Significance
In the transition from a fossil-based to a bio-based economy, bioethanol will be generated from the lignocellulosic biomass of second-generation biofuel crops, such as poplar. The lignin polymers in the plant cell walls represent the main factor determining the recalcitrance of biomass to enzymatic processing. We have grown genetically modified poplars, down-regulated for cinnamoyl-CoA reductase (CCR), an enzyme in the lignin biosynthetic pathway, in field trials in Belgium and France. We show that wood samples derived from the transgenic trees are more easily processed into ethanol. However, strong down-regulation also affected biomass yield. In conclusion, CCR down-regulation may become a successful strategy to improve biomass processing if the yield penalty can be overcome.
Keywords: bioethanol, GM, second-generation bioenergy
Abstract
Lignin is one of the main factors determining recalcitrance to enzymatic processing of lignocellulosic biomass. Poplars (Populus tremula x Populus alba) down-regulated for cinnamoyl-CoA reductase (CCR), the enzyme catalyzing the first step in the monolignol-specific branch of the lignin biosynthetic pathway, were grown in field trials in Belgium and France under short-rotation coppice culture. Wood samples were classified according to the intensity of the red xylem coloration typically associated with CCR down-regulation. Saccharification assays under different pretreatment conditions (none, two alkaline, and one acid pretreatment) and simultaneous saccharification and fermentation assays showed that wood from the most affected transgenic trees had up to 161% increased ethanol yield. Fermentations of combined material from the complete set of 20-mo-old CCR–down-regulated trees, including bark and less efficiently down-regulated trees, still yielded ∼20% more ethanol on a weight basis. However, strong down-regulation of CCR also affected biomass yield. We conclude that CCR down-regulation may become a successful strategy to improve biomass processing if the variability in down-regulation and the yield penalty can be overcome.
Global warming and the depletion of fossil fuels provide a major impetus for the increased interest in renewable energy sources. Liquid biofuels, bioethanol in particular, are currently produced from the freely accessible sucrose in sugarcane and from starch in maize grain but suffer from the unfavorable ramifications of the “food versus fuel” debate. Second-generation biofuels derive from lignocellulosic biomass in dedicated energy crops (poplar, switchgrass, Miscanthus sp., and others) that can be grown on marginal lands with less fertilizer for multiple annual cycles, or from the currently underused lignocellulosic residues from crop plants, such as corn, wheat, and sugarcane bagasse. Therefore, the energy output and carbon savings are expected to be much higher than those of first-generation biofuel crops (1, 2). However, the polysaccharides in lignocellulosic biomass are not readily enzyme-accessible mainly because of the presence of lignin. To improve the accessibility of the polysaccharides for enzymatic digestion, the biomass is pretreated, typically with acid or alkali, to disrupt the bonds between lignin and hemicelluloses, or to break down and/or remove the lignin itself (3). Engineering plants to produce less lignin or a lignin structure enriched in easily degradable bonds has become an important research objective (4). Lignin is a heteropolymer formed mainly from the monolignols coniferyl and sinapyl alcohol and to a lesser extent from p-coumaryl alcohol, resulting in guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) units when incorporated into the lignin polymer, respectively (5–7). Cinnamoyl-CoA reductase (CCR) catalyzes the first step of the monolignol-specific pathway. It converts the hydroxycinnamoyl-CoA esters to their corresponding hydroxycinnamaldehydes (mainly feruloyl-CoA to coniferaldehyde), and down-regulation of CCR typically results in reduced lignin content (8–13). CCR–down-regulated poplars are characterized by an orange to wine-red coloration of the xylem that often appears in patches along the stem. This pronounced coloration is associated with a reduction in lignin amount and the incorporation of low levels of ferulic acid into the polymer (13, 14).
As lignin is the most important factor limiting the conversion of plant biomass to fermentable sugars (15–17), we have evaluated whether wood from transgenic poplar, down-regulated in CCR, is easier to process into ethanol. Field trials were established in Belgium and France after a long process of obtaining regulatory permission (18). Field trials are an essential step in translating fundamental knowledge generated in the laboratory to conditions closer to industrial exploitation because greenhouse-derived data cannot a priori be extrapolated to field-grown trees without experimentation. For example, greenhouse-grown trees do not experience the annual cycles of growth and dormancy, implying that they continuously develop a relatively large zone of lignifying—i.e., not fully lignified—xylem, whereas xylem of field-grown trees matures and becomes structurally different at the end of the season. Furthermore, field trials allow the trees to be grown as short-rotation coppice, a practice that is preferred when trees are cultivated for bioenergy purposes (19). Publicly accessible databases reveal that more than 700 field-trial applications with genetically modified (GM) trees (including forest trees, fruit trees, and wood perennials) have been made worldwide (20), but data on very few field trials have been published (21). Only one field trial, with transgenic 4-coumarate:CoA ligase (4CL)–down-regulated poplar, has been evaluated for biofuel applications, but no improvement in saccharification efficiency was observed (22). Improvements in ethanol yields have been demonstrated for C3H-deficient and F5H-overexpressing poplars although these were greenhouse-grown (23). Here, we show that reducing CCR expression in field-grown poplar in two locations in Europe improves biomass processing into glucose and ethanol, but the associated yield penalty has to be overcome to advance translation to industrial applications.
Results
Down-Regulation of CCR Improves Saccharification Yield.
