Significance
Growth hormone-releasing hormone (GHRH) and its agonistic analogs, besides augmenting the release of growth hormone from the pituitary, can exert direct stimulatory effects on various extrapituitary tissues. Present oncologic evaluation of growth of a glioblastoma cell line revealed that the combination of GHRH agonist, JI-34, with doxorubicin (DOX) produced greater inhibition in vivo than either drug alone. In vitro, JI-34 also potentiated the effects of DOX, decreased the expression of the neuroectodermal stem-cell antigen, nestin, and up-regulated the glial maturation marker, GFAP. These findings indicate that GHRH agonists can induce differentiation of cancer cells, increasing the response to DOX. These observations expand the known spectrum of activities of GHRH and its analogs and have potential clinical implications for therapy.
Keywords: peptide analogs, targeted therapy
Abstract
The dismal prognosis of malignant brain tumors drives the development of new treatment modalities. In view of the multiple activities of growth hormone-releasing hormone (GHRH), we hypothesized that pretreatment with a GHRH agonist, JI-34, might increase the susceptibility of U-87 MG glioblastoma multiforme (GBM) cells to subsequent treatment with the cytotoxic drug, doxorubicin (DOX). This concept was corroborated by our findings, in vivo, showing that the combination of the GHRH agonist, JI-34, and DOX inhibited the growth of GBM tumors, transplanted into nude mice, more than DOX alone. In vitro, the pretreatment of GBM cells with JI-34 potentiated inhibitory effects of DOX on cell proliferation, diminished cell size and viability, and promoted apoptotic processes, as shown by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide proliferation assay, ApoLive-Glo multiplex assay, and cell volumetric assay. Proteomic studies further revealed that the pretreatment with GHRH agonist evoked differentiation decreasing the expression of the neuroectodermal stem cell antigen, nestin, and up-regulating the glial maturation marker, GFAP. The GHRH agonist also reduced the release of humoral regulators of glial growth, such as FGF basic and TGFβ. Proteomic and gene-expression (RT-PCR) studies confirmed the strong proapoptotic activity (increase in p53, decrease in v-myc and Bcl-2) and anti-invasive potential (decrease in integrin α3) of the combination of GHRH agonist and DOX. These findings indicate that the GHRH agonists can potentiate the anticancer activity of the traditional chemotherapeutic drug, DOX, by multiple mechanisms including the induction of differentiation of cancer cells.
Glioblastoma multiforme (GBM) is one of the most aggressive human cancers, and the afflicted patients inevitably succumb. The dismal outcome of this malignancy demands great efforts to find improved methods of treatment (1). Many compounds have been synthesized in our laboratory in the past few years that have proven to be effective against diverse malignant tumors (2–14). These are peptide analogs of hypothalamic hormones: luteinizing hormone-releasing hormone (LHRH), growth hormone-releasing hormone (GHRH), somatostatin, and analogs of other neuropeptides such as bombesin and gastrin-releasing peptide. The receptors for these peptides have been found to be widely distributed in the human body, including in many types of cancers (2–14). The regulatory functions of these hypothalamic hormones and other neuropeptides are not confined to the hypothalamo–hypophyseal system or, even more broadly, to the central nervous system (CNS). In particular, GHRH can induce the differentiation of ovarian granulosa cells and other cells in the reproductive system and function as a growth factor in various normal tissues, benign tumors, and malignancies (2–4, 6, 11, 14–18). Previously, we also reported that antagonistic cytototoxic derivatives of some of these neuropeptides are able to inhibit the growth of several malignant cell lines (2–14).
Our earlier studies showed that treatment with antagonists of LHRH or GHRH rarely effects complete regression of glioblastoma-derived tumors (5, 7, 10, 11). Previous studies also suggested that growth factors such as EGF or agonistic analogs of LHRH serving as carriers for cytotoxic analogs and functioning as growth factors may sensitize cancer cells to cytotoxic treatments (10, 19) through the activation of maturation processes. We therefore hypothesized that pretreatment with one of our GHRH agonists, such as JI-34 (20), which has shown effects on growth and differentiation in other cell lines (17, 18, 21, 22), might decrease the pluripotency and the adaptability of GBM cells and thereby increase their susceptibility to cytotoxic treatment.
In vivo, tumor cells were implanted into athymic nude mice, tumor growth was recorded weekly, and final tumor mass was measured upon autopsy. In vitro, proliferation assays were used for the determination of neoplastic proliferation and cell growth. Changes in stem (nestin) and maturation (GFAP) antigen expression was evaluated with Western blot studies in vivo and with immunocytochemistry in vitro. The production of glial growth factors (FGF basic, TGFβ) was verified by ELISA. Further, using the Human Cancer Pathway Finder real-time quantitative PCR, numerous genes that play a role in the development of cancer were evaluated. We placed particular emphasis on the measurement of apoptosis, using the ApoLive-Glo Multiplex Assay kit and by detection of the expression of the proapoptotic p53 protein. This overall approach permitted the evaluation of the effect of GHRH agonist, JI-34, on the response to chemotherapy with doxorubicin.
Results
In Vivo Effects of the GHRH Agonist JI-34 Alone or in Combination with DOX on the Growth of Glioblastoma U-87 MG Xenografts in Nude Mice.
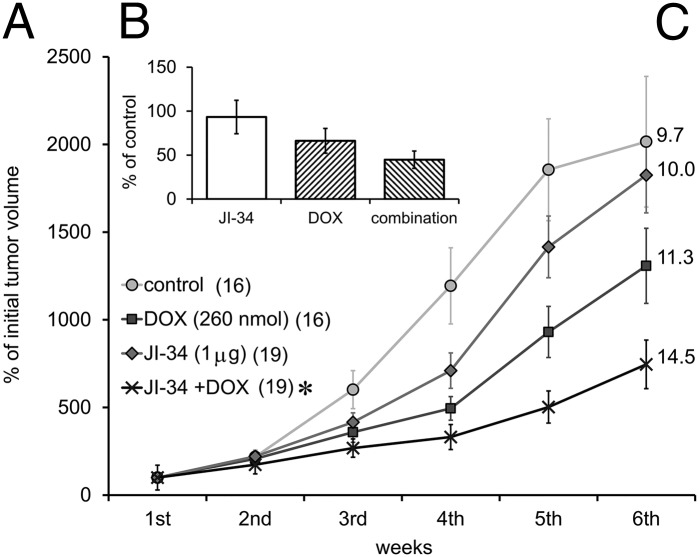
Nude mice bearing U-87 MG tumors were treated with the GHRH agonist, JI-34 (50 µg/kg/d), DOX (13 µmol/kg/wk), or with their combination for 6 wk. Control animals received daily saline injections. The administration of DOX reduced growth of U-87 MG tumors by 35%. The combination of JI-34 with DOX elicited an even greater (63%) inhibition of tumor growth (Fig. 1A) and repeated measure ANOVA revealed a significant difference (F3,66 = 1.962, P < 0.01, Fisher’s post hoc test: P < 0.05 between J-34 + DOX and control). Similar suppressive effects of these therapies were observed on the basis of the reduction of final tumor weights at sacrifice (Fig. 1B) and increases in tumor doubling times (Fig. 1C), although these results did not prove to be statistically significant.
Fig. 1.
(A) The effect of treatment with the GHRH agonist JI-34 (50 µg/kg/d or 1 µg/mouse/d) (J) and doxorubicin (DOX; D) (13 µmol/kg/wk) alone and in combination on the growth of U-87 MG, human GBM tumors xenotransplanted to nude mice. Numbers at labels represent the number of successfully implanted tumors. (B) Final weights of necropsied tumor samples compared with the control. (C) Numbers at the end of each curve show the tumor doubling times. *P < 0.05 vs. control.
GHRH Agonist JI-34 Augments the Inhibitory Effects of DOX on Proliferation and Growth of U-87 GM Cells in Vitro.
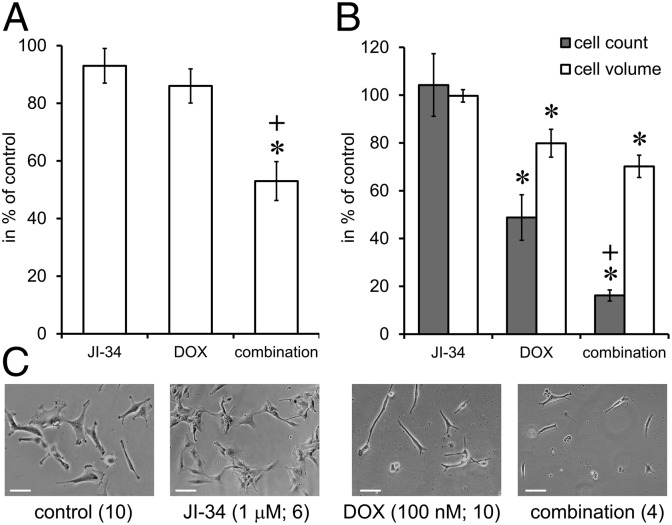
The effect of JI-34, DOX, or their combination on the proliferation rate of U-87 MG cells was tested using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Exposure to GHRH agonist, JI-34, alone at 1 µM concentration or 100 nM DOX alone as separate drugs, had no significant effect on the proliferation of U-87 MG cells in vitro. When cells were pretreated with 1 µM JI-34 for 24 h before exposure to the combination of DOX + JI-34 for 48 h, the inhibitory effect (47% inhibition) of the combination treatment proved to be statistically significant (Fig. 2A) (ANOVA; F3,20 = 11.37, Tukey’s post hoc: P < 0.01 vs. both control and DOX).
Fig. 2.
The effect of single exposure (A) or repeated exposure (B) to GHRH agonist JI-34 (1 µM) (J) doxorubicin (100 nM DOX; D), and their combination on the proliferation of U-87 MG cells in vitro. *P < 0.05 vs. control, +P < 0.05 vs. DOX. For conditions see Materials and Methods and Results. (C) Phase-contrast images of the U-87 MG cell cultures. Images are shown at 400× magnification with 20-megapixel resolution. Representative sections of the visual field were cropped and fitted. (Scale bars, 100 µm.)
In a different experiment, cells were cultured in T-25 flasks, and drugs were applied repeatedly (1 µM JI-34 daily and DOX weekly at 100 nM) for 3 wk (Fig. 2B). Cell number and cell volume were then determined by cell counting and cell volumetric assays, respectively. JI-34 alone had no significant effect, but both DOX and its combination with JI-34 significantly decreased the number of cells (52% and 84% decrease, respectively; F3,26 = 19.51 and Tukey’s post hoc: P < 0.01 for DOX vs. control and combination vs. control, whereas Fisher’ post hoc: P < 0.05 for combination vs. DOX). DOX and its combination with JI-34 also reduced the volume of the cells (20% and 30% decrease, respectively; F3,26 = 9.87 and Tukey’s post hoc: P < 0.01 for DOX vs. control and combination vs. control) as assessed by intracellular water content. Fig. 2C shows representative microscopic images of all four treatment groups. The treatment with GHRH agonist resulted in adherent cultures of neuroectodermal cells with more prominent glial projections. Conversely, DOX treatment elicited characteristic fusiform changes in morphology due to arrested mitoses and the mitotic collapse that precedes apoptosis. The combination exerted a devastative effect, perhaps due to the mitotic synchronizing and sensitizing activity of JI-34.
Effect of GHRH Agonist JI-34 and Its Combination with DOX on the Expression of Genes Related to Cell Proliferation, Apoptosis, Cell Cycle, Angiogenesis, Invasion, and Metastasis.
Quantitative real-time PCR array studies revealed a significant effect, of the combination of JI-34 and DOX treatment for 6 wk, on the expression of several markers of tumor growth, invasion, and metastasis formation in U-87 cell-derived tumors (Table 1). The combination of JI-34 and DOX increased the expression of the proapoptotic BCL-2-associated agonist of cell death (BAD) and decreased the expression of the anti-apoptotic B-cell lymphoma 2 (Bcl-2). The increase in BAD seems to be of special importance as DOX itself had an opposite effect on BAD expression. The combination treatment also attenuated the expression of the contact activator integrin α3 subunit (by approximately twofold). In addition, an almost threefold decrease was observed in the metastasis promoting S100 calcium binding protein A4 (S100-A4).
Table 1.
Relative expression of genes related to tumor growth
| Treatment genes | DOX | JI-34 + DOX |
| BCL-2–associated agonist of cell death (BAD) | −1.1* | 1.11 |
| B-cell CLL/lymphoma 2 (Bcl-2) | −1.8* | −1.86* |
| Integrin α3 (CD49C) | −1.65 | −1.85* |
| S100 calcium binding protein A4 (S100-A4) | −2.34 | −2.75* |
Three parallel experiments were run for both groups. *P < 0.05 vs. control.
GHRH Agonist, JI-34 Facilitates the Apoptotic Activity and Increases the Intracellular Retention of DOX.
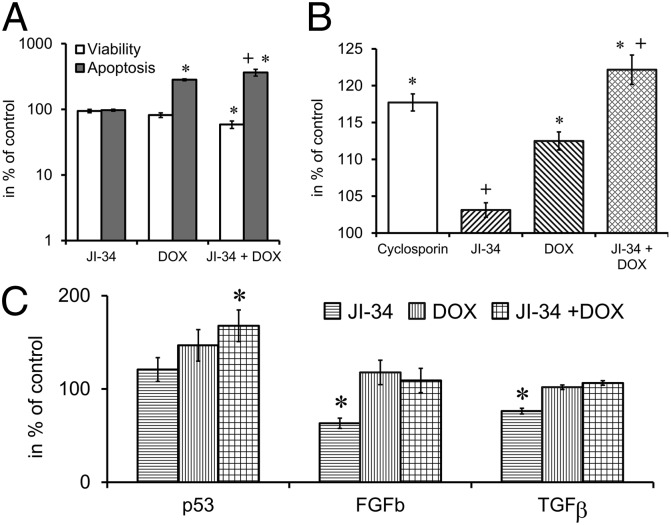
GHRH agonist JI-34 alone (1 µM) had no effect on apoptosis, but DOX at the 100-nM concentration and the combination of DOX 100 nM and JI-34 1 µM elicited a significant increase (182% and 263%, respectively) in apoptosis as measured by the ApoLive-Glo Multiplex assay after 24 h of treatment (Fig. 3A; F3,17 = 41.31; Tukey’s P < 0.01 for DOX vs. control and combination vs. control and Fisher’s P < 0.05 combination vs. DOX). The combination of the DOX and JI-34 treatment also decreased the viability of the cells by 41% (F3,17 = 7.00, Tukey’s P < 0.01 vs. control and Fisher’s P < 0.05 vs. DOX).
Fig. 3.
The effect of the combination treatment with the GHRH agonist JI-34 + DOX on viability and apoptosis (A), calcein retention (B), and the expression of bFGF, TGFβ, and the tumor suppressor p53 (C), in vitro. For conditions see Materials and Methods and Results. *P < 0.05 vs. control. DOX, doxorubicin; FGFb, fibroblast growth factor basic; TGFβ, transforming growth factor β. *P < 0.05 vs. control, +P < 0.05 vs. DOX.
In the multidrug resistance (MDR) assay both cyclosporin-A (manufacturer’s recommended control) and DOX treatment increased calcein retention (17% and 7%, respectively; Fig. 3B; F4,86 = 28.856, P < 0.01; Tukey’s post hoc test: P < 0.01 vs. control). The JI-34 pretreatment significantly augmented calcein retention in the combination group by 23% compared with the control (F4,86 = 28.856, P < 0.01; Tukey’s post hoc test: P < 0.01 vs. control and P < 0.01 vs. DOX). It appears that JI-34 pretreatment leads to decreased expression of MDR transporter proteins and increased calcein and DOX retention.
Effects of GHRH Agonist JI-34, DOX, and Their Combination on the Levels of Intracellular p53 or Secreted FGFb and TGFβ.
The changes in the expression of these regulators of tumor growth and differentiation in vitro were detected by ELISA experiments from either cell culture supernatants or from homogenates of U-87 cells treated with 100 nM DOX, 1 µM JI-34, or their combination (Fig. 3C). Using homogenized U-87 MG cell culture samples, we detected a significant (68%) increase in the level of the tumor suppressor p53 upon exposure of the cells to the treatment with combination of JI-34 + DOX (F3,12 = 3.6; Tukey’s P < 0.05 vs. control). The levels of both the glial growth factor, bFGF (23, 24) (F3,16 = 5.15; Fisher’s P < 0.05 JI-34 vs. control), and the dedifferentiation and tumor promoting factor TGFβ (25) (F3,10 = 4.5; Fisher’s P < 0.05 JI-34 vs. control), were significantly lower (37% and 24%, respectively) in the supernatant of the cell cultures exposed to JI-34 treatment, demonstrating the inhibitory effects of JI-34 on the production of these cytokines.
Effects of GHRH Agonist JI-34 and DOX, Alone and in Combination, on the Expression of GHRH Receptors, Nestin and Glial Fibrillary Acidic Protein.
Western blot studies (Fig. 4) verified the expression of pituitary type GHRH receptor (pGHRH-R) and its splice variant, SV1, in samples of U-87 MG xenografts. None of the treatments significantly altered the expression of these receptors. The GHRH agonist decreased and the treatment with DOX increased the expression of the neuroectodermal stem-cell marker, nestin, but the effects on the maturation antigen glial fibrillary acid protein (GFAP) (26) were opposite. In the case of both intermediary filaments, statistically significant differences could be observed according to the integrated density values. The combination treatment produced an 80% decrease in nestin expression compared with the control (F3,8 = 7.991; Tukey’s P < 0.01 for JI-34 + DOX vs. DOX, P < 0.05 for JI-34 + DOX vs. control and Tukey’s P < 0.05 for JI-34 vs. DOX), whereas the JI-34 pretreatment caused a 70% increase in GFAP expression compared with the control (F3,8 = 7.991; Fisher’s P < 0.01 for JI-34 vs. DOX, P < 0.05 for JI-34 vs. control and Fisher’s P < 0.05 for JI-34 + DOX vs. DOX).
Fig. 4.
Western blot images (A) and integrated density values (IDVs) (B) for the expression of GHRH receptors and differentiation antigens in necropsied in vivo samples. GHRH-R, pituitary type growth hormone releasing hormone receptor; SV1, splice variant-1 of GHRH receptor; DOX or D, doxorubicin; J, JI-34; GFAP, glial fibrillary acidic protein. *P < 0.05 vs. control.
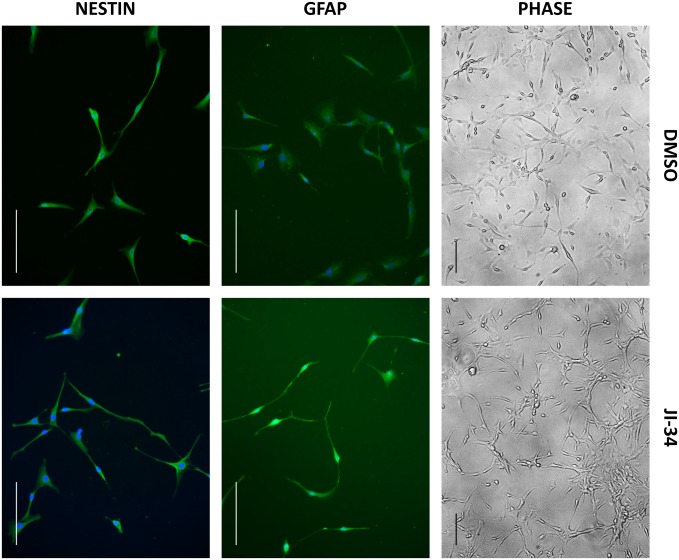
When U-87 MG cells were treated with the GHRH agonist, JI-34 (1 µM) in reduced serum-containing growth medium, cells underwent morphological changes, having more/longer astrocytic processes than cells in the control group. Representative phase-contrast images from control and GHRH agonist-treated groups are shown in Fig. 5. To further demonstrate the change in differentiation state following the administration of GHRH agonist, cells were fixed and stained for the detection of the astrocyte marker, GFAP, and the neuroectodermal stem cell marker, nestin. The elevation in the intensity of GFAP-labeling indicates that the GHRH agonist-treated cells attained a higher differentiation level than the untreated cells. The fluorescence staining with nestin antibody, however, did not show a significant change in the level of nestin following the administration of GHRH agonist.
Fig. 5.
GHRH agonist induces the differentiation of U-87 MG cells in reduced serum-containing medium in vitro. Cells were treated with GHRH agonist JI-34 (1 µM) or DMSO in 0.1% FBS-containing growth medium. Phase-contrast images of live cells were taken after 2 d and then cells were fixed and stained for the differentiation markers, GFAP and nestin. The GHRH agonist-treated cells possess a higher tendency toward outgrowth of projections; moreover, high levels of GFAP were detected in these cells, indicative of glial differentiation. The protein level of nestin did not change notably following the treatment with GHRH agonist. Nuclei were stained with DAPI. (Scale bars, 100 µm.)
Discussion
Our results successfully demonstrate that the concurrent administration of an agonistic analog of GHRH (20) and a traditional cytotoxic drug, DOX, augments the antineoplastic action of the latter. Our findings show that the treatment with the combination of the GHRH agonist and DOX inhibits the in vivo growth of xenotransplanted U-87 MG tumors as well as decreasing the multiplication and growth of these glioblastoma cells in vitro. This effect may be attributed to the ability of the GHRH agonist to induce changes in maturation state consequently decreasing the pluripotency of the neoplastic cells. Traditional cancer therapies frequently fail because of a phenomenon which may be called “survival of the fittest” (27), based upon the natural selection of cancer cells under evolutionary pressure exerted by the treatment itself, and which is analogous to the development of bacterial antimicrobial resistance under antibiotic therapy. This phenomenon may imply the presence of “cancer stem cells” (26, 27). These cancer stem cells can provide an inexhaustible pool of cellular adaptation upon challenge, when there is no time for dedifferentiation, and the induction of the expression of resistance genes.
Previously, our group reported that EGF, a well-known growth factor in both physiological and pathological states, is able to sensitize cancer cells to cytotoxic treatment (19). Our prior results also suggested that an agonistic LHRH analog could have some sensitizing activity, either as a carrier molecule for a cytotoxic agent or as a cotreatment with a cytotoxic agent (10), but it is more likely that the increased therapeutic efficacy is due to targeting capacity to the receptors. Based on our present findings, we can postulate that this sensitizing activity may be related to a maturation effect on the glioblastoma cells, a process characterized by the down-regulation of levels of nestin (a common neuroectodermal marker), and the up-regulation of levels of GFAP (28). Whereas DOX elicited relative increases in nestin and decrease in expression of GFAP, GHRH agonist JI-34 elicited opposite changes in the expression pattern of intermediary filaments in the present study.
Previously we demonstrated that antagonistic analogs of GHRH potentitate the inhibitory effects of docetaxel on growth of human MX-1 and MDA-MB-231 breast cancers and H460 nonsmall cell lung cancers in vivo (29–31). We believed that the increased efficacy of the response was due to the combined effect of GHRH antagonists and docetaxel or to their synergistic action. However, to our knowledge, no beneficial effects of GHRH agonists on tumor growth have been reported previously.
Abrogation of cancer stem cells would be an initial step, a very special one, leading to the successful treatment of GBM and other malignancies by decreasing the resilience of the tumor and its ability to generate recurrence. Facilitation of maturation has already been demonstrated to be effective in the treatment of certain malignancies: destruction of the nonsense fusion protein denoted PML-RARα with arsenic-trioxide (AsO3); liberation of RARα; administration of the strong maturation factor, retinoic acid, brings about long-lasting regression in acute promyelocytic leukemia (32). Retinoic acid is required for the differentiation of many epithelial cells (33). In addition, retinoids, as a sole treatment of tumor phenotypes with bioavailable receptors, have proven efficacy in basal cell carcinoma and Verruca vulgaris (34). Retinoids play an important role in the maturation of glial cells (28) and have already been successfully used in the therapy of gliomas (35).
The down-regulation of GHRH receptors by our potent GHRH agonist (20), which could lead to the development of resistance to the treatment, may be excluded as indicated by our Western blot studies. This finding supports our view (2, 3, 10, 11) that GHRH and LHRH analogs may manifest a broad range of activities and their pharmacologic profile cannot be limited to the down-regulation of their receptors, as occurs in prostate tumors and contributes to the well-known therapeutic effect of pharmacological castration (12).
The combination treatment of GHRH agonist and DOX also stimulated apoptosis and decreased the viability of the otherwise immortal U-87 cells in vitro (36, 37). Activation of programmed cell death may be related to several initiating events, such as the synthesis of the most important tumor suppressor p53, a protein whose gene is often mutated in GBMs (38, 39). Furthermore, we observed a decrease in the expressions of the anti-apoptotic Bcl-2 and increase in the proapoptotic BAD genes (39, 40). The impact of the combination on BAD, which triggers apoptosis by inactivating Bcl-2 and Bcl-xL (41), was completely opposite to the effect of DOX alone.
Moreover, in sharp contrast with DOX, JI-34 elicited a down-regulation of two important glial growth factors (42, 43), FGF basic and TGFβ. This may also reflect stem cell maturation and a tendency to increased cellular mortality, because growth promoting cytokines (42) are able to stimulate the survival cascades (44). The decrease in TGFβ is especially important, because this cytokine not only stimulates malignant transformation and growth (43), but also promotes angiogenesis, epithelial–mesenchymal transition (EMT), invasion, and suppresses peritumoral immune responses (45). Further, deprivation of growth factors (2) may suspend the constitutive stimulatory activity of extracellular signal-regulated kinases/mitogen activated protein kinases (ERK/MAPKs) over Bcl-2 (37). The combination significantly mitigated the transcription of the contact activator integrin domain, integrin α3 subunit. This molecule, as are other integrins, is frequently overexpressed in GMB tumors and plays an important role in the migration of neuroectodermal cells in both physiological and pathological circumstances (46). Upon binding laminin, fibronectin, or vitronectin (47), the integrin facilitates cell proliferation, EMT, and invasion through the activation of integrin-linked (48) and focal adhesion kinases (FAKs) (49). The down-regulation of the transcription of S100 calcium binding protein A4 (S100-A4) gene, which regulates microtubule polymerization and migration, may also contribute to the inhibition of cancer cell motility and metastatic spread. Moreover, S100-A4, beside activating motility, plays additional roles in proteolysis, EMT, angiogenesis, and cell survival (50).
Our demonstration that a change in the differentiation state of U-87 MG cells triggered by a GHRH agonist highly increases the susceptibility to cytotoxic treatments is particularly important for determining future strategies in the treatment of glioblastoma. GHRH analogs are promising candidates in this respect, as they readily bypass the blood–brain barrier (51). These findings suggest the potential for the development of new therapeutic paradigms in the treatment of brain cancers using agonistic as well as antagonistic analogs of hypothalamic GHRH. These GHRH analogs (2, 4, 6, 11, 20–22), may augment the direct cytotoxic effect of traditional chemotherapeutic drugs, by stimulation and maturation of stem cells and eliciting cellular synchronization of the cell cycle. To further investigate this phenomenon, other studies are necessary to assess the outcome of the combination of GHRH analogs and improved forms of chemotherapeutic agents such as liposomal doxorubicin.
More sophisticated pretreatment or cotreatment with GHRH agonists may further augment the effect of chemotherapeutic agents on malignancies. The impact on cell cycle is especially important, in this regard, because synchronization of dormant cells may make the tumor more susceptible to intercalating agents, such as DOX (52). To clarify these possibilities, further studies are needed.
Materials and Methods
Peptides and Chemicals.
The GHRH agonist, JI-34, was synthesized in our laboratory by solid-phase method and purified by reversed-phase HPLC as described previously (20). The structure of JI-34 is [Dat1, Orn12,21, Abu15,Nle27, Asp28, Agm29]hGH-RH-(1–29) (Abu, α-aminobutyric acid; Agm, agmatine; Dat, desaminotyrosine; Nle, norleucine; Orn, ornithine). For studies in vivo, we used daily s.c. injections of JI-34 dissolved in 0.1% DMSO (Sigma) in 10% (vol/vol) aqueous propylene glycol (vehicle solution). For in vitro experiments, JI-34 was dissolved in 0.1% DMSO and diluted with incubation medium. DOX (Chemtec Leuna) was administered i.v. and dissolved in 0.01 N acetic acid and diluted with 5% (wt/vol) mannitol.
Animal Experiments.
Six-wk-old nude mice (Ncr nu/nu) were obtained from the National Cancer Institute (Bethesda, MD). The animals were housed in sterile cages in a temperature-controlled room with a 12-h light/12-h dark schedule and were fed with autoclaved chow and water, ad libitum. The Institutional Animal Care and Use Committee, VA Medical Center Miami, fully approved the animal protocols. For the therapy study, 106 cells per mouse of the human glioblastoma cell line, U-87 MG (American Type Culture Collection, ATCC), were injected into the flanks of four nude mice (Ncr nu/nu) under isoflurane anesthesia (Baxter). A month later, the resulting tumors were harvested and minced into ∼3-mm3 pieces and transplanted into flanks of nude mice, under anesthesia, using a minitrocar. When the tumors reached ∼32 mm3, the mice were randomized into four groups. The animals received the following treatments for 6 wk: (Group 1) control, 16 tumors, vehicle solution; (group 2) agonist JI-34, 1 μg/20 g, s.c., daily, 19 tumors; (group 3) DOX, 260 nmol/20 g, i.v., weekly on Thursdays, 19 tumors; and (group 4) JI-34 (1 μg/20 g daily) + DOX (260 nmol/20 g), 16 tumors. Tumor volume was measured with microcalipers once a week and calculated using the formula: (length × width × height × π)/6. Tumor doubling time was calculated using the formula: [study duration × logarithm (LOG) 2]/(LOG final tumor volume − LOG initial tumor volume). At the end of the experiment, the mice were killed under pentobarbital anesthesia, tumors were excised and weighed; necropsy was performed. Samples free of necrotic debris were immediately snap frozen in liquid nitrogen and stored at −80 °C for further analyses.
Cell Maintenance and Cell Counting.
U-87 MG cells were cultured in Eagle's minimum essential medium (ATCC) medium [supplemented with 10% FBS (ATCC) and 0.1% penicillin/streptomycin] at 37 °C and 5% CO2 atmosphere. For cell count and cell size determination, 103 cells were seeded into T-25 flasks. The medium was changed two to three times a week. The following treatment in vitro groups were set up: (Group 1) control, vehicle solution; (group 2) JI-34 at 1 μM final concentration, daily; (group 3) DOX at 100 nM final concentration, weekly on Thursdays; and (group 4) JI-34, 1 μM and DOX, 100 nM. After 3 wk, cell count and cell size were determined by Z Series Coulter Counter (Beckman Coulter).
Cell Volume Determination.
Cell volume was estimated by measuring the intracellular water space by the method of Kletzein et al. (53), as modified by Bender and Norenberg (54). Briefly, 1 mM 3-O-methylglucose (3-OMG) and 0.5 µCi/mL [3H]-3-OMG were added to the culture 6 h before the volume assay. At the end of the incubation period, culture medium was aspirated, and an aliquot was saved for radioactivity determination. Cells were washed rapidly six times with ice-cold buffer containing 229 mM sucrose, 1 mM Tris-nitrate, 0.5 mM calcium nitrate, and 0.1 mM phloretin, pH 7.4. Cells were harvested into 0.5 mL of 1 N sodium hydroxide. Radioactivity in the cell extracts and media was determined, and an aliquot of the cell extract was used for protein estimation with the Bio-Rad bicinchoninic acid kit. Values were normalized to protein level, and cell volume was expressed as microliters/milligram protein.
Proliferation Assays in Vitro.
For proliferation studies, 104 cells per well were seeded in 100 μL media, in a 96-well plate, and incubated for 24 h in a humidified incubator at 37 °C. Then, culture medium was replaced with FBS-free medium (starvation) for 24 h. After 24 h, the cells received full medium containing the following treatments: Groups 1 (control) and 3, vehicle solution; groups 2 and 4, JI-34 (1 μM final concentration). After another 24 h, the cells received the following treatments: group 1, vehicle solution; group 2, JI-34; group 3, DOX (100 nM final concentration); and group 4, JI-34 + DOX. Then, they were incubated for 48 h and the effect on cell proliferation was evaluated by using the MTT assay (CellTiter 96 Non-Radioactive Cell Proliferation Assay; Promega), according to the manufacturer's instructions with the help of a Victor3 multilabel counter (Perkin-Elmer) (13).
Apoptosis Assay.
Determinations of viability and apoptosis were performed from freshly seeded cell samples (104 cells per well, in 100 μL media, in a 96-well plate) with the help of ApoLive-Glo Multiplex Assay (Promega), according to the manufacturer’s instructions. First the cells received full medium containing the following treatments: Groups 1 (control) and 3, vehicle solution; groups 2 and 4, JI-34 (1 μM final concentration). After 24 h, the cells received the following treatments: group 1, (vehicle solution); group 2, JI-34; group 3, DOX (100 nM final concentration); and group 4, JI-34 + DOX. After another 24 h of incubation, viability reagent was added to the wells and fluorescence was measured by Victor3 multilabel counter. Then Caspase-Glo 3/7 apoptosis reagent was added and luminescence was determined.
Immunocytochemistry.
Cells were seeded onto coverslips in six-well plates (50,000 cells per well) in 10% FBS-containing growth medium. This medium was replaced with serum-free medium the following day for 24 h. Thereafter, GHRH agonist (JI-34, 1 µM) was added in medium supplemented with 0.1% FBS and remained for the consecutive 2 d. Control cells received vehicle (DMSO). At the end of the treatment, phase-contrast images of live cells were collected and cells were fixed in ice-cold acetone for 10 min, washed with PBS three times, and blocked with 2% goat serum in PBS for 30 min. GFAP (Abcam; 1:500 dilution) or nestin (BD Transduction Laboratories; 1:75 dilution) antibodies were added in PBS for 1 h. Anti-rabbit and anti-mouse secondary antibodies (Alexa Fluor 488; Jackson Immunoresearch) were also applied for 1 h. Coverslips were mounted in Vectashield mounting medium containing DAPI for nuclear staining (Vector Laboratories). Images were acquired on a Nikon Eclipse Ti fluorescence microscope (Nikon Instruments).
MDR Assay.
The multidrug resistance assays were performed according to the manufacturer’s instructions (Cayman Chemical). U-87 MG cells were seeded, 5 × 104 cells per well density in 100 μL medium in 96-well, black, clear-bottom plates and grown overnight in a humidified incubator at 37 °C. The next day the medium was discarded, and the cells were treated according to the following protocol: Groups 1 (control) and 3, vehicle solution; groups 2 and 4, JI-34 (1 μM final concentration). After another 24 h, the cells received the following treatments: Group 1, vehicle solution; group 2, JI-34; group 3, DOX (100 nM final concentration); and group 4, JI-34 + DOX. During the second treatment, half of the control wells were treated with cyclosporin-A solution in 1/1,000 dilution as positive control according to the manufacturer’s description. Afterward, the cells were incubated for 1 h, then calcein AM/Hoechst dye combined staining solution was added. Fifteen minutes later, both cell density (at excitation and emission wavelengths of 355 nm and 465 nm, respectively) and calcein retention (at excitation and emission wavelengths of 485 nm and 535 nm, respectively) were detected with the help of a Victor3 multilabel counter. Relative calcein retention values were expressed as a function of cell density.
Total RNA Isolation and Reverse Transcription.
Total RNA was isolated from representative, DNase treated, U-87 MG dissected tumor samples using a NucleoSpin kit according to the manufacturer’s instructions (Macherey-Nagel). Four tumor samples from each group were analyzed. The yield and the quality of RNA samples were determined spectrophotometrically using 260 nm, and 260/280- and 260/230-nm ratio. The synthesis of cDNA was performed as described (55). Briefly, 1 µg of RNA from each sample was reverse transcribed into cDNA by a RT First Strand kit (Qiagen). Reverse transcription was done in a Veriti 96-well thermal cycler (Applied Biosystems).
Cancer Pathway Finder Quantitative PCR Array.
The Human Cancer PathwayFinder quantitative PCR array (PAHS-033A; Qiagen) used in our study contains 84 unique genes related to cell proliferation, apoptosis, cell cycle, angiogenesis, invasion, and metastasis. All PCR arrays were performed using the iQ5 Multicolor Real-Time Detections system (Bio-Rad). All genes represented by the array showed a single peak on the melting curve characteristic of the specific products. Experiments were run in triplicate for each study group. Analysis of gene expression data was performed using Excel-based PCR Array Data Analysis software provided by the manufacturer (Qiagen). Fold changes in gene expression were calculated using the ΔΔCt method and five stably expressed housekeeping genes (B2M, HPRT1, RPL13A, GAPDH, and ACTB) were used for normalization of the results.
Statistical Analyses.
Statistical analyses were performed using either t test for independent samples, univariate analysis of variance (ANOVA), or repeated measure ANOVA (RMANOVA). ANOVA was followed by Tukey's or Fisher's post hoc test, while RMANOVA was followed by Fisher's post hoc test. Results are expressed as the means ± SEM. Differences, compared with the control, with P < 0.05 considered as statistically significant. Statistical analyses and data reductions were performed by SigmaPlot 11.0 (Systat software) and IBM SPSS Statistics 20.0.
Additional information is provided in SI Text.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Service of the Veterans Affairs Department (A.V.S.); Departments of Pathology and Medicine, Division of Hematology/Oncology, University of Miami, Miller School of Medicine (A.V.S.); and the South Florida Veterans Affairs Foundation for Research and Education (A.V.S.) and the L. Austin Weeks Endowment for Urologic Research (N.L.B.). The work of F.G.R. was supported in part by a grant from the Urology Care Foundation Research Scholars Program and the American Urological Association, Southeastern Section. The support of the European Union and the government of Hungary, cofinanced by the European Social Fund in the framework of Tarsadalmi Megujulas Operativ Program 4.2.2-A-11/1/KONV-2012-0052 (to M.J.), is also acknowledged. P.P. was supported by a stipend program of the Department of Medicine, Dresden and by the Helmholtz Alliance Imaging and Curing Environmental Metabolic Diseases through the Initiative and Networking Fund of the Helmholtz Association.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322622111/-/DCSupplemental.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4(1):33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 3.Engel J, Emons G, Pinski J, Schally AV. AEZS-108: A targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21(6):891–899. doi: 10.1517/13543784.2012.685128. [DOI] [PubMed] [Google Scholar]
- 4.Schally AV, et al. Hypothalamic hormones and cancer. Front Neuroendocrinol. 2001;22(4):248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 5.Schally AV, Nagy A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol Metab. 2004;15(7):300–310. doi: 10.1016/j.tem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40(5):315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 7.Nagy A, Schally AV. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biol Reprod. 2005;73(5):851–859. doi: 10.1095/biolreprod.105.043489. [DOI] [PubMed] [Google Scholar]
- 8.Stangelberger A, Schally AV, Djavan B. New treatment approaches for prostate cancer based on peptide analogues. Eur Urol. 2008;53(5):890–900. doi: 10.1016/j.eururo.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Szepeshazi K, et al. Receptor-targeted therapy of human experimental urinary bladder cancers with cytotoxic LH-RH analog AN-152 [AEZS- 108] Oncotarget. 2012;3(7):686–699. doi: 10.18632/oncotarget.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaszberenyi M, et al. Inhibition of U-87 MG glioblastoma by AN-152 (AEZS-108), a targeted cytotoxic analog of luteinizing hormone-releasing hormone. Oncotarget. 2013;4(3):422–432. doi: 10.18632/oncotarget.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaszberenyi M, et al. Suppression of the proliferation of human U-87 MG glioblastoma cells by new antagonists of growth hormone-releasing hormone in vivo and in vitro. Target Oncol. 2013;8(4):281–290. doi: 10.1007/s11523-013-0264-y. [DOI] [PubMed] [Google Scholar]
- 12.Engel JB, Schally AV. Drug insight: Clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat Clin Pract Endocrinol Metab. 2007;3(2):157–167. doi: 10.1038/ncpendmet0399. [DOI] [PubMed] [Google Scholar]
- 13.Pozsgai E, Schally AV, Halmos G, Rick F, Bellyei S. The inhibitory effect of a novel cytotoxic somatostatin analogue AN-162 on experimental glioblastoma. Horm Metab Res. 2010;42(11):781–786. doi: 10.1055/s-0030-1261955. [DOI] [PubMed] [Google Scholar]
- 14.Kiaris H, Schally AV, Varga JL, Groot K, Armatis P. Growth hormone-releasing hormone: An autocrine growth factor for small cell lung carcinoma. Proc Natl Acad Sci USA. 1999;96(26):14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciampani T, Fabbri A, Isidori A, Dufau ML. Growth hormone-releasing hormone is produced by rat Leydig cell in culture and acts as a positive regulator of Leydig cell function. Endocrinology. 1992;131(6):2785–2792. doi: 10.1210/endo.131.6.1332849. [DOI] [PubMed] [Google Scholar]
- 16.Moretti C, Bagnato A, Solan N, Frajese G, Catt KJ. Receptor-mediated actions of growth hormone releasing factor on granulosa cell differentiation. Endocrinology. 1990;127(5):2117–2126. doi: 10.1210/endo-127-5-2117. [DOI] [PubMed] [Google Scholar]
- 17.Barabutis N, Schally AV. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 18.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22(8):311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Krebs LJ, et al. Regulation of targeted chemotherapy with cytotoxic lutenizing hormone-releasing hormone analogue by epidermal growth factor. Cancer Res. 2000;60(15):4194–4199. [PubMed] [Google Scholar]
- 20.Izdebski J, et al. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1995;92(11):4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107(28):12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107(6):2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi F, Saya H, Bruner JM, Morrison RS. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci USA. 1994;91(2):484–488. doi: 10.1073/pnas.91.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison RS, et al. Basic fibroblast growth factor and fibroblast growth factor receptor I are implicated in the growth of human astrocytomas. J Neurooncol. 1994;18(3):207–216. doi: 10.1007/BF01328955. [DOI] [PubMed] [Google Scholar]
- 25.Joseph JV, Balasubramaniyan V, Walenkamp A, Kruyt FA. TGF-β as a therapeutic target in high grade gliomas: Promises and challenges. Biochem Pharmacol. 2013;85(4):478–485. doi: 10.1016/j.bcp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 27.Eyler CE, Rich JN. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26(17):2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying M, et al. Regulation of glioblastoma stem cells by retinoic acid: Role for Notch pathway inhibition. Oncogene. 2011;30(31):3454–3467. doi: 10.1038/onc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohla F, et al. Synergistic inhibition of growth of lung carcinomas by antagonists of growth hormone-releasing hormone in combination with docetaxel. Proc Natl Acad Sci USA. 2006;103(39):14513–14518. doi: 10.1073/pnas.0605309103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchholz S, et al. Potentiation of mammary cancer inhibition by combination of antagonists of growth hormone-releasing hormone with docetaxel. Proc Natl Acad Sci USA. 2007;104(6):1943–1946. doi: 10.1073/pnas.0610860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitz S, et al. Combination of GHRH antagonists and docetaxel shows experimental effectiveness for the treatment of triple-negative breast cancers. Oncol Rep. 2013;30(1):413–418. doi: 10.3892/or.2013.2435. [DOI] [PubMed] [Google Scholar]
- 32.de Thé H, Chen Z. Acute promyelocytic leukaemia: Novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10(11):775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 33.Kufe DW, Holland JF, Frei E. Cancer Medicine 6. 6th Ed. Canada: BC Decker, Hamilton, ON; 2003. American Cancer Society. [Google Scholar]
- 34.Stüttgen G. Historical perspectives of tretinoin. J Am Acad Dermatol. 1986;15(4 Pt 2):735–740. doi: 10.1016/s0190-9622(86)70228-4. [DOI] [PubMed] [Google Scholar]
- 35.Mawson AR. Retinoids in the treatment of glioma: A new perspective. Cancer Manag Res. 2012;4:233–241. doi: 10.2147/CMAR.S32449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443(7113):796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igney FH, Krammer PH. Death and anti-death: Tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 38.Cox LS. Multiple pathways control cell growth and transformation: Overlapping and independent activities of p53 and p21Cip1/WAF1/Sdi1. J Pathol. 1997;183(2):134–140. doi: 10.1002/(SICI)1096-9896(199710)183:2<134::AID-PATH960>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 39.Stegh AH, DePinho RA. Beyond effector caspase inhibition: Bcl2L12 neutralizes p53 signaling in glioblastoma. Cell Cycle. 2011;10(1):33–38. doi: 10.4161/cc.10.1.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Lisanti MP, Liao DJ. Reviewing once more the c-myc and Ras collaboration: Converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle. 2011;10(1):57–67. doi: 10.4161/cc.10.1.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurley SN, et al. Mechanism of anti-glioma activity and in vivo efficacy of the cannabinoid ligand KM-233. J Neurooncol. 2012;110(2):163–177. doi: 10.1007/s11060-012-0958-5. [DOI] [PubMed] [Google Scholar]
- 42.Jeuken J, van den Broecke C, Gijsen S, Boots-Sprenger S, Wesseling P. RAS/RAF pathway activation in gliomas: The result of copy number gains rather than activating mutations. Acta Neuropathol. 2007;114(2):121–133. doi: 10.1007/s00401-007-0239-0. [DOI] [PubMed] [Google Scholar]
- 43.Golestaneh N, Mishra B. TGF-beta, neuronal stem cells and glioblastoma. Oncogene. 2005;24(37):5722–5730. doi: 10.1038/sj.onc.1208925. [DOI] [PubMed] [Google Scholar]
- 44.Rosenwald IB. The role of translation in neoplastic transformation from a pathologist’s point of view. Oncogene. 2004;23(18):3230–3247. doi: 10.1038/sj.onc.1207552. [DOI] [PubMed] [Google Scholar]
- 45.Wick W, Naumann U, Weller M. Transforming growth factor-beta: A molecular target for the future therapy of glioblastoma. Curr Pharm Des. 2006;12(3):341–349. doi: 10.2174/138161206775201901. [DOI] [PubMed] [Google Scholar]
- 46.Tsuji T. Physiological and pathological roles of alpha3beta1 integrin. J Membr Biol. 2004;200(3):115–132. doi: 10.1007/s00232-004-0696-5. [DOI] [PubMed] [Google Scholar]
- 47.Ritchie CK, Giordano A, Khalili K. Integrin involvement in glioblastoma multiforme: Possible regulation by NF-kappaB. J Cell Physiol. 2000;184(2):214–221. doi: 10.1002/1097-4652(200008)184:2<214::AID-JCP9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 48.Koul D, et al. Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol Cancer Ther. 2005;4(11):1681–1688. doi: 10.1158/1535-7163.MCT-05-0258. [DOI] [PubMed] [Google Scholar]
- 49.Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am J Pathol. 2005;167(5):1379–1387. doi: 10.1016/S0002-9440(10)61225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boye K, Maelandsmo GM. S100A4 and metastasis: A small actor playing many roles. Am J Pathol. 2010;176(2):528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaeger LB, Banks WA, Varga JL, Schally AV. Antagonists of growth hormone-releasing hormone cross the blood-brain barrier: A potential applicability to treatment of brain tumors. Proc Natl Acad Sci USA. 2005;102(35):12495–12500. doi: 10.1073/pnas.0504163102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang XL, Wang AH. Structural studies of atom-specific anticancer drugs acting on DNA. Pharmacol Ther. 1999;83(3):181–215. doi: 10.1016/s0163-7258(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 53.Kletzien RF, Pariza MW, Becker JE, Potter VR. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- 54.Bender AS, Norenberg MD. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J Neurosci Res. 1998;54(5):673–680. doi: 10.1002/(SICI)1097-4547(19981201)54:5<673::AID-JNR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Rick FG, et al. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108(9):3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.