to 1990 (Fig. 2). Recovery from this freeze event was variable in space, but, on regional scales, mangrove area in all of the 0.25° latitudinal bands recovered to pre-1989 levels within 9 y.
Fig. 1.
(A) Map of study area showing the long-term increase (black) or decrease (red) in mangrove area for each 0.25° latitudinal band from 25.25° to 29.75°N. Long-term change was defined as the relative change in 5-y mean mangrove area from 1984–1988 to 2007–2011. (B) Relationship between latitude and relative change in mangrove area. Solid line represents a piecewise regression and the vertical dotted line gives the breakpoint (26.75°N) of the piecewise regression.
Fig. 2.
A 28-y time series of mangrove area separated into 0.25° latitudinal bands. Mangrove area is displayed relative to the first year in the time series (1984). (Right) A value of 1 indicates no change (100% of 1984 area), 0 indicates total loss of mangroves, and 2 indicates a doubling of mangrove area. Data were calculated from Landsat imagery collected each year between April and July. White areas indicate that no cloud-free Landsat data were available.
Between 1984 and 2011, coastal weather stations along the same coastline recorded a general increase in temperature: mean annual temperature increased at five of eight weather stations and mean winter temperature increased at seven of eight stations (Table S2). However, only the northern stations documented significant decreases in the frequency of extreme cold events (i.e., days colder than −4 °C; Table S2). For example, in Titusville (28°31′N) and Daytona Beach (29°10′N), there were 1.2 and 1.4 fewer days colder than −4 °C per year in 2006 to 2011 compared with 1984 to 1989. In contrast, there was no change in the number of days colder than −4 °C in West Palm Beach (26°41′N) or Miami Beach (25°46′N). In these southern stations, extreme cold events were rare or nonexistent throughout the study period.
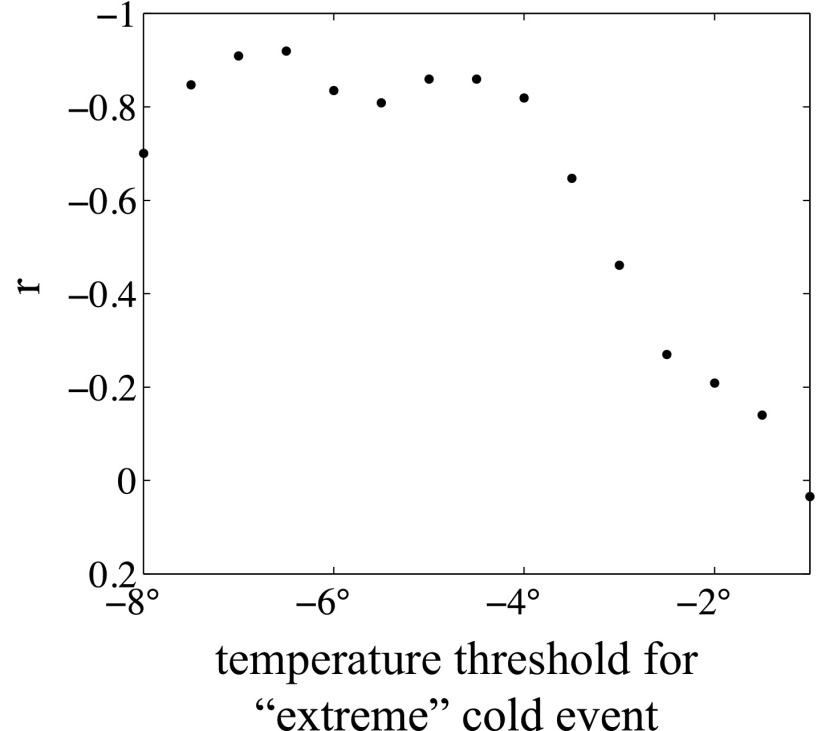
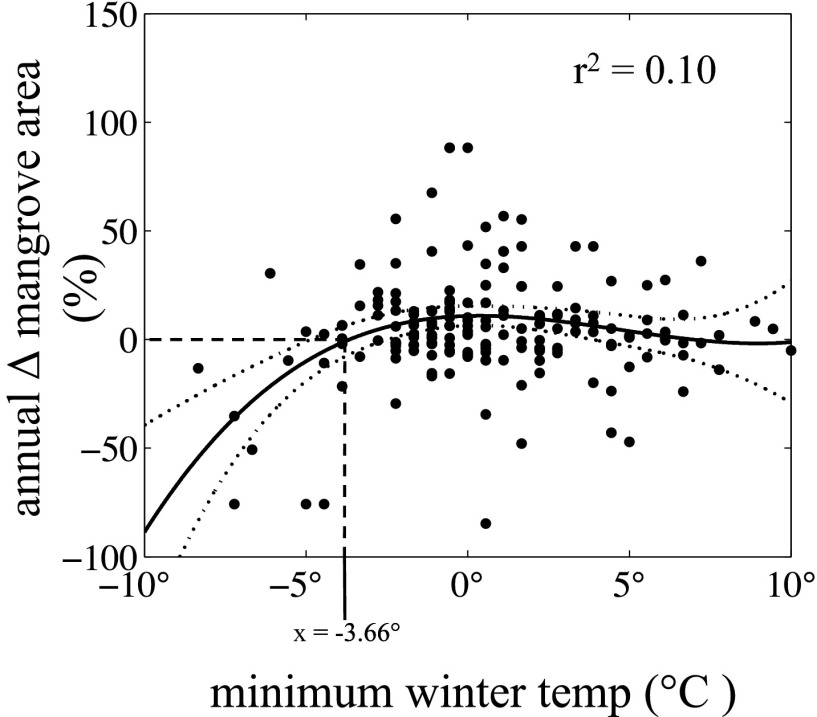