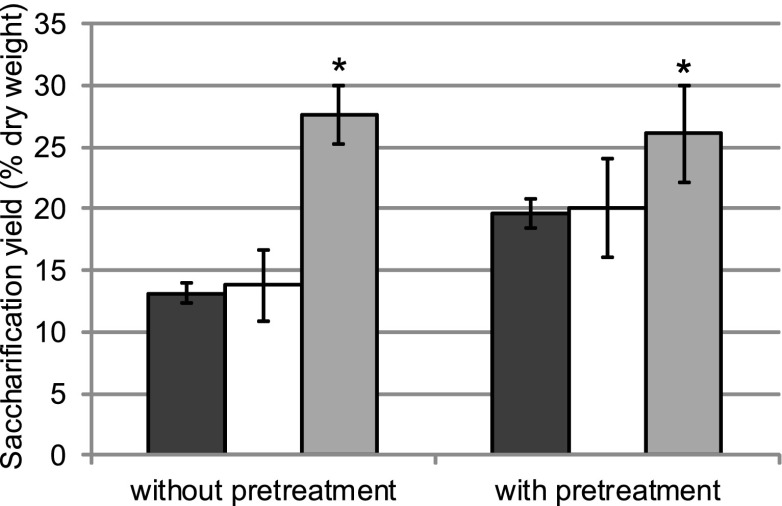
Down-regulation of CCR was obtained by transforming poplar with sense (lines FS3 and FS40) and antisense constructs (line FAS13) (13). These lines, along with the wild type (WT) controls, were micropropagated and grown in the greenhouse for 6 mo. Upon removing the bark, the typical red coloration of the xylem was visible and appeared in patches in some trees of line FAS13, whereas trunks of FS3 and FS40 were more uniform in color. Accordingly, preliminary saccharification experiments of debarked stems without and with an acid (HCl) pretreatment indicated that FS3 and FS40 had the highest saccharification yields (Fig. S1). To compare saccharification yields in red versus white areas of the same trunk, separate white- and red-colored xylem of FAS13 was scraped from the stems and compared with scraped xylem from the WT. Whether or not a pretreatment was performed, the saccharification yield of white xylem from the CCR-deficient trees did not differ substantially from that of WT (Fig. 1). In contrast, saccharification of red xylem yielded significantly more glucose compared with white xylem and xylem of WT, both with and without pretreatment. As expected, and in agreement with Leplé et al. (13), lignin content in the scraped red xylem (10.9 ± 1.1%) was 19% reduced compared with that of WT (13.5 ± 0.2%), whereas that of white xylem (13.4 ± 1.6%) was not reduced.
Fig. 1.
Saccharification yields of xylem. Saccharification yields after 48 h, without and with acid (HCl) pretreatment, of scrapings of greenhouse-grown WT poplar (n = 2) and white and red xylem of greenhouse-grown FAS13 (n = 7). Error bars represent SDs. *P < 0.01. Dark gray, WT; white, white xylem of FAS13; light gray, red xylem of FAS13.
Field Trial in Belgium.
Next, we requested regulatory permission to establish a field trial with lines FS3, FS40, and WT. The field was established in Belgium and consisted of six randomized blocks for each line, each block consisting of 20 clonally propagated trees (Fig. 2A and Fig. S2A). Ten months after planting, stems were cut back at the end of the winter, and ∼20 cm of the basal part of the stems was sampled, debarked, and scored for the red phenotype. As already observed in greenhouse-grown trees, the red xylem appeared often in patches, because of unequal levels of gene silencing in red and white areas (13). The data obtained from greenhouse-grown trees had indicated that saccharification yield was only increased in red xylem, but was comparable to that of WT in white xylem. Therefore, to investigate whether the red xylem that had developed in field-grown transgenic trees was also more easily converted into fermentable sugars, we first grouped the wood samples into six different redness classes, depending on the red surface area of the stem pieces (Fig. 2C). For FS40, every single debarked trunk piece had a red phenotype, either in patches or fully red, whereas FS3 appeared to be less down-regulated in CCR and had more trees that were completely white. Then, the saccharification yield was analyzed for debarked wood samples of WT and the three most red classes (class 3, 4, and 5) of both transgenic lines, thus bypassing the issue of unequal gene silencing. The highest saccharification yields, for both transgenic lines and independent of the applied treatment, were usually observed for debarked wood of fully red stems (i.e., class 5) (Fig. S3A). However, the harsher the NaOH pretreatment was, the lower was the increase in saccharification yield compared with the WT (Table 1 and Fig. S3B).
Fig. 2.
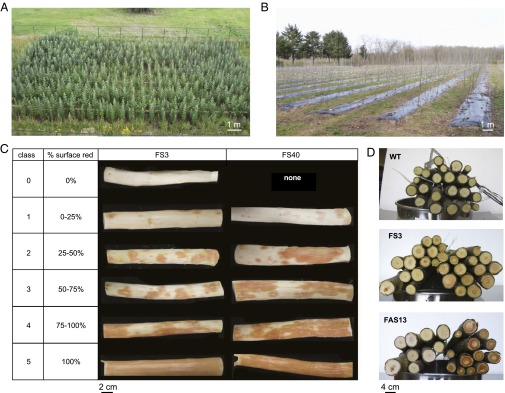
Pictures of the Belgian and French field trials. (A) Field trial in Belgium (July 2009). (B) Field trial in France just before harvest (March 2010). (C) Classification and illustration of the variegated red phenotype observed for the transgenic trees in the Belgian field trial. (D) Illustration of the variegated red coloration in cross-sections for WT (Top), FS3 (Middle), and FAS13 (Bottom) from the French field trial.
Table 1.
Saccharification data of wood from both field trials
| Line | Pretreatment | Saccharification yield, % dry weight | Increase in saccharification yield compared to WT, % | Cellulose conversion, % cellulose | Increase in cellulose conversion compared to WT, % |
| Belgian trial | |||||
| FS3 | None | 10.5 ± 2.5 | 14.1 | 23.3 ± 3.9 | 39.5 |
| 0.4 M H2SO4 | 26.5 ± 4.3 | 18.8 | 55.7 ± 5.2 | 28.3 | |
| 6.25 mM NaOH | 32.0 ± 5.4 | 14.7 | 67.6 ± 7.0 | 31.8 | |
| 62.5 mM NaOH | 39.3 ± 4.7 | 11.3 | 79.3 ± 6.8 | 15.1 | |
| FS40 | None | 12.8 ± 2.3 | 39.1 | 25.4 ± 4.6 | 52.1 |
| 0.4 M H2SO4 | 28.3 ± 3.2 | 26.9 | 56.7 ± 5.0 | 30.6 | |
| 6.25 mM NaOH | 33.8 ± 4.9 | 21.1 | 68.7 ± 9.7 | 33.9 | |
| 62.5 mM NaOH | 39.3 ± 4.8 | 11.3 | 79.7 ± 9.3 | 15.7 | |
| WT | None | 9.2 ± 1.2 | 16.7 ± 1.7 | ||
| 0.4 M H2SO4 | 22.3 ± 3.5 | 43.4 ± 4.5 | |||
| 6.25 mM NaOH | 27.9 ± 3.3 | 51.3 ± 4.1 | |||
| 62.5 mM NaOH | 35.3 ± 2.6 | 68.9 ± 4.0 | |||
| French trial | |||||
| FS3 | None | 9.9 ± 2.2 | 25.3 | 19.9 ± 7.2 | 17.1 |
| 0.4 M H2SO4 | 18.1 ± 3.5 | 33.1 | 36.2 ± 10.4 | 23.5 | |
| 6.25 mM NaOH | 23.6 ± 3.1 | 24.9 | 46.4 ± 12.4 | 14.3 | |
| 62.5 mM NaOH | 28.6 ± 3.3 | 14.9 | 56.4 ± 14.5 | 5.6 | |
| FAS13 | None | 18.9 ± 3.8 | 139.2 | 34.7 ± 8.2 | 104.1 |
| 0.4 M H2SO4 | 26.5 ± 2.9 | 94.9 | 48.3 ± 5.7 | 64.8 | |
| 6.25 mM NaOH | 33.7 ± 2.6 | 78.3 | 60.6 ± 5.6 | 49.3 | |
| 62.5 mM NaOH | 36.2 ± 2.1 | 45.4 | 65.2 ± 5.4 | 22.1 | |
| WT | None | 7.9 ± 1.9 | 17.0 ± 5.2 | ||
| 0.4 M H2SO4 | 13.6 ± 2.1 | 29.3 ± 6.8 | |||
| 6.25 mM NaOH | 18.9 ± 2.6 | 40.6 ± 10.1 | |||
| 62.5 mM NaOH | 24.9 ± 2.3 | 53.4 ± 12.0 |
Saccharification yields are the mean (± SD) for all trees of redness class 5 for the Belgian field trial whereas cellulose measurements were performed on selected individual trees only (Table S1) (WT, n = 6; FS3, n = 6; FS40, n = 7). To calculate cellulose conversions, the specific saccharification yields of those individual trees for which cellulose was measured were used. For each of the five blocks in the French field trial, saccharification yield was determined for the five most red trees per transgenic line and five randomly chosen WT trees. For the French field trial, cellulose conversion was determined for pooled trees (Table S1) (WT, n = 10; FS3, n = 10; FAS13, n = 10). The cell-wall residue (expressed as % dry weight) was taken into account to calculate the cellulose conversions. Bold, significantly increased compared with WT.
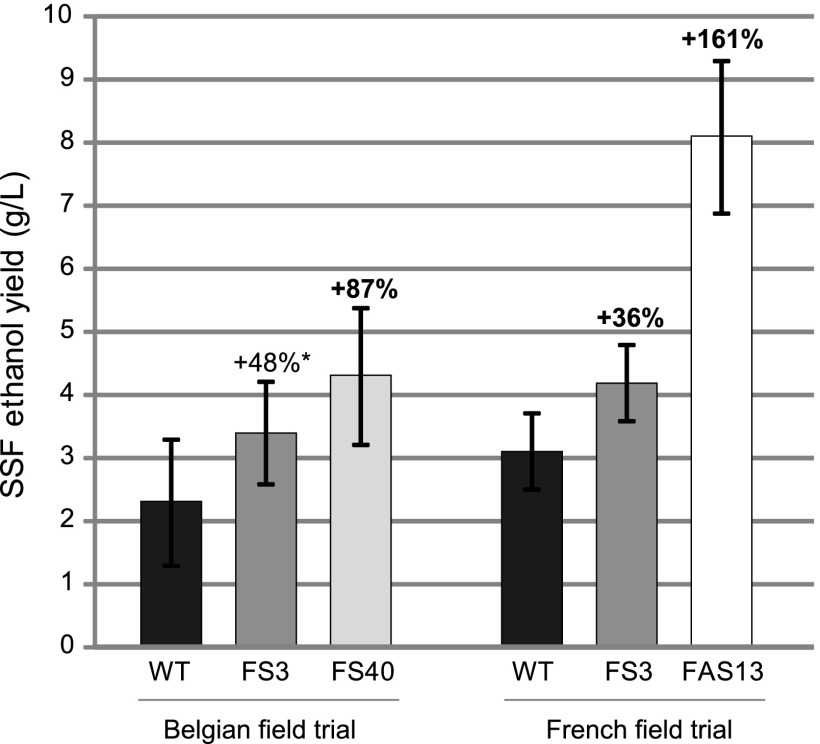
To test whether and to what extent (upper bound) the improved saccharification translated into higher ethanol yields, a selection was made of fully red tree samples, which were as much as possible derived from trees spread over the entire field (Table S1). These samples were analyzed by a simultaneous saccharification and fermentation (SSF) assay. The ethanol yields of FS3 and FS40 were 48% and 87% increased over that of the WT, respectively (Fig. 3).
Fig. 3.
Ethanol yield (g/L) for the Belgian and French field trials. For both locations, SSF was performed on pooled trees (Table S1) (Belgian trial: WT, n = 6; FS3, n = 5; FS40, n = 6; French trial: WT, n = 10; FS3, n = 10; FAS13, n = 10). Ethanol yields were measured after 31 h and 28 h of SSF for the Belgian and French field trials, respectively. Error bars represent SDs. Bold, significantly increased compared to WT. *P = 0.08.
Only trees that had fully red stems (i.e., class 5) were significantly reduced in height by 27% (FS3) and 17% (FS40), and the diameter was only decreased for stems of class 5 of line FS3, by 21% (Fig. S4 A and B). The biomass with or without bark of the basal 20-cm stem segments was only decreased for FS3 class 5 by 48% and 51%, respectively (Fig. S4 C and D). If the reduction in biomass of the 20-cm stem segments used for SSF was taken into account, FS3 and FS40 yielded the same amount of ethanol as WT.
Field Trial in France.
CCR-deficient poplars were grown in another field trial in France (Fig. 2B). One transgenic line, FS3, was common to both field trials. The French field trial also included FAS13 and WT poplar. The field itself was divided into five blocks with 24 clonal replicates in each block for each line (Fig. S2B). Twenty months after planting, individual stems were harvested in a similar way as those from the Belgian field trial. Again, to circumvent the inequality of gene silencing and to determine the upper limit of improvement, for each of the five blocks in the field, the five most red trees for both transgenic lines and five WT trees were selected for saccharification and SSF analysis (Fig. 2D).
Similarly to in the Belgian field trial, both transgenic lines, FS3 and FAS13, had considerably increased saccharification yields per gram dry biomass compared with WT for all pretreatment conditions (Table 1). The increase in saccharification yield for FS3 was higher than that observed for FS3 trees from the Belgian field trial. However, FAS13 outperformed the other lines and even reached more than a doubling in saccharification yield without pretreatment. The increased saccharification yield translated into increased ethanol yields of 36% for FS3 up to 161% for FAS13, as demonstrated by SSF (Fig. 3). The weight of the main stems of all individual trees per line and per block was determined (Fig. S4E). The transgenic lines FS3 and FAS13 showed an average reduction in biomass yield of 16% and 24%. Considering the biomass penalty only for those trees that had been selected for the SSF experiments, i.e., 18% (FS3) and 40% (FAS13), and taking the SSF ethanol yield of the stem segments of these trees as a proxy for the SSF yield of the entire tree, the biomass yield penalty of FS3 outweighed the improvement in ethanol yield per gram biomass, similarly to FS3 grown in the Belgian field. However, FAS13 still had a 57% increase in ethanol yield compared with WT.
The Improved Saccharification Yield Is Due to a Higher Cellulose Conversion and Correlates with the Abundance of Ferulic Acid Markers.
The improved saccharification yield observed could be due to higher cellulose levels in the transgenic lines or to a higher cellulose conversion. The cellulose content of the transgenic trees from the Belgian and French trials was similar to that of the WT (Table 2). Therefore, the cellulose fraction of all transgenic lines was more efficiently hydrolyzed than that of WT (Table 1). FAS13 had, under each pretreatment condition, the highest increase in cellulose conversion compared with WT and reached a doubled cellulose conversion when no pretreatment was included in the saccharification assays.
Table 2.
Wood composition of transgenics and WT from both field trials
| Composition | Belgian trial |
French trial |
||||
| FS3 | FS40 | WT | FS3 | FAS13 | WT | |
| ABSL (% CWR) | 16.4 ± 1.2 | 16.6 ± 0.6 | 16.5 ± 0.9 | 18.2 ± 0.6 | 16.3 ± 1.1 | 18.6 ± 0.6 |
| Klason (% CWR) | 19.6 ± 1.9 | 19.1 ± 1.8 | 20.7 ± 1.9 | 19.8 ± 1.9 | 16.6 ± 1.6 | 21.7 ± 1.9 |
| H + G + S (µmol/g Klason) | 2096.4 ± 278.4 | 2078.0 ± 267.1 | 2305.8 ± 285.6 | 2254.0 ± 247.5 | 1588.0 ± 224.7 | 2430.4 ± 286.1 |
| H/G/S (mol%) | 1.3/33.6/65.1 ± 0.7/2.2/2.0 | 1.2/33.5/65.2 ± 0.5/2.4/2.1 | 1.7/33.7/64.6 ± 0.6/1.7/1.5 | 0.8/32.3/66.9 ± 0.2/1.1/1.2 | 0.8/34.4/64.8 ± 0.1/0.7/0.7 | 0.8/32.8/66.4 ± 0.0/1.7/1.7 |
| FA x 10−4 (area/(IS x mg CWR)) | 3.0 ± 2.1 | 6.2 ± 1.9 | 1.0 ± 0.3 | 4.3 ± 0.7 | 18.0 ± 4.1 | 1.2 ± 2.0 |
| AG x 10−4 (area/(IS x mg CWR)) | 9.4 ± 7.3 | 16.1 ± 6.6 | 3.7 ± 1.3 | 13.8 ± 4.6 | 25.6 ± 11.1 | 4.4 ± 1.8 |
| Cellulose (μg/mg CWR) | 538.0 ± 63.7 | 516.9 ± 60.3 | 509.0 ± 48.3 | 535.7 ± 55.8 | 560.5 ± 47.1 | 523.5 ± 49.3 |
| Hemicelluloses (μg/mg CWR) | 197.1 ± 20.3 | 208.8 ± 10.7 | 196.8 ± 16.0 | 197.2 ± 13.1 | 207.4 ± 18.1 | 192.4 ± 15.2 |
Wood composition (± SD) of transgenics and WT from both field trials. For the Belgian field trial, wood composition was determined on the individual trees that were selected for SSF (Table S1) (WT, n = 6; FS3, n = 6; FS40, n = 7). For the French field trial, wood composition was determined on pooled trees (Table S1) (WT, n = 10; FS3, n = 10; FAS13, n = 10). FA, ferulic acid; AG, CCR marker; IS, internal standard; bold, significantly increased compared with WT; underlined, significantly decreased compared with WT.
Surprisingly, the acetyl bromide soluble lignin (ABSL) content was only lower for FAS13 (by 12%), but not for the other lines (Table 2). In contrast, for all transgenic lines in both field trials, Klason lignin content was significantly lower (by 5–24%) than that of WT (Table 2). All three transgenic lines incorporated elevated amounts of ferulic acid into lignin (Table 2). The ferulic acid markers (14) are good indicators of the altered lignin structure because they have been fixed in the lignin polymer at the time of lignin deposition. In this sense, they even reflect the altered wood composition more accurately than the red coloration, which is only visible at the surface of the trunk. The abundance of the ferulic acid markers correlated with the saccharification yield for all pretreatment conditions, with Pearson correlation coefficients ranging from 0.53 to 0.92 (Fig. S5).
Saccharification and Fermentation of Samples with Bark.
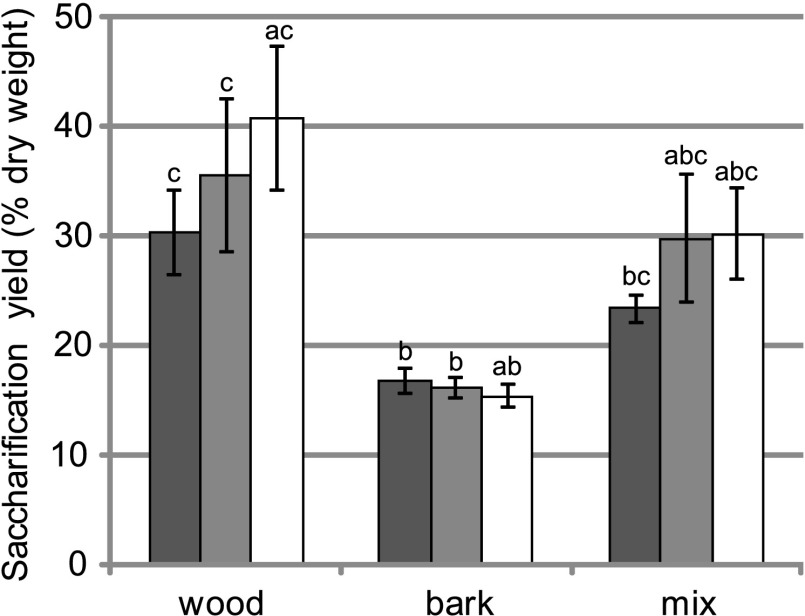
We next performed saccharifications of stems with bark. Saccharifications of wood–bark mixtures were performed with one alkaline pretreatment (6.25 mM NaOH) on the same samples as selected for SSF for both transgenic lines FS3 and FS40 of the Belgian field trial. The saccharification yield of the bark was roughly half of that of xylem tissue. Accordingly, when wood and bark material were combined, saccharification resulted in intermediate yields. However, the wood–bark mix still had a substantial increase in saccharification yield (+27% and +29% for FS3 and FS40) compared with that of WT (Fig. 4).
Fig. 4.
Saccharification yields of wood and bark analyzed separately and of wood–bark mixtures of transgenic trees and WT of the Belgian field trial. The same trees that were selected for SSF and wood compositional analysis were used (WT, n = 6; FS3, n = 6; FS40, n = 7). A pretreatment with 6.25 mM NaOH was applied. Error bars represent SDs. Dark gray, WT; light gray, FS3; white, FS40. aSignificantly different compared with WT within the same tissue; bsignificantly different compared with wood within the same line; csignificantly different compared with bark within the same line.
In addition, complete stems of the entire 20-mo-old French field trial, including bark, apical parts, and less red trees, were combined in three pools per line, saccharified, and fermented by SSF. After 36 h of SSF, WT, FS3, and FAS13 had ethanol yields of 3.8 (± 0.03), 4.7 (± 0.12), and 4.3 (± 0.08) g/L, respectively; i.e., the transgenic trees had a 14–26% higher ethanol yield than the WT. However, the biomass penalty (Fig S4E) outweighed the improved ethanol yields. For traits with a significant line effect, broad-sense heritabilities are shown in Table S2.
Discussion
Lignin is one of the most important factors limiting the processing of plant biomass into fermentable sugars (15–17). Here, we have evaluated CCR–down-regulated poplars, grown under greenhouse and field conditions, for their saccharification and ethanol yields. The fact that CCR down-regulation was associated with a visible phenotype allowed us to collect samples that were uniformly down-regulated in CCR from both the Belgian and the French field trials, thus bypassing the unequal gene silencing issue. This red phenotype-based sampling allowed us to answer our primary question on whether wood properties of dormant field-grown CCR–down-regulated trees were similarly improved as actively growing trees from the greenhouse.
CCR transgenic trees were evaluated at two field sites, which is necessary because of possible genotype × environment interactions (21). Although saccharification yields and cellulose conversions (Table 1) for the common FS3 line varied between the two field trials, a substantially increased saccharification and ethanol yield was obtained for red wood harvested from both field trials, indicating that the effects of CCR down-regulation on wood processing observed in the greenhouse were maintained in the two field environments. The one caveat is that the analyzed wood was still juvenile (10-mo-old for the Belgian and 20-mo-old for the French field trial) and not derived from a full 3-y rotation.
Wood from the fully red field-grown trees from both field trials had higher saccharification yields than the WT for all transgenic lines under all tested saccharification pretreatment conditions, except for FS3 without pretreatment in the Belgian field trial (Table 1). Moreover, it is important to note that wood from CCR transgenic trees processed under a given pretreatment saccharified equally well as WT wood processed with a harsher treatment; for example, untreated FAS13 saccharified equally well compared with 6.25 mM NaOH-treated WT in the French field trial. Because pretreatment is one of the biggest costs in the production of biofuels (24), the processing of lignin-reduced or lignin-modified trees has the potential to decrease the amount of chemicals and water used and, thus, the overall production cost and environmental impact.
Previous publications and wet-chemistry assays on greenhouse-grown trees have shown that down-regulation of CCR resulted in a reduced lignin content, at least in the red-colored wood (13). Remarkably, ABSL content in field-grown CCR–down-regulated poplars was not (for FS3 and FS40) or only modestly (for FAS13) decreased (Table 2), even in samples that were red throughout the stem as seen in cross-sections. This result is in contrast with the Klason lignin content, which was significantly reduced in agreement with the improved saccharification and ethanol yield. Similarly, the abundance of oligolignols, also a proxy for lignin amount (25), was reduced in the field-grown samples (Fig. S6). It is possible that the high ABSL levels were caused by increased amounts of UV-absorbing substances that are detected by the acetyl bromide method used to quantify lignin (22), potentially by increased xylan degradation in tissues with lower lignin content (26). The increased saccharification yields correlated well with the abundance of the ferulic acid markers. At this point, we cannot conclude to what extent the incorporation of the ferulic acid contributes to the improved processing of the wood samples.
SSF of wood derived from FAS13 yielded 161% more ethanol than WT (Fig. 3). This experiment was performed on a selection of completely red, debarked basal trunks, thus avoiding stability issues of gene silencing. The value of 161% increase in ethanol yield can thus be considered as the maximum obtained in the ideal case of a uniformly and stably CCR–down-regulated tree and with focus on wood tissue only. Taking the yield penalty for these trees into account, FAS13 still yielded 57% more ethanol than WT. On the other hand, the experiment in which all trees from the French field trial were pooled and analyzed by SSF, resulted in a 14–26% increase in ethanol yield compared with WT. This value can be considered as the lower bound because, in this experimental design, trees that were less efficiently down-regulated for CCR, were also included in the analyzed pool and not only wood, but complete trees with bark and apical parts. However, the 14–26% increase in ethanol yield per gram of dry wood was outweighed by the overall yield penalty. Obviously, one of the main future objectives will be to overcome the yield penalty that is often associated with modified lignin (27). Among the likely causes of the yield penalty are the structural abnormalities of the plant cell wall, such as collapsed xylem (Fig. S7), as was previously noticed in greenhouse-grown CCR–down-regulated poplars (13). In 4CL–down-regulated poplar, the reduced growth has been attributed to collapsed xylem and the formation of tyloses in the vascular system, and it was shown that vascular tissue in the red areas is defective in its transport capacity (22, 28). One option to circumvent the negative consequences of lignin down-regulation on plant performance while still achieving strong down-regulation is to specifically target the down-regulation to fibers but leaving the vessels intact. Such strategies have already proven successful in Arabidopsis thaliana (29, 30).
In conclusion, although we have observed variability in the level of down-regulation in individual trunk parts, we were able to demonstrate a relationship between CCR down-regulation and saccharification yield because the visible phenotype allowed us to classify the material into highly and less down-regulated samples. We conclude that CCR down-regulation may become a successful strategy to improve wood processing (to fermentable sugars) if it can be stabilized and targeted to fibers only.
Materials and Methods
Belgian Field Trial.
FS3, FS40, and WT trees were transferred to the field in May 2009, under a GM field trial authorization (B/BE/07/V2) provided by the Belgian competent authorities after a positive ruling from the Belgian Biosafety Advisory Council. Beginning of March 2010, the bottom ∼20 cm of the stems was harvested, debarked, and immediately photographed.
French Field Trial.
FS3, FAS13, and WT poplars were planted in the field in July 2008, after obtaining suitable authorization (Application B/FR/07/06/01, Authorization 07/015) from the “Direction Générale de l’Alimentation” from the French “Ministère de l’Agriculture et de la Pêche” (on September 21, 2007 for a 5-y period) after a positive ruling from the French “Commission du Génie Biomoléculaire”. In March 2010, an ∼20-cm segment at the base of each stem was harvested, debarked, and photographed.
Saccharification Assays.
For a detailed description of the saccharification assay for greenhouse-grown samples, see SI Materials and Methods. For field-grown poplar samples, the basal part of the harvested stems were handled with the iWALL custom-designed robot (Labman Automation Ltd.) (31).
Simultaneous Saccharification and Fermentation.
Poplar biomass was pretreated with lime, saccharified, and fermented as described (32, 33).
Wood Compositional Analysis.
Lignin analyses included the acetyl bromide (34) and Klason method (35) and thioacidolysis (14, 36); polysaccharides were analyzed by the Updegraff method and the alditol-acetate assay (37).
Metabolomics.
Gradient elution and MS analysis were performed as described (38).
Statistical Analyses.
Associated P values were adjusted with the Dunnett test (two-sided). Differences with a Dunnett adjusted P value <0.05 were considered significant.
Details on all methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the technical staff for managing and sampling both field trials, Ingeborg Stals and Dieter Depuydt for help in SSF analyses, and René Custers for requesting regulatory permission for the Belgian field trial. This work was supported by grants from the Multidisciplinary Research Partnership “Biotechnology for a sustainable economy” of Ghent University, the European Commission’s Seventh Framework Program (ENERGYPOPLAR FP7-211917 and MultiBioPro 270089), European Cooperation in Science and Technology Action FP0905, and Hercules Foundation Grant AUGE/014. R.V.A. is indebted to the Agency for Innovation by Science and Technology for a predoctoral fellowship. N.S., C.E.F., and J.R. were funded by the US Department of Energy’s Great Lakes Bioenergy Research Center (Department of Energy Office of Science Grant BER DE–FC02–07ER64494).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321673111/-/DCSupplemental.
References
- 1.Yuan JS, Tiller KH, Al-Ahmad H, Stewart NR, Stewart CN., Jr Plants to power: Bioenergy to fuel the future. Trends Plant Sci. 2008;13(8):421–429. doi: 10.1016/j.tplants.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Solomon BD. Biofuels and sustainability. Ann N Y Acad Sci. 2010;1185:119–134. doi: 10.1111/j.1749-6632.2009.05279.x. [DOI] [PubMed] [Google Scholar]
- 3.Vanholme B, et al. Towards a carbon-negative sustainable bio-based economy. Front Plant Sci. 2013;4:174. doi: 10.3389/fpls.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanholme R, et al. Metabolic engineering of novel lignin in biomass crops. New Phytol. 2012;196(4):978–1000. doi: 10.1111/j.1469-8137.2012.04337.x. [DOI] [PubMed] [Google Scholar]
- 5.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 6.Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. Lignin biosynthesis and structure. Plant Physiol. 2010;153(4):895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonawitz ND, Chapple C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu Rev Genet. 2010;44:337–363. doi: 10.1146/annurev-genet-102209-163508. [DOI] [PubMed] [Google Scholar]
- 8.Laskar DD, et al. The Arabidopsis cinnamoyl CoA reductase irx4 mutant has a delayed but coherent (normal) program of lignification. Plant J. 2006;48(5):674–686. doi: 10.1111/j.1365-313X.2006.02918.x. [DOI] [PubMed] [Google Scholar]
- 9.Ruel K, et al. Impact of CCR1 silencing on the assembly of lignified secondary walls in Arabidopsis thaliana. New Phytol. 2009;184(1):99–113. doi: 10.1111/j.1469-8137.2009.02951.x. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LA, et al. Improving saccharification efficiency of alfalfa stems through modification of the terminal stages of monolignol biosynthesis. BioEnerg Res. 2008;1(3-4):180–192. [Google Scholar]
- 11.Tamasloukht B, et al. Characterization of a cinnamoyl-CoA reductase 1 (CCR1) mutant in maize: Effects on lignification, fibre development, and global gene expression. J Exp Bot. 2011;62(11):3837–3848. doi: 10.1093/jxb/err077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauwe R, et al. Molecular phenotyping of lignin-modified tobacco reveals associated changes in cell-wall metabolism, primary metabolism, stress metabolism and photorespiration. Plant J. 2007;52(2):263–285. doi: 10.1111/j.1365-313X.2007.03233.x. [DOI] [PubMed] [Google Scholar]
- 13.Leplé J-C, et al. Downregulation of cinnamoyl-coenzyme A reductase in poplar: Multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure. Plant Cell. 2007;19(11):3669–3691. doi: 10.1105/tpc.107.054148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralph J, et al. Identification of the structure and origin of a thioacidolysis marker compound for ferulic acid incorporation into angiosperm lignins (and an indicator for cinnamoyl CoA reductase deficiency) Plant J. 2008;53(2):368–379. doi: 10.1111/j.1365-313X.2007.03345.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25(7):759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 16.Studer MH, et al. Lignin content in natural Populus variants affects sugar release. Proc Natl Acad Sci USA. 2011;108(15):6300–6305. doi: 10.1073/pnas.1009252108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Acker R, et al. Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in Arabidopsis thaliana. Biotechnol Biofuels. 2013;6:46. doi: 10.1186/1754-6834-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Custers R. First GM trial in Belgium since 2002. Nat Biotechnol. 2009;27(6):506. doi: 10.1038/nbt0609-506. [DOI] [PubMed] [Google Scholar]
- 19.Dickmann DI. Silviculture and biology of short-rotation woody crops in temperate regions: Then and now. Biomass Bioenergy. 2006;30(8-9):696–705. [Google Scholar]
- 20.Walter C, Fladung M, Boerjan W. The 20-year environmental safety record of GM trees. Nat Biotechnol. 2010;28(7):656–658. doi: 10.1038/nbt0710-656. [DOI] [PubMed] [Google Scholar]
- 21.Pilate G, Déjardin A, Leplé J-C. Field trials with lignin-modified transgenic trees. Adv Bot Res. 2012;61:1–36. [Google Scholar]
- 22.Voelker SL, et al. Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 2010;154(2):874–886. doi: 10.1104/pp.110.159269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansfield SD, Kang K-Y, Chapple C. Designed for deconstruction—poplar trees altered in cell wall lignification improve the efficacy of bioethanol production. New Phytol. 2012;194(1):91–101. doi: 10.1111/j.1469-8137.2011.04031.x. [DOI] [PubMed] [Google Scholar]
- 24.Lynd LR, et al. How biotech can transform biofuels. Nat Biotechnol. 2008;26(2):169–172. doi: 10.1038/nbt0208-169. [DOI] [PubMed] [Google Scholar]
- 25.Vanholme R, et al. A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell. 2012;24(9):3506–3529. doi: 10.1105/tpc.112.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatfield RD, Grabber J, Ralph J, Brei K. Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: Some cautionary notes. J Agric Food Chem. 1999;47(2):628–632. doi: 10.1021/jf9808776. [DOI] [PubMed] [Google Scholar]
- 27.Bonawitz ND, Chapple C. Can genetic engineering of lignin deposition be accomplished without an unacceptable yield penalty? Curr Opin Biotechnol. 2013;24(2):336–343. doi: 10.1016/j.copbio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Kitin P, et al. Tyloses and phenolic deposits in xylem vessels impede water transport in low-lignin transgenic poplars: A study by cryo-fluorescence microscopy. Plant Physiol. 2010;154(2):887–898. doi: 10.1104/pp.110.156224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang F, et al. Engineering secondary cell wall deposition in plants. Plant Biotechnol J. 2013;11(3):325–335. doi: 10.1111/pbi.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen PD, et al. Engineering of plants with improved properties as biofuels feedstocks by vessel-specific complementation of xylan biosynthesis mutants. Biotechnol Biofuels. 2012;5:84. doi: 10.1186/1754-6834-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro N, et al. A high-throughput platform for screening milligram quantities of plant biomass for lignocellulose digestibility. Bioenerg Res. 2010;3(1):93–102. [Google Scholar]
- 32.Chang VS, Kaar WE, Burr B, Holtzapple MT. Simultaneous saccharification and fermentation of lime-treated biomass. Biotechnol Lett. 2001;23(16):1327–1333. [Google Scholar]
- 33.Chang VS, Nagwani M, Kim C-H, Holtzapple MT. Oxidative lime pretreatment of high-lignin biomass. Appl Biochem Biotechnol. 2001;94(1):1–28. doi: 10.1385/abab:94:1:01. [DOI] [PubMed] [Google Scholar]
- 34.Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass). Part I: Lignin. J Vis Exp. 2010;37:e1745. doi: 10.3791/1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dence CW. In: Methods in Lignin Chemistry. Lin SY, Dence CW, editors. Berlin: Springer; 1992. pp. 33–61. [Google Scholar]
- 36.Yue F, Lu F, Sun R-C, Ralph J. Syntheses of lignin-derived thioacidolysis monomers and their uses as quantitation standards. J Agric Food Chem. 2012;60(4):922–928. doi: 10.1021/jf204481x. [DOI] [PubMed] [Google Scholar]
- 37.Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass). Part II: Carbohydrates. J Vis Exp. 2010;37:e1837. doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunewald W, et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc Natl Acad Sci USA. 2012;109(5):1554–1559. doi: 10.1073/pnas.1121134109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.