Significance
The Fenton reaction, Fe2+ + H2O2, plays fundamental roles in vivo and in advanced oxidation processes. Its mechanism and the identity of the intermediates involved, however, remain controversial. Here we present direct, mass-specific evidence of the prompt formation of mono- and poly-iron FeIV=O (ferryl) species on the surface of aqueous FeCl2 microjets exposed to gaseous H2O2 or O3 beams. Remarkably, Fe2+ ions at the aqueous surface react with H2O2 and O3 >103 times faster than Fe(H2O)62+ in bulk water. Our results suggest that interfacial Fenton and Fenton-like chemistries could play a more significant role than hitherto envisioned.
Keywords: metal ions, reactive oxygen species, aerosols, advanced oxidation processes, nanoparticles
Abstract
In a fundamental process throughout nature, reduced iron unleashes the oxidative power of hydrogen peroxide into reactive intermediates. However, notwithstanding much work, the mechanism by which Fe2+ catalyzes H2O2 oxidations and the identity of the participating intermediates remain controversial. Here we report the prompt formation of O=FeIVCl3− and chloride-bridged di-iron O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5− ferryl species, in addition to FeIIICl4−, on the surface of aqueous FeCl2 microjets exposed to gaseous H2O2 or O3 beams for <50 μs. The unambiguous identification of such species in situ via online electrospray mass spectrometry let us investigate their individual dependences on Fe2+, H2O2, O3, and H+ concentrations, and their responses to tert-butanol (an ·OH scavenger) and DMSO (an O-atom acceptor) cosolutes. We found that (i) mass spectra are not affected by excess tert-butanol, i.e., the detected species are primary products whose formation does not involve ·OH radicals, and (ii) the di-iron ferryls, but not O=FeIVCl3−, can be fully quenched by DMSO under present conditions. We infer that interfacial Fe(H2O)n2+ ions react with H2O2 and O3 >103 times faster than Fe(H2O)62+ in bulk water via a process that favors inner-sphere two-electron O-atom over outer-sphere one-electron transfers. The higher reactivity of di-iron ferryls vs. O=FeIVCl3− as O-atom donors implicates the electronic coupling of mixed-valence iron centers in the weakening of the FeIV–O bond in poly-iron ferryl species.
High-valent FeIV=O (ferryl) species participate in a wide range of key chemical and biological oxidations (1–4). Such species, along with ·OH radicals, have long been deemed putative intermediates in the oxidation of FeII by H2O2 (Fenton’s reaction) (5, 6), O3, or HOCl (7, 8). The widespread availability of FeII and peroxides in vivo (9–12), in natural waters and soils (13), and in the atmosphere (14–18) makes Fenton chemistry and FeIV=O groups ubiquitous features in diverse systems (19). A lingering issue regarding Fenton’s reaction is how the relative yields of ferryls vs. ·OH radicals depend on the medium. For example, by assuming unitary ·OH radical yields, some estimates suggest that Fenton’s reaction might account for ∼30% of the ·OH radical production in fog droplets (20). Conversely, if Fenton’s reaction mostly led to FeIV=O species, atmospheric chemistry models predict that their steady-state concentrations would be ∼104 times larger than [·OH], thereby drastically affecting the rates and course of oxidative chemistry in such media (20). FeIV=O centers are responsible for the versatility of the family of cytochrome P450 enzymes in catalyzing the oxidative degradation of a vast range of xenobiotics in vivo (21–28), and the selective functionalization of saturated hydrocarbons (29). The bactericidal action of antibiotics has been linked to their ability to induce Fenton chemistry in vivo (9, 30–34). Oxidative damage from exogenous Fenton chemistry likely is responsible for acute and chronic pathologies of the respiratory tract (35–38).
Despite its obvious importance, the mechanism of Fenton’s reaction is not fully understood. What is at stake is how the coordination sphere of Fe2+ (39–46) under specific conditions affects the competition between the one-electron transfer producing ·OH radicals (the Haber–Weiss mechanism) (47), reaction R1, and the two-electron oxidation via O-atom transfer (the Bray–Gorin mechanism) into FeIVO2+, reaction R2 (6, 23, 26, 27, 45, 48–51):
Ozone reacts with Fe2+ via analogous pathways leading to (formally) the same intermediates, reactions R3a, R3b, and R4 (8, 49, 52, 53):
At present, experimental evidence about these reactions is indirect, being largely based on the analysis of reaction products in bulk water in conjunction with various assumptions. Given the complex speciation of aqueous Fe2+/Fe3+ solutions, which includes diverse poly-iron species both as reagents and products, it is not surprising that classical studies based on the identification of reaction intermediates and products via UV-absorption spectra and the use of specific scavengers have fallen short of fully unraveling the mechanism of Fenton’s reaction. Herein we address these issues, focusing particularly on the critically important interfacial Fenton chemistry that takes place at boundaries between aqueous and hydrophobic media, such as those present in atmospheric clouds (16), living tissues, biomembranes, bio-microenvironments (38, 54, 55), and nanoparticles (56, 57).
We exploited the high sensitivity, surface selectivity, and unambiguous identification capabilities of a newly developed instrument based on online electrospray mass spectrometry (ES-MS) (58–62) to identify the primary products of reactions R1–R4 on aqueous FeCl2 microjets exposed to gaseous H2O2 and O3 beams under ambient conditions [in N2(g) at 1 atm at 293 ± 2 K]. Our experiments are conducted by intersecting the continuously refreshed, uncontaminated surfaces of free-flowing aqueous microjets with reactive gas beams for τ ∼10–50 μs, immediately followed (within 100 μs; see below) by in situ detection of primary interfacial anionic products and intermediates via ES-MS (Methods, SI Text, and Figs. S1 and S2). We have previously demonstrated that online mass spectrometric sampling of liquid microjets under ambient conditions is a surface-sensitive technique (58, 62–67).