We found a strong relationship between increases in mangrove area and decreases in the frequency of extreme cold events (Table 1). However, expansion of mangroves was not associated with changes in mean annual or mean winter temperatures. In addition, the relationship between mangrove expansion and decreases in the frequency of extreme cold events was only significant if an extreme cold event was defined as colder than −4 °C; the relationship disappeared when the temperature threshold was raised a small amount (Fig. 3 and Table 1). This <−4 °C threshold also was apparent on annual time scales. Our model of the relationship between annual change in mangrove area and minimum temperature during the intervening winter estimated no change in mangrove area with a minimum temperature of −3.66 °C (Fig. 4). Change in mangrove area was negative for winters with minimum temperatures colder than −3.66 °C.
Table 1.
Regression statistics for bivariate relationships between change in mangrove area and change in environmental variables
| Predictor | r | P Value |
| Δ Winter temperature | 0.20 | 0.62 |
| Δ Annual temperature | 0.32 | 0.40 |
| Δ No. of days | ||
| <−1° | 0.03 | 0.93 |
| <−2° | −0.21 | 0.59 |
| <−3° | −0.46 | 0.21 |
| <−4° | −0.82* | <0.01* |
| <−5° | −0.86* | <0.01* |
| <−6° | −0.83* | <0.01* |
| <−7° | −0.91* | <0.001* |
| <−8° | −0.70* | 0.03* |
| Δ Precipitation | −0.23 | 0.56 |
| Δ Urban landcover | 0.29 | 0.23 |
| Δ Agriculture landcover | 0.40 | 0.10 |
Significant relationship with change in mangrove area (P < 0.05).
Fig. 3.
The correlation between change in mangrove cover and the change in the total number of days with minimum temperature lower than the stated threshold on the x axis. The x axis gives the definition of an extreme cold event: daily minimum temperature must be below this value.
Fig. 4.
Relationship between percent change in mangrove area between successive summers and the minimum temperature during the intervening winter. Solid line, polynomial model; dotted lines, 95% CIs; vertical dashed line, minimum winter temperature associated with no change in mangrove area.
Discussion
Our results indicate that mangroves are expanding poleward along the east coast of North America, and further suggest that this expansion is associated with recent warming. However, the observed expansion on the eastern coast of Florida has been facilitated not by increases in mean temperature, but by decreases in the frequency of discrete cold events. We found that the number of days with minimum temperatures colder than −4 °C represents an ecological threshold for mangrove forests: years in which temperature decreased to less than −4 °C exhibited decreases in mangrove cover (Fig. 4), whereas increases in mangrove area occurred in regions that experienced small reductions in the frequency of days colder than −4 °C between 1984 and 2011 (Table 1). Our empirically derived threshold is similar to the Florida citrus industry’s definition of a severe freeze [−5 to −6.7 °C (31)]. Moreover, experimental studies demonstrated significant mortality of mangrove (Avicennia) seedlings exposed to a temperature of 6.5 °C below zero, but negligible mortality at temperatures near 0 °C (32, 33). Our results demonstrate that a temperature threshold is already evident in the mangrove–salt marsh ecotone of Florida. Relatively small future decreases in the frequency of extreme cold events could lead to further increases in mangrove cover near the current poleward limits of mangrove forests in Florida. Over the past 50 y, daily minimum temperature has increased faster than daily mean or maximum temperature (34). If this asymmetrical warming trend continues, models that use average daily or monthly temperature may underestimate the response of mangrove–salt marsh ecosystems to future warming. Furthermore, mangrove expansion in response to climate change will likely be rapid relative to many terrestrial plant species, as water dispersed plants can often travel further than those dispersed by wind or animals (35).
Other authors have documented expansion of mangrove into saltmarsh on local scales in Florida (25, 26), Louisiana (27), and Australia (28, 29), but with uncertainty regarding the mechanisms behind these expansions. None of the aforementioned studies quantitatively linked mangrove expansions to changes in climatic conditions, or to regional observations of the dynamics of mangrove ecosystems. Here we directly link reductions in the frequency of extreme cold events to increases in mangrove area. A recent study that developed habitat suitability models for 12 species of mangroves around the world found that temperature-related variables were important predictors of distribution for all 12 species (24). As a result, it is likely that cold thresholds act as a barrier to the poleward extension of mangroves in other parts of the world besides Florida. However, in areas with very low precipitation or freshwater input (e.g., Baja Peninsula, Saudi Arabia, Western Australia), the abundance and extent of mangroves may be limited by factors such as drought tolerance, and so the response of mangroves to future warming will likely be different. In addition, there is evidence that cold tolerance varies among species of mangroves (36); therefore, we expect the specific temperature thresholds that define an extreme cold event to also vary among species.
In tests of alternative hypotheses for the expansion of mangroves, we found no significant relationships with changes in precipitation, urban land cover, and agricultural land cover (Table 1). We examined changes in land cover to determine whether eutrophication from agricultural or urban runoff could be linked to increases in mangrove area. This analysis would also capture large-scale revegetation of abandoned agricultural lands by mangroves if it were occurring. It is unlikely that regional-scale increases in mangrove area would arise from local scale changes in nutrient inputs, sedimentation, or hydrology, as these local drivers typically have local scale impacts (37, 38). Another alternative is that sea level rise has contributed to inland expansion of mangroves across our study area (30). However, this mechanism does not create the positive relationship between latitude and increase in mangrove area we observed. No significant latitudinal variability in sea-level changes along the east coast of Florida has been recorded over the past 50 y. According to National Oceanic and Atmospheric Administration (NOAA) data, mean sea level trends for tidal stations in Miami Beach, FL (25.8°N), Daytona Beach, FL (29.2°N), and Mayport, FL (30.4°N), have been 2.39 ± 0.43 mm/y, 2.32 ± 0.63 mm/y, and 2.40 ± 0.31 mm/y, respectively (39). In addition, there was no latitudinal trend in the amount of low-elevation habitat vulnerable to inland expansion by mangroves (Methods and Fig. S1A) and no significant relationship between change in mangrove area and the amount of this low-elevation habitat (r = 0.19; P = 0.44; Fig. S1B). In fact, the coast of southern Florida is expected to experience particularly large increases in mangrove area as a result of sea level rise (40). However, we found the opposite pattern: over the past 30 y, mangrove area has decreased marginally in southeast Florida and increased dramatically in northeast Florida.
Although reductions in the frequency of extreme cold events are facilitating an expansion of mangrove forests poleward, these forests face a number of other local- and regional-scale threats such that the net change in global mangrove area may be negative in the near term. Coastal development, aquaculture, and timber production are driving mangrove deforestation losses of 1% to 2% per year (19, 20). Other aspects of climate change are expected to cause reductions in mangrove area. For example, decreases in precipitation and increases in temperature may decrease mangrove survival in some arid regions, and rising sea levels may threaten 10–20% of mangroves by 2100 (41). To the extent that inland habitat is suitable, these losses may be offset by landward migration of mangroves. However, accelerating coastal development may eliminate the potential for landward migration in many areas. Replacement of coastal wetland foundation species, whether salt marsh or mangrove, will dramatically alter the structural complexity, community composition, food web dynamics, nutrient cycling, carbon sequestration, water filtration, and stability of these sensitive coastal environments.
Methods
Our study area included the entire east coast of Florida from 25°N to 30°N (Fig. 1A). Most of this area lies within the Indian River Lagoon, a drowned river valley where subtropical and temperate climatic zones converge. Coastal wetlands in this region receive large nutrient inputs from runoff, canals, and rivers draining agricultural and urban development. Salt marsh wetlands in the region are vegetated by short-statured herbaceous plants, including Spartina alterniflora (smooth cordgrass) and Spartina bakeri (sand cordgrass) in the low intertidal and Distichlis spicata (saltgrass), Salicornia virginica (glasswort), Batis maritima (saltwort) and Suaeda spp. (seablite) in the high intertidal. Meadows of Juncus roemerianus (needlegrass rush) and Borrichia frutescens (bushy sea ox-eye) are also common in the high intertidal. Mangrove wetlands in this area are dominated by one to three species of short to medium-sized trees, including Rhizophora mangle (red mangrove), Avicennia germinans (black mangrove), and Laguncularia racemosa (white mangrove).
We measured mangrove area along the east coast of Florida (25°N to 30°N) each summer from 1984 to 2011 by using 30-m resolution multispectral Landsat 5 Thematic Mapper satellite imagery. All three species of mangroves found in Florida (A. germinans, L. racemosa, and R. mangle) were grouped together in our analysis. We were unable to obtain imagery of the entire coastline in 1985, 1992, 2009, and 2010 because of excessive cloud cover during the Landsat acquisitions. After atmospherically correcting the Landsat images (42), we identified potential mangrove habitat from a 2010 mangrove atlas (43). We then used spectral mixture analysis to determine how much potential habitat was covered by mangrove forest in each image (44). Each pixel of potential mangrove habitat was modeled as a linear combination of three endmembers: photosynthetic vegetation (which we assume to be mangrove), water, and bare soil. These endmembers were represented by pure pixels of each of the three land cover types. The result of this process was a measure of the fraction of each pixel that was covered by mangrove forest. To validate our Landsat based estimates of mangrove area, we identified nine distinct contiguous stands of mangroves and compared our Landsat estimates of the area of each stand to area estimates made from high-resolution (1 m) IKONOS imagery. Mangrove canopy area was identified manually on the IKONOS image; mangrove trees were easily distinguished at this resolution. We also conducted a field survey in March 2013 to validate our interpretation of the IKONOS image. During the survey, we used GPS to identify locations of pure mangrove and salt marsh stands. The GPS points matched our manual interpretation of the IKONOS image, and the Landsat estimates of the area of nine distinct mangrove stands aligned closely with the high-resolution IKONOS estimates (Fig. S2). In addition, a number of previous studies in mangroves have demonstrated strong relationships between satellite-based vegetation indices, canopy closure estimates, and other estimates of canopy cover (45–48).
Mangrove area (as estimated by Landsat) was summed into 0.25° latitudinal bins (∼30 km) to examine latitudinal changes in mangrove extent. To determine long-term changes in mangrove area, we calculated the percent change in the 5-y mean of mangrove area from 1984–1988 to 2007–2011 for each latitudinal bin. We used 5-y means to average out short-term variability in mangrove area. We compared changes in mangrove area to latitude by using piecewise linear regression (49) in the R package “segmented” (50).
We obtained direct observations of daily temperature from 1984 to 2011 from 9 coastal National Weather Service stations that ranged in latitude from 25°46′N to 30°20′N (Table S2). We calculated three temperature metrics for each year of the time series: mean annual (July to June) temperature, mean winter (December to February) temperature, and the number of extreme cold events each year (July to June). Extreme cold events were defined as days when the temperature decreased below some threshold. We created 15 different extreme cold event variables by varying this threshold from −1 to −8 °C in 0.5° increments. We then compared changes in these temperature variables to changes in mangrove area by using linear regression and correlation analyses (Figs. S3 and S4). We also compared annual changes in mangrove area to the minimum winter temperature experienced during the intervening winter.
To test hypotheses other than temperature that may explain variation in mangrove area changes, we also compared changes in mangrove area to changes in precipitation and land use (Fig. S4). We obtained mean annual precipitation data from the same weather stations from which we acquired temperature data. We used Landsat-based land cover products developed by the Florida Fish and Wildlife Conservation Commission for the years 1985 and 2003 to examine land use change across our study area (51). Although the temporal coverage of our land use change data did not precisely match that of our mangrove data (1984–2011), most of the increases in mangrove area in the northern latitudes of our study area occurred before 2003 (Fig. 2). As a result, if land use change was a driver of mangrove change, its impact would likely be apparent by 2003. For each 0.25° coastal latitudinal bin, we calculated the change in area of urban and agricultural land cover from 1985 to 2003 for the watershed (52) that contained that 0.25° of coastline. Changes in urban and agricultural land cover were normalized by watershed area. Changes in the number of extreme cold events were not significantly correlated with changes in mean annual temperature, mean winter temperature, annual precipitation, urban land cover, or agricultural land cover (P > 0.10 for all pairwise comparisons). To test for a latitudinal trend in the amount of coastline vulnerable to inland mangrove expansion as a result of sea-level rise, we estimated the amount of coastal habitat with an elevation between −0.1 and 1 m above sea level for each 0.25° latitudinal bin. We obtained digital elevation data from the NOAA National Geophysical Data Center’s US Coastal Relief Model (53).
Supplementary Material
Acknowledgments
The authors thank Mark Spalding for permission to use the digital version of his World Atlas of Mangroves. Funding for this work was provided by National Aeronautics and Space Administration Climate and Biological Response Program NNX11AO94G and National Science Foundation Macrosystems Biology Program EF 1065821 and 1065098.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315800111/-/DCSupplemental.
References
- 1.Rosenzweig C, et al. Attributing physical and biological impacts to anthropogenic climate change. Nature. 2008;453(7193):353–357. doi: 10.1038/nature06937. [DOI] [PubMed] [Google Scholar]
- 2.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333(6045):1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 3.Jentsch A, Kreyling J, Beierkuhnlein C. A new generation of climate-change experiments: Events, not trends. Front Ecol Environ. 2007;5(7):365–374. [Google Scholar]
- 4.Wernberg T, et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat Clim Change. 2012;3(1):78–82. [Google Scholar]
- 5.Lenton TM, et al. Tipping elements in the Earth’s climate system. Proc Natl Acad Sci USA. 2008;105(6):1786–1793. doi: 10.1073/pnas.0705414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monaco CJ, Helmuth B. Tipping Points, Thresholds and the Keystone Role of Physiology in Marine Climate Change Research. London: Academic Press; 2011. [DOI] [PubMed] [Google Scholar]
- 7.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 8.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318(5857):1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 9.Wethey DS, Woodin SA. Ecological hindcasting of biogeographic responses to climate change in the European intertidal zone. Hydrobiologia. 2013;606(1):139–151. [Google Scholar]
- 10.Helmuth B, et al. Climate change and latitudinal patterns of intertidal thermal stress. Science. 2002;298(5595):1015–1017. doi: 10.1126/science.1076814. [DOI] [PubMed] [Google Scholar]
- 11.Folke C, et al. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- 12.Andersen T, Carstensen J, Hernández-García E, Duarte CM. Ecological thresholds and regime shifts: Approaches to identification. Trends Ecol Evol. 2009;24(1):49–57. doi: 10.1016/j.tree.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Groffman PM, et al. Ecological thresholds: the key to successful environmental management or an important concept with no practical application? Ecosystems (N Y) 2006;9(1):1–13. [Google Scholar]
- 14.Loarie SR, et al. The velocity of climate change. Nature. 2009;462(7276):1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 15.Agardy T, et al. Coastal Systems. In: Hassan R, Scholes R, Ash N, editors. The Millennium Ecosystem Assessment: Ecosystems and Human Well-Being: Current State and Trends. Vol 1. Washington, DC: Island; 2005. pp. 515–549. [Google Scholar]
- 16.Ewel KC, Twilley RR, Ong JE. Different kinds of mangrove forests provide different goods and services. Global Ecol Biogeogr Lett. 1998;7:83–94. [Google Scholar]
- 17.Aburto-Oropeza O, et al. Mangroves in the Gulf of California increase fishery yields. Proc Natl Acad Sci USA. 2008;105(30):10456–10459. doi: 10.1073/pnas.0804601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costanza R, et al. The value of the world’s ecosystem services and natural capital. Nature. 1997;387(6630):253–260. [Google Scholar]
- 19.Alongi DM. Present state and future of the world’s mangrove forests. Environ Conserv. 2002;29(3):331–349. [Google Scholar]
- 20.Duke NC, et al. A world without mangroves? Science. 2007;317(5834):41–42. doi: 10.1126/science.317.5834.41b. [DOI] [PubMed] [Google Scholar]
- 21.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328(5985):1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 22.Kangas PC, Lugo AE. The distribution of mangroves and saltmarsh in Florida. Trop Ecol. 1990;31(1):32–39. [Google Scholar]
- 23.Osland MJ, Enwright N, Day RH, Doyle TW. Winter climate change and coastal wetland foundation species: Salt marshes vs. mangrove forests in the southeastern United States. Glob Change Biol. 2013;19(5):1482–1494. doi: 10.1111/gcb.12126. [DOI] [PubMed] [Google Scholar]
- 24.Record S, Charney N, Zakaria R, Ellison A. Projecting global mangrove species and community distributions under climate change. Ecosphere. 2013;4(3):1–23. [Google Scholar]
- 25.Stevens PW, Fox SL, Montague CL. The interplay between mangroves and saltmarshes at the transition between temperate and subtropical climate in Florida. Wetlands Ecol Manage. 2006;14(5):435–444. [Google Scholar]
- 26.Michener WK, Blood ER, Bildstein KL, Brinson MM, Gardner LR. Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol Appl. 1997;7(3):770–801. [Google Scholar]
- 27.Michot T, Day R, Wells C. Increase in black mangrove abundance in coastal Louisiana. Louisiana Natural Resources News (Newsletter of the Louisiana Association of Professional Biologists) 2010:4–5. [Google Scholar]
- 28.Mitchell ML, Adam P. The decline of saltmarsh in Botany Bay. Wetlands (Australia) 2009;8(2):55–60. [Google Scholar]
- 29.Saintilan N, Williams RJ. Mangrove transgression into saltmarsh environments in south-east Australia. Glob Ecol Biogeogr. 1999;8(2):117–124. [Google Scholar]
- 30.López–Medellín X, et al. Oceanographic anomalies and sea-level rise drive mangroves inland in the Pacific coast of Mexico. J Veg Sci. 2011;22(1):143–151. [Google Scholar]
- 31.Miller KA, Downton MW. The freeze risk to Florida citrus. I: Investment decisions. J Clim. 1993;6(2):354–363. [Google Scholar]
- 32.McMillan C, Sherrod CL. The chilling tolerance of black mangrove, Avicennia germinans, from the Gulf of Mexico coast of Texas, Louisiana and Florida. Contrib Mar Sci. 1986;29:9–16. [Google Scholar]
- 33.Pickens CN, Hester MW. Temperature tolerance of early life history stages of black mangrove Avicennia germinans: Implications for range expansion. Estuaries Coasts. 2011;34(4):824–830. [Google Scholar]
- 34.Intergovernmental Panel on Climate Change . In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 35.Ridley HN. The Dispersal of Plants Throughout the World. Kent, UK: Ashford; 1930. [Google Scholar]
- 36.Stuart SA, Choat B, Martin KC, Holbrook NM, Ball MC. The role of freezing in setting the latitudinal limits of mangrove forests. New Phytol. 2007;173(3):576–583. doi: 10.1111/j.1469-8137.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 37.Feller IC, Whigham DF, McKee KL, Lovelock CE. Nitrogen limitation of growth and nutrient dynamics in a disturbed mangrove forest, Indian River Lagoon, Florida. Oecologia. 2003;134(3):405–414. doi: 10.1007/s00442-002-1117-z. [DOI] [PubMed] [Google Scholar]
- 38.Young BM, Harvey EL. A spatial analysis of the relationship between mangrove (Avicennia marinavar. australasica) physiognomy and sediment accretion in the Hauraki Plains, New Zealand. Estuar Coast Shelf Sci. 1996;42:231–246. [Google Scholar]
- 39. National Oceanographic and Atmospheric Administration, National Ocean Service (2009) Sea Level Variations of the United States 1854-2006. (NOAA, Silver Spring, MD), NOAA Technical Report NOS CO-OPS 53.
- 40.Doyle TW, Girod GF, Books MA. Preparing for a Changing Climate: the Potential Consequence of Climate Variability and Change: Gulf Coast Region. Baton Rouge, LA: Gulf Coast Climate Change Assessment Council; 2003. Modeling Mangrove Forest Migration Along the Southwest Coast of Florida Under Climate Change; pp. 211–222. [Google Scholar]
- 41.Alongi DM. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci. 2008;76(1):1–13. [Google Scholar]
- 42.Furby S, Campbell N. Calibrating images from different dates to ‘like-value’ digital counts. Remote Sens Environ. 2001;77(2):186–196. [Google Scholar]
- 43.Spalding M, Kainuma M, Collins L. World Atlas of Mangroves. New York: Earthscan; 2010. [Google Scholar]
- 44.Adams J, Smith M, Gillespie A. Remote Geochemical Analysis: Elemental and Mineralogical Composition. Vol 7. Cambridge, UK: Cambridge Univ Press; 1993. Imaging spectroscopy: Interpretation based on spectral mixture analysis; pp. 145–166. [Google Scholar]
- 45.Ramsey EW, Jensen JR. Remote sensing of mangrove wetlands: Relating canopy spectra to site-specific data. Photogramm Eng Remote Sensing. 1996;62(8):939–948. [Google Scholar]
- 46.Green EP, Clark CD, Mumby PJ, Edwards AJ, Ellis A. Remote sensing techniques for mangrove mapping. Int J Remote Sens. 1998;19(5):935–956. [Google Scholar]
- 47.Kovacs JM, Flores-Verdugo F, Wang J, Aspden LP. Estimating leaf area index of a degraded mangrove forest using high spatial resolution satellite data. Aquat Bot. 2004;80(1):13–22. [Google Scholar]
- 48.Kovacs JM, Wang J, Flores-Verdugo F. Mapping mangrove leaf area index at the species level using IKONOS and LAI-2000 sensors for the Agua Brava Lagoon, Mexican Pacific. Estuar Coast Shelf Sci. 2005;62(1):377–384. [Google Scholar]
- 49.Toms JD, Lesperance ML. Piecewise regression: A tool for identifying ecological thresholds. Ecology. 2003;84:2034–2041. [Google Scholar]
- 50.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 51.Kautz R, Stys B, Kawula R. Florida vegetation 2003 and land use change between 1985-89 and 2003. Fla Sci. 2007;70:12–23. [Google Scholar]
- 52.US Geological Survey and US Department of Agriculture Natural Resources Conservation Service . Federal Standards and Procedures for the National Watershed Boundary Dataset (WBD) Reston, VA: US Geological Survey; 2012. [Google Scholar]
- 53. NOAA National Geophysical Data Center (2013) U.S. Coastal Relief Model. Available at www.ngdc.noaa.gov/mgg/coastal/crm.html. Accessed October 28, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.