Results and Discussion
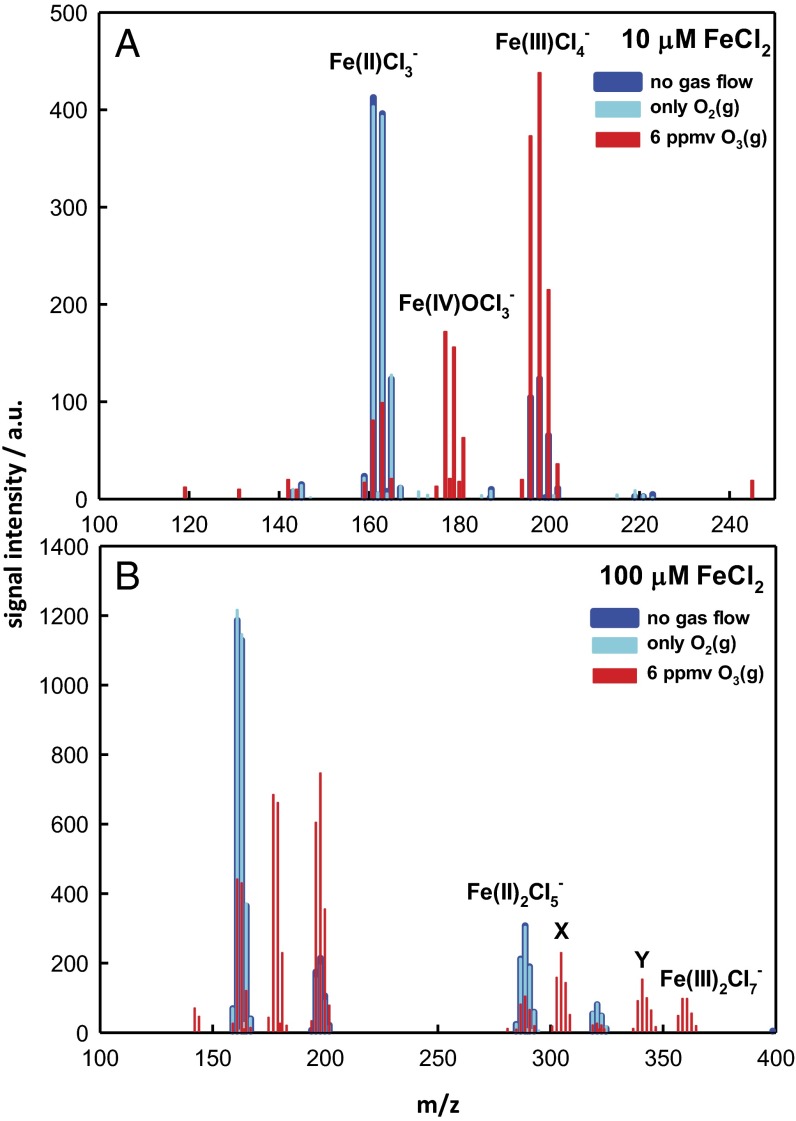
Fig. 1 A and B shows negative ion ES mass spectra of the surface of 10 μM and 100 μM FeCl2 aqueous microjets, respectively, while being exposed to O2(g) and O3(g)/O2(g) mixtures for contact times τ of the order of a few tens of microseconds. Gas–liquid contact times τ correspond to the estimated lifetimes of the microjets, i.e., before they are broken up by the nebulizer gas into submicrometer-sized droplets carrying net excess charges (58) (SI Text). A hard upper bound to τ can be derived from the fact that microdroplets carried by the nebulizer gas issuing from the injector nozzle at typical v ∼2 × 104 cm⋅s−1 velocities would reach the inlet to the detection section of the mass spectrometer ∼2 cm away from the tip of the nozzle in <100 μs. The net charges produced during the aerodynamic breakup of the liquid jet represent the raw information acquired by the mass spectrometer (58). We confirmed experimentally that in our instrument charge separation among the anions and cations present in interfacial layers is largely driven pneumatically (rather than electrostatically/electrochemically) (68) by showing that signal intensities and anion fractionation increase at higher nebulizer gas velocities v and extrapolate to zero as v → 0 (58). In Fig. 1A, the ES mass spectral multiplets at m/z = 161, 163, and 165 correspond to FeIICl3−. The characteristic multiplet patterns arising from natural abundance 35Cl (75%) and 37Cl (25%) chlorine isotopes let us establish the number of Cl− contained in each detected species and, hence, the molecular composition of these singly charged ions (Fig. S3). In the presence of O3(g)/O2(g) mixtures, new ES mass spectral clusters appear at m/z = 177, 179, and 181 and m/z = 196, 198, and 200, which can be readily assigned to O=FeIVCl3− and FeIIICl4−, respectively. We verified that Cl− is inert toward O3(g) and does not participate in the oxidation process, in accord with the small value of k(Cl− + O3) = 0.1 M−1⋅s−1 in bulk water (69). Thus, ∼50% FeII is oxidized by 6 parts per million by volume (ppmv) (6 × 10−6 atm) O3(g) into FeIII and FeIV at the air–water interface within τ ∼10–50 μs. This is a remarkable result because from (i) the (maximum) equilibrium concentration of dissolved O3 in the experiments of Fig. 1—[O3(aq)] = 6 × 10−8 M [from Henry’s law constant for O3(g) in bulk water at ambient temperature H = 0.01 M atm−1] (70)—and (ii) the rate coefficients of reactions R3a—k3a = (1.7 ± 0.4) × 105 M−1⋅s−1—and R4—k4 = (8.2 ± 0.3) × 105 M−1⋅s−1—in bulk water (7), we estimate that less than 0.1% Fe2+ should have been consumed under present conditions. In other words, reactions R3a and R4 proceed ∼103–104 times faster at the gas–water interface than in bulk water. The modest concentration enhancements of many gases at the air–water interface predicted by theoretical simulation (70) and demonstrated experimentally (71–74) would not substantially alter the above statement. We tentatively ascribe the significant acceleration of reaction R4 at the gas–water interface to the enhanced lability and/or distorted geometry (75) of the hydration shell of Fe2+ at the interface relative to bulk water, a condition that would facilitate the substitution of O3 for hydration waters and, hence, the direct interaction with the metal center required by O-atom transfer during subnanosecond gas–liquid encounters (76) (see below).
Fig. 1.
Negative ion ES mass spectra of 10 μM (A) and 100 μM (B) FeCl2 aqueous microjets exposed to O2(g) or O3(g)/O2(g) for ∼10–50 μs. X and Y correspond to O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5−, respectively. See text for details.
Fig. 1B shows additional peaks at higher masses. The ES mass signals at m/z = 287, 289, and 291 correspond to FeII2 Cl5−. The group at m/z = 303, 305, and 307, hereafter labeled X, can be assigned to O=FeIV·Cl·FeIICl4− on the basis of peak masses and the characteristic Cl5-multiplet pattern. Similarly, we assign the group at m/z = 339, 341, 343, 345, and 347, hereafter labeled Y, to O=FeIV·Cl·FeIIICl5−. Our results are qualitatively consistent with previous reports based on the UV-absorption detection of O=FeIV species during the bulk ozonolysis of acidic Fe2+ (8, 23, 49, 52).
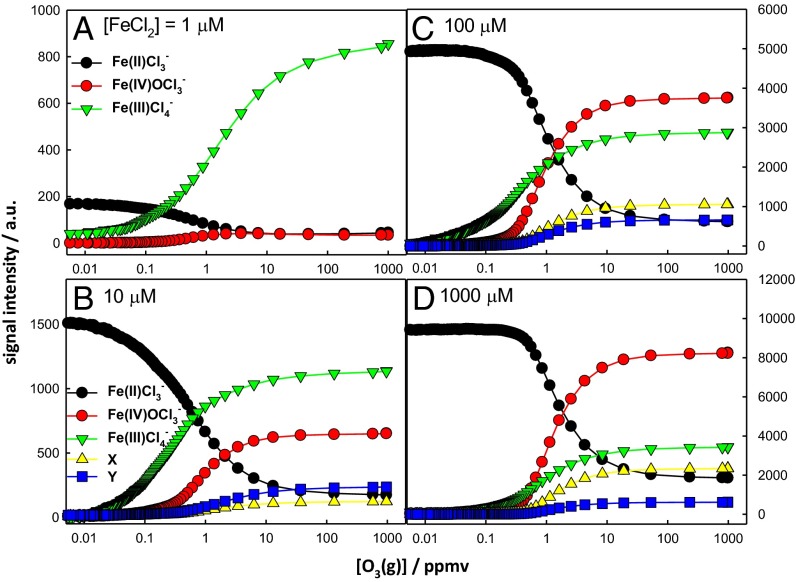
Fig. 2 A–D shows the evolution of reactant and products as functions of [O3(g)] at FeCl2 concentrations spanning the 1–1,000-μM range. It is apparent that although all signal intensities increase with [FeCl2], the ratio α = O=FeIVCl3−/FeIIICl4− is not constant, as expected for the products of concurrent reactions R3a and R4, but both depend on O3(g) and FeCl2 concentrations (8, 51). It should be pointed out that mass signal intensities are not linear functions of bulk concentrations throughout, because the interfacial concentrations detected herein will plateau as the interface becomes saturated. Also, reactant signals may bottom out rather than vanish at sufficiently large O3(g) [or H2O2(g)] concentrations because interfacial layers are continuously replenished by diffusion from the bulk liquid (66). Because the O=FeIVCl3− intermediate reacts further with Fe2+ via reaction R5,
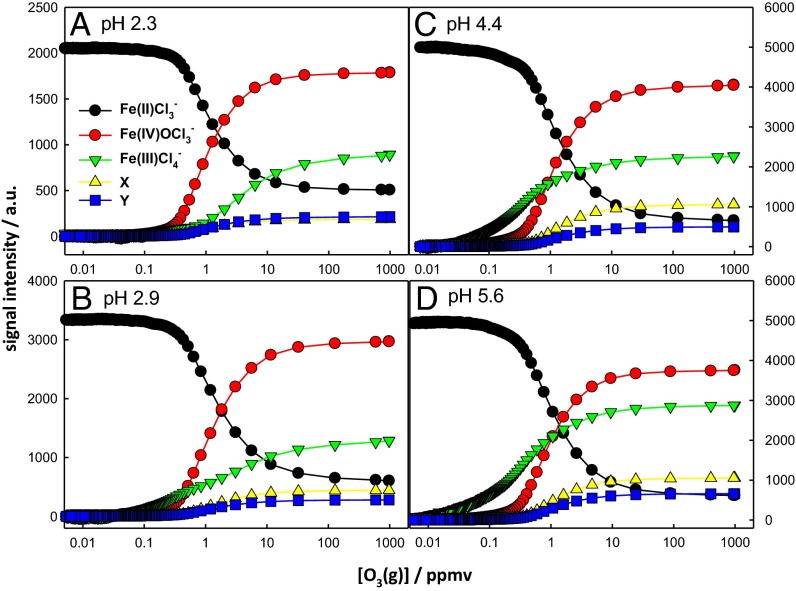
the observed increase of α at higher O3(g) and FeCl2 concentrations is not the result of secondary chemistry (see below). Note that (i) the higher-mass products X, Y, and FeIII2Cl7− appear at [FeCl2] ≥10 μM, and (ii) α depends weakly on pH (Fig. 3), in contrast to previous reports that O=FeIV is formed only under very acidic (pH ≤2) bulk conditions (6, 8, 77). The more extensive hydrolysis of Fe2+ in more basic solutions prevented us from performing experiments above pH ∼6.5. It should be realized, however, that the acid–base properties of the air–water interface are quite different from those of bulk water. Whereas bulk water is neutral at pH 7, the aerial surface is neutral on bulk water at pH ∼3.5 (63, 65, 78). This caveat prevents direct comparisons from being made between the pH-dependences observed herein and those previously reported for similar experiments in bulk solution. We wish to emphasize that the concentration dependences observed in our experiments strongly support our assumption that the detected species are produced on the surface of the intact jet (whose composition is identical to that of the injected solution) rather than on the ensemble of daughter droplets (whose compositions will span the broad distributions generated by random solvent evaporation) (63, 65) (see also SI Text).
Fig. 2.
ES mass spectral signal intensities of reactant and products at the surface of 1 μM (A), 10 μM (B), 100 μM (C), and 1,000 μM (D) FeCl2 aqueous microjets as functions of the O3(g) mixing ratio (1 ppmv = 2.5 × 1013 molecules per centimeter−3 at 1 atm, 293 K). All experiments in 1 atm N2(g) at 293 K. Background signal was subtracted for Fe(III)Cl4−. X and Y correspond to O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5−, respectively.
Fig. 3.
ES mass spectral signal intensities of reactant and products from 100 μM FeCl2 aqueous microjets at pH 2.3 (A), pH 2.9 (B), pH 4.4 (C), and pH 5.6 (D) as functions of the O3(g) mixing ratio (1 ppmv = 2.5 × 1013 molecules per centimeter−3 at 1 atm, 293 K). All experiments in 1 atm N2(g) at 293 K. Background signal was subtracted for Fe(III)Cl4−. X and Y correspond to O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5−, respectively.
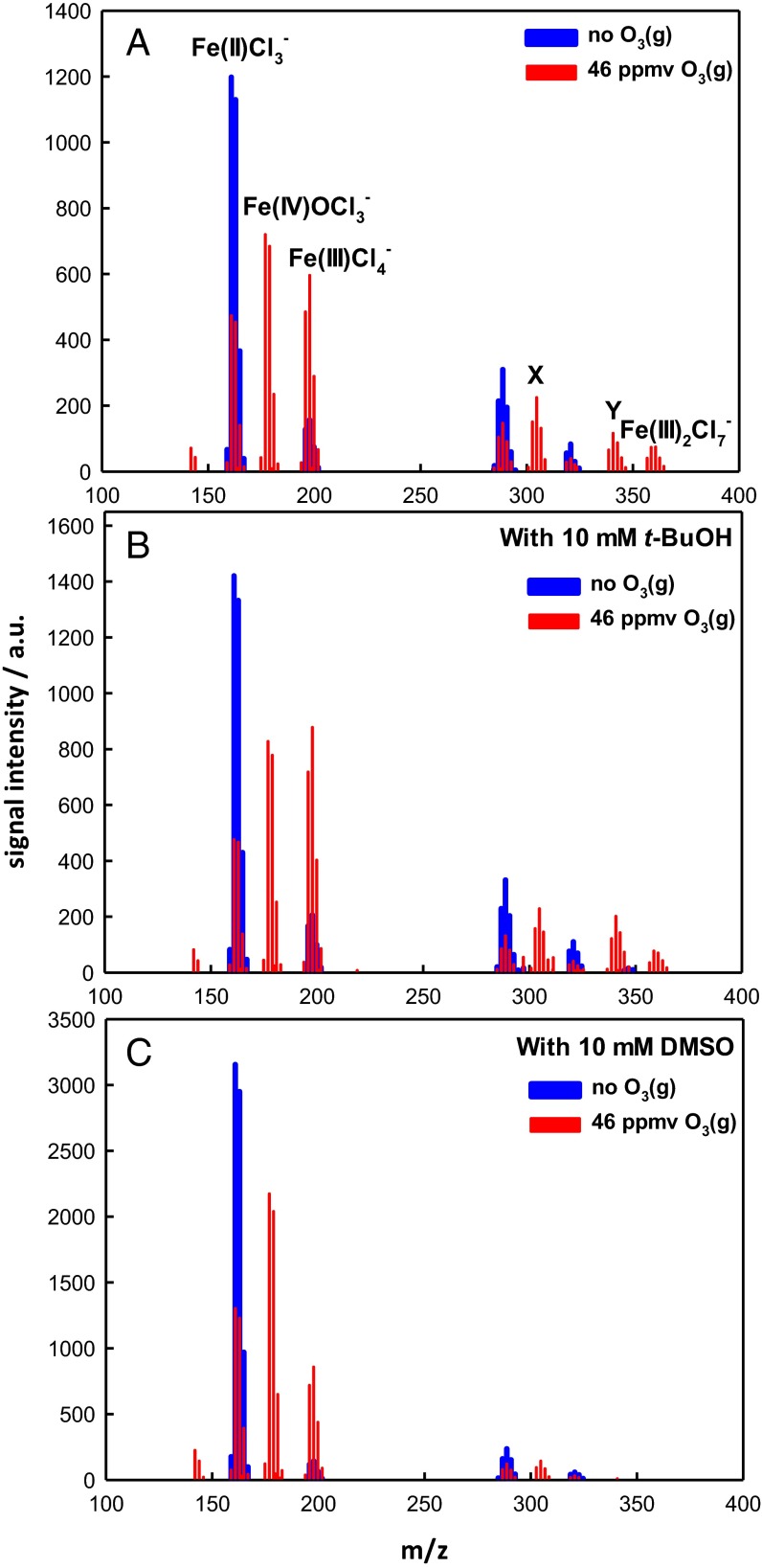
We tested the effects of tert-butanol (t-BuOH) and DMSO additions to FeCl2 microjets exposed to O3(g). t-BuOH is an efficient scavenger of ·OH radicals (k·OH+t-BuOH = 5 × 108 M−1⋅s−1 in bulk water) (79), whereas DMSO functions as both an ·OH scavenger and an O-atom acceptor (6, 49, 52). Fig. 4 shows negative ion mass spectra of aqueous 100 μM FeCl2 microjets containing large excesses (100 × [FeCl2]) of t-BuOH or DMSO upon exposure to O3(g). Notably, the addition of t-BuOH has no effect whatsoever on mass spectra, thereby implying that ·OH radicals do not participate in the formation of the observed products. Because ·OH radicals, if present, also would react rapidly with Fe2+ [k·OH+Fe(II) = 3.2 × 108 M−1⋅s−1] (80) to produce more Fe3+, we infer that the decomposition of the their O3·- precursor, reaction R3b, is too slow under present conditions.
Fig. 4.
Negative ion ES mass spectra of aqueous microjet containing 100 μM FeCl2 in the absence (A) and presence (B) of 10 mM t-BuOH and in the presence of 10 mM DMSO (C), exposed to O3(g) for ∼10–50 μs. X and Y correspond to O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5−, respectively.
The addition of DMSO as a cosolute, in contrast, has marked effects on product distribution. DMSO quenches most (but not all) ozonation products, such as O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5−, and all higher-mass poly-iron species. Remarkably, the mono-iron ferryl O=FeIVCl3− (and, as expected, FeIIICl4−) is not affected.
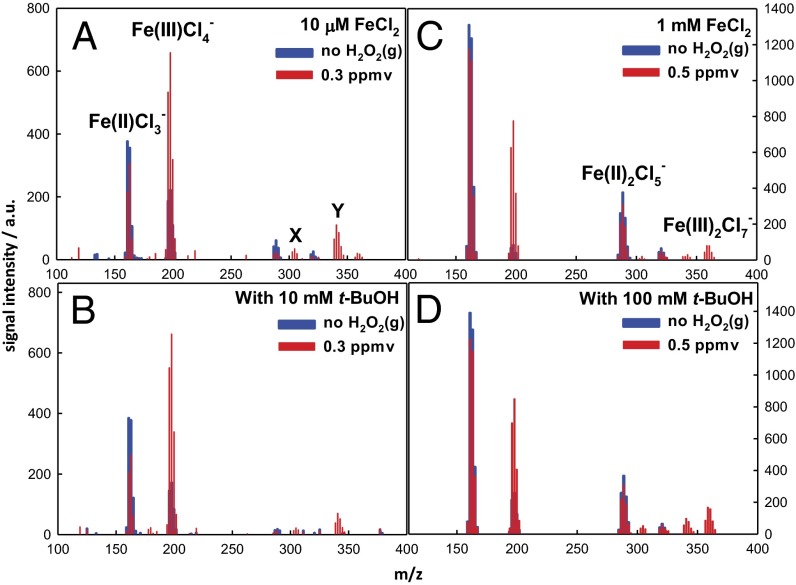
Similar experiments in which aqueous microjets containing 10 μM and 1 mM FeCl2, in the absence and presence of t-BuOH as a cosolute, were exposed to H2O2(g)/N2(g) mixtures led to the products shown in Fig. 5. A comparison of Figs. 1 and 5 confirms that H2O2 and O3 react with interfacial Fe2+ along reactions R1–R2 and R3a,b–R4, respectively, leading to (formally) the same products, albeit in different proportions. Note again that from Henry’s law constant for H2O2(g) in bulk water—H = 105 M⋅atm−1 (∼107 times larger than for O3) (81)—the maximum equilibrium concentration of dissolved H2O2 in the experiments of Fig. 5A is [H2O2(aq)] = 0.03 M. Therefore, from k(FeII + H2O2) ∼ 50 M−1⋅s−1, we estimate that Fe2+ half-lives would be on the order of seconds if reactions R1–R2 took place in bulk water, rather than a few tens of microseconds, as observed in our experiments (81). Note that in contrast to the O3 case, O=FeIVCl3− is undetectable (compare Fig. 5 vis-a-vis Fig. 1), an event we ascribe to the slowness of R2 relative to R4 under present conditions, because O=FeIVCl3− would be consumed at the same rates via R5 in both systems. We confirmed that the addition of DMSO as a cosolute in these experiments (Fig. S4) has an effect similar to those displayed in Fig. 4C.
Fig. 5.
Negative ion ES mass spectra of 10 μM (A and B) and 1 mM (C and D) FeCl2 aqueous microjets in the absence/presence of excess t-BuOH as a cosolute exposed to H2O2(g) for ∼10–50 μs. X and Y correspond to O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5−, respectively.
The prompt formation of products in our experiments, at rates several orders of magnitude larger than those estimated for the same reactants dissolved in bulk water, and the peculiar variation in the ratio of the products of parallel reactions R3a and R4 (FeIIICl4− and O=FeIVCl3−) as a function of ozone and Fe2+ concentrations reveal the exceptional characteristics of Fenton-like chemistry at the air–water interface. These phenomena, which could be typical of water–hydrophobe interfaces in general, reveal (i) the enhanced reactivity of interfacial Fe2+ as an O-atom acceptor from O3 and H2O2 and (ii) the progressive emergence of such species to the outermost interfacial layers at higher Fe2+ bulk concentrations. Extensive ab initio molecular dynamics (Car–Parrinello) calculations that incorporate the water solvent explicitly would be required to elucidate the molecular details of these unique events at the gas–water interface (26, 48, 82). The possibility that doubly charged Fe2+ cations might be present in shallow interfacial layers is supported by molecular dynamics calculations, which predict that the concentration of doubly charged Mg2+ peaks at ∼4 Å below the Gibbs dividing surface at values twice as large as its concentration in bulk water (83). Recent X-ray reflectivity studies confirmed the existence of nonmonotonic cation density profiles within ∼1-nm interfacial layers of aqueous electrolyte solutions (84). We cannot rule out the possibility that impinging gases are hydrated before colliding with the liquid surface (85, 86), but we deem it inconsequential because O3–(H2O)n and H2O2–(H2O)n complexes represent a very small fraction of O3 and H2O2 gas flows under present conditions.
By considering that O-atom transfer, in contrast to electron transfer (reactions R1 and R3a), requires direct contact of the O-atom donors (H2O2 and O3) with the metal ion, and that the hydration waters of Fe(H2O)62+ in bulk water are exchanged approximately every 0.5 μs (87), whereas O3(g) remains trapped on the water surface for only 0.1 ns (76), our results imply that either (i) dissociative ligand substitution in interfacial Fe(H2O)62+ (IF) is much faster than in Fe(H2O)62+ in bulk water (B) or (ii) interfacial Fe(H2O)62+ has a distorted octahedral geometry, on account of the broken symmetry, that lets O3 approach the Fe2+ center via low-energy associative interchange pathways (88). Against this backdrop, our findings reveal that the dynamics and thermodynamics of ion hydration at aqueous interfaces are quite different from those in bulk water (75, 89). Thus, the roles and behavior of ions in many physical, chemical, and biological interfacial processes may not be predicted (or analyzed) from the properties of the corresponding ions in bulk water.
The dissimilar evolutions of O=FeIVCl3− and FeIIICl4− in Figs. 2 and 3 are consistent with IF(z) depth profiles at the interface that depend on total [Fe2+]. Everything happens as though the IF species involved in O-atom transfer are preferentially pushed to the surface of more concentrated Fe2+ solutions. In this context, it is relevant to point out that we recently found that hydronium (H3O+) emerges at the surface of water less than pH 4 as a “superacid” that protonates impinging gases having proton affinities larger than water (62, 64, 90). Thermodynamics dictates that this is possible only if interfacial H3O+ is weakly hydrated. If Fe2+ behaves similarly, the enhanced reactivity of IF relative to B and its emergence at the surface of more concentrated solutions could be alternatively ascribed to an incomplete hydration shell of IF.
We associate the significantly enhanced reactivity of poly-iron ferryls relative to O=FeIVCl3− as O-atom donors to DMSO with the weakening of the O=FeIV bond, and ascribe such weakening to electronic rather than inductive effects. Our view is based on the fact that strong electron-donating ligands, such as thiolate (24), in the axial position are known to weaken and elongate the O=FeIV bond in low-spin (S = 1) complexes by increasing the population of its σ- and π-antibonding molecular orbitals (45). Because a Cl− ligand coordinatively bound to FeII (as in X) or FeIII (as in Y) should be less nucleophilic than unbound Cl−, the enhanced reactivity of poly-iron ferryls appears to be a result of the electronic coupling of the iron centers via Cl− bridges. Further experimental work and high-level quantum chemistry calculations are needed to fully elucidate the molecular basis of the O-donating power of poly-iron ferryls.
In summary, we present compelling evidence of the prompt formation of mono- and poly-iron FeIV species on the surface of aqueous FeIICl2 microjets exposed to gaseous H2O2 or O3 beams. The exceedingly fast reactions of interfacial Fe2+ with gas-phase H2O2 and O3 [103–104 times faster than similar reactions of Fe(H2O)62+ in bulk aqueous media] are ascribed to a labile/incomplete hydration shell that favors inner-sphere O-atom transfers over outer-sphere one-electron transfers. The finding that di-iron ferryls O=FeIV·Cl·FeIICl4− and O=FeIV·Cl·FeIIICl5− are fully scavenged by the O-atom acceptor DMSO, whereas O=FeIVCl3− is not, implicates the electronic coupling of mixed-valence iron centers in weakening the FeIV=O bond. Present results suggest a more significant role than hitherto envisioned for the FeIV species produced in Fenton and Fenton-like chemistries at aqueous interfaces opposite hydrophobic media, such as air in atmospheric aerosols and clouds, proteins in living tissues, biomembranes, and bio-microenvironments.
Methods
Our experiments involve the injection of aqueous FeCl2 jets into the spraying chamber of an ES mass spectrometer (Agilent 6130 Quadrupole LC/MS Electrospray System) flushed with N2(g) at 1 atm, 293 K. Jets are exposed therein to orthogonal gas-phase O3 or H2O2 beams. The species produced on the surface of such jets are analyzed in situ via online ES-MS. The present experimental setup essentially is the same as the one reported elsewhere (62, 64, 65). Solutions are pumped (100 μL⋅min−1) into the spraying chamber through a grounded stainless steel needle (100-μm bore) coaxial with a sheath-issuing nebulizer N2(g) at a high gas velocity vg (∼160 m/s). The species detected by ES-MS are assumed to be produced in collisions of gaseous H2O2 or O3 with the surface of the intact aqueous jets containing microdroplets (D0 > 1 μm) as they emerge from the nozzle, i.e., before they are broken up into submicrometer-sized droplets (58) (see also SI Text). These smaller droplets already carry net charge of either sign. It should be emphasized that charge separation is a one-time event driven by the conversion of kinetic energy of the nebulizer gas into surface and electrostatic energies of submicrometer-sized droplets (58). We have demonstrated the surface specificity of our experiments by showing that (i) anion signal intensities in the mass spectra of equimolar salt solutions adhere to a normal Hofmeister series (rather than being identical) (91, 92), (ii) the depth of the interfacial layers sampled is controllable as a function of nebulizer gas velocity v (58), and (iii) they allow the detection of products of gas–liquid reactions that could be formed only at the air–water interface (62, 64–66, 71).
Gaseous hydrogen peroxide, H2O2(g), was injected into the spraying chamber carried by ultrapure (>99.999%) N2(g) sparging hydrogen peroxide solution [extra pure reagent, 30% (wt/wt) in water; Nacalai Tesque] kept in a trap held at 293 K in a temperature-controlled bath (TRC-4C; Thomas). Carrier gas flow rates were regulated by calibrated digital mass flow controllers (SEC-400 Mark 3; Horiba STEC) up to 1 standard liter per minute (Fig. S1). [H2O2(g)] was derived from the reported H2O2 vapor pressures of H2O2:H2O mixture at the temperatures (93). We verified that Cl− is inert toward H2O2(g) under present conditions (Fig. S5). Teflon gas lines were cleaned and dried daily with ultrapure nitrogen gas. Ozone was generated by flowing ultrapure O2(g) (>99.998%; Kyoto Teisan) through a silent discharge ozonizer (KSQ-050; Kotohira) and quantified via online UV-visible absorption spectrophotometry (Agilent 8453; Agilent Technologies) at 250 and 300 nm [absorption cross sections σ(250 nm) = 1.1 × 10−17, σ(300 nm) = 3.9 × 10−19 cm2⋅molecule−1 at 298 K] before entering the reaction chamber (Fig. S2). Throughout, the reported [O3(g)] values, which correspond to the concentrations actually sensed by the microjets in the reaction chamber, are estimated to be ∼13 times smaller than the values determined from UV absorbance because of further dilution by the drying gas. The gas molecule hitting the surface of the pH-adjusted (by concentrated HCl/NaOH, and the pH was already measured by a calibrated pH meter, Horiba LAQUA F-74, before the experiments) aqueous microjet can stick to it by accommodation, reacting therein, or rebound (94, 95). See Supporting Information for more details.
Supplementary Material
Acknowledgments
We are grateful to Dr. Himanshu Mishra and Profs. Michael Hoffmann, William Goddard, and Harry Gray of the California Institute of Technology for valuable discussions. S.E. thanks the Japan Science and Technology Agency PRESTO program, Grant for Environmental Research Projects from The Sumitomo Foundation, and Steel Foundation for Environmental Protection Technology. Y.S. thanks the Grant-in-Aid for Japan Society for the Promotion of Science Fellows for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314885111/-/DCSupplemental.
References
- 1.Groves JT. High-valent iron in chemical and biological oxidations. J Inorg Biochem. 2006;100(4):434–447. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon RA, Kochi JK. Metal-Catalyzed Oxidation of Organic Compounds. New York: Academic; 1981. [Google Scholar]
- 3.Bakac A. Oxygen activation with transition-metal complexes in aqueous solution. Inorg Chem. 2010;49(8):3584–3593. doi: 10.1021/ic9015405. [DOI] [PubMed] [Google Scholar]
- 4.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 5.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 6.Bataineh H, Pestovsky O, Bakac A. pH-induced mechanistic changeover from hydroxyl radicals to iron(IV) in the Fenton reaction. Chem. Sci. 2012;3(5):1594–1599. [Google Scholar]
- 7.Conocchioli TJ, Hamilton EJ, Sutin N. Formation of iron(IV) in the oxidation of iron(II) J Am Chem Soc. 1965;87(4):926–927. [Google Scholar]
- 8.Logager T, Holcman J, Sehested K, Pedersen T. Oxidation of ferrous-ions by ozone in acidic solutions. Inorg Chem. 1992;31(17):3523–3529. [Google Scholar]
- 9.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Mishra S, Imlay J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch Biochem Biophys. 2012;525(2):145–160. doi: 10.1016/j.abb.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouault TA. Cell biology. An ancient gauge for iron. Science. 2009;326(5953):676–677. doi: 10.1126/science.1181938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, et al. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ Sci Technol. 2008;42(13):4927–4933. doi: 10.1021/es800408u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris E, et al. Enhanced role of transition metal ion catalysis during in-cloud oxidation of SO2. Science. 2013;340(6133):727–730. doi: 10.1126/science.1230911. [DOI] [PubMed] [Google Scholar]
- 15.Charrier JG, Anastasio C. Impacts of antioxidants on hydroxyl radical production from individual and mixed transition metals in a surrogate lung fluid. Atmos Environ. 2011;45(40):7555–7562. doi: 10.1016/j.atmosenv.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deguillaume L, et al. Transition metals in atmospheric liquid phases: Sources, reactivity, and sensitive parameters. Chem Rev. 2005;105(9):3388–3431. doi: 10.1021/cr040649c. [DOI] [PubMed] [Google Scholar]
- 17.Jacob DJ. Heterogeneous chemistry and tropospheric ozone. Atmos Environ. 2000;34(12-14):2131–2159. [Google Scholar]
- 18.Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. 2nd Ed. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 19.Dunford HB. Oxidations of iron(II)/(III) by hydrogen peroxide: From aquo to enzyme. Coord Chem Rev. 2002;233:311–318. [Google Scholar]
- 20.Deguillaume L, Leriche M, Chaumerliac N. Impact of radical versus non-radical pathway in the Fenton chemistry on the iron redox cycle in clouds. Chemosphere. 2005;60(5):718–724. doi: 10.1016/j.chemosphere.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Cho KB, et al. Evidence for an alternative to the oxygen rebound mechanism in C-H bond activation by non-heme Fe(IV)O complexes. J Am Chem Soc. 2012;134(50):20222–20225. doi: 10.1021/ja308290r. [DOI] [PubMed] [Google Scholar]
- 22.Prat I, et al. Observation of Fe(V)=O using variable-temperature mass spectrometry and its enzyme-like C-H and C=C oxidation reactions. Nat Chem. 2011;3(10):788–793. doi: 10.1038/nchem.1132. [DOI] [PubMed] [Google Scholar]
- 23.Pestovsky O, Bakac A. Reactivity of aqueous Fe(IV) in hydride and hydrogen atom transfer reactions. J Am Chem Soc. 2004;126(42):13757–13764. doi: 10.1021/ja0457112. [DOI] [PubMed] [Google Scholar]
- 24.Green MT, Dawson JH, Gray HB. Oxoiron(IV) in chloroperoxidase compound II is basic: Implications for P450 chemistry. Science. 2004;304(5677):1653–1656. doi: 10.1126/science.1096897. [DOI] [PubMed] [Google Scholar]
- 25.Munro AW, Girvan HM, Mason AE, Dunford AJ, McLean KJ. What makes a P450 tick? Trends Biochem Sci. 2013;38(3):140–150. doi: 10.1016/j.tibs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Ensing B, Buda F, Gribnau MCM, Baerends EJ. Methane-to-methanol oxidation by the hydrated iron(IV) oxo species in aqueous solution: A combined DFT and Car-Parrinello molecular dynamics study. J Am Chem Soc. 2004;126(13):4355–4365. doi: 10.1021/ja038865a. [DOI] [PubMed] [Google Scholar]
- 27.Decker A, et al. Spectroscopic and quantum chemical studies on low-spin FeIV=O complexes: Fe-O bonding and its contributions to reactivity. J Am Chem Soc. 2007;129(51):15983–15996. doi: 10.1021/ja074900s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srnec M, Wong SD, England J, Que L, Jr, Solomon EI. π-Frontier molecular orbitals in S = 2 ferryl species and elucidation of their contributions to reactivity. Proc Natl Acad Sci USA. 2012;109(36):14326–14331. doi: 10.1073/pnas.1212693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton DHR, Doller D. The selective functionalization of saturated-hydrocarbons—Gif chemistry. Acc Chem Res. 1992;25(11):504–512. [Google Scholar]
- 30.Yeom J, Imlay JA, Park W. Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. J Biol Chem. 2010;285(29):22689–22695. doi: 10.1074/jbc.M110.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juárez-Hernández RE, Miller PA, Miller MJ. Syntheses of siderophore-drug conjugates using a convergent thiol-maleimide system. ACS Med Chem Lett. 2012;3(10):799–803. doi: 10.1021/ml300150y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freinbichler W, et al. Highly reactive oxygen species: Detection, formation, and possible functions. Cell Mol Life Sci. 2011;68(12):2067–2079. doi: 10.1007/s00018-011-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keren I, Wu YX, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339(6124):1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 34.Luo YZ, Han ZX, Chin SM, Linn S. Three chemically distinct types of oxidants formed by iron-mediated Fenton reactions in the presence of DNA. Proc Natl Acad Sci USA. 1994;91(26):12438–12442. doi: 10.1073/pnas.91.26.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akatsuka S, et al. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS One. 2012;7(8):e43403. doi: 10.1371/journal.pone.0043403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang L, et al. Iron overload signature in chrysotile-induced malignant mesothelioma. J Pathol. 2012;228(3):366–377. doi: 10.1002/path.4075. [DOI] [PubMed] [Google Scholar]
- 37.Jomova K, Baros S, Valko M. Redox active metal-induced oxidative stress in biological systems. Transit Met Chem. 2012;37(2):127–134. [Google Scholar]
- 38.Wang B, et al. Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: Catalytic activities mediated by surface chemical states. J Phys Chem C. 2013;117(1):383–392. [Google Scholar]
- 39.Bernasconi L, Baerends EJ. The EDTA complex of oxidoiron(IV) as realisation of an optimal ligand environment for high activity of FeO2+ Eur J Inorg Chem. 2008;2008(10):1672–1681. [Google Scholar]
- 40.Latifi R, Sainna MA, Rybak-Akimova EV, de Visser SP. Does hydrogen-bonding donation to manganese(IV)-oxo and iron(IV)-oxo oxidants affect the oxygen-atom transfer ability? A computational study. Chemistry. 2013;19(12):4058–4068. doi: 10.1002/chem.201202811. [DOI] [PubMed] [Google Scholar]
- 41.Ye SF, Geng CY, Shaik S, Neese F. Electronic structure analysis of multistate reactivity in transition metal catalyzed reactions: The case of C-H bond activation by non-heme iron(IV)-oxo cores. Phys Chem Chem Phys. 2013;15(21):8017–8030. doi: 10.1039/c3cp00080j. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz H. Chemistry with methane: Concepts rather than recipes. Angew Chem Int Ed Engl. 2011;50(43):10096–10115. doi: 10.1002/anie.201006424. [DOI] [PubMed] [Google Scholar]
- 43.Fu Q, et al. Interface-confined ferrous centers for catalytic oxidation. Science. 2010;328(5982):1141–1144. doi: 10.1126/science.1188267. [DOI] [PubMed] [Google Scholar]
- 44.McDonald AR, Que L. High-valent nonheme iron-oxo complexes: Synthesis, structure, and spectroscopy. Coord Chem Rev. 2013;257(2):414–428. [Google Scholar]
- 45.Jackson TA, et al. Axial ligand effects on the geometric and electronic structures of nonheme oxoiron(IV) complexes. J Am Chem Soc. 2008;130(37):12394–12407. doi: 10.1021/ja8022576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofstetter TE, Armentrout PB. Threshold collision-induced dissociation and theoretical studies of hydrated Fe(II): Binding energies and Coulombic barrier heights. J Phys Chem A. 2013;117(6):1110–1123. doi: 10.1021/jp3044829. [DOI] [PubMed] [Google Scholar]
- 47.Weiss J. Reaction mechanism of oxidation-reduction processes. Nature. 1934;133:648–649. [Google Scholar]
- 48.Ensing B, Buda F, Blöchl P, Baerends EJ. Chemical Involvement of Solvent Water Molecules in Elementary Steps of the Fenton Oxidation Reaction. Angew Chem Int Ed Engl. 2001;40(15):2893–2895. doi: 10.1002/1521-3773(20010803)40:15<2893::AID-ANIE2893>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 49.Pestovsky O, Bakac A. Aqueous ferryl(IV) ion: Kinetics of oxygen atom transfer to substrates and oxo exchange with solvent water. Inorg Chem. 2006;45(2):814–820. doi: 10.1021/ic051868z. [DOI] [PubMed] [Google Scholar]
- 50.Kremer ML. Mechanism of the Fenton reaction. Evidence for a new intermediate. Phys Chem Chem Phys. 1999;1(15):3595–3605. [Google Scholar]
- 51.Bossmann SH, et al. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J Phys Chem A. 1998;102(28):5542–5550. [Google Scholar]
- 52.Pestovsky O, et al. Aqueous Fe-IV=O: Spectroscopic identification and oxo-group exchange. Angew Chem Int Ed. 2005;44(42):6871–6874. doi: 10.1002/anie.200502686. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto Y, Enami S, Tonokura K. Enhancement of gaseous iodine emission by aqueous ferrous ions during the heterogeneous reaction of gaseous ozone with aqueous iodide. J Phys Chem A. 2013;117(14):2980–2986. doi: 10.1021/jp308407j. [DOI] [PubMed] [Google Scholar]
- 54.Terman A, Kurz T. Lysosomal iron, iron chelation, and cell death. Antioxid Redox Signal. 2013;18(8):888–898. doi: 10.1089/ars.2012.4885. [DOI] [PubMed] [Google Scholar]
- 55.Watt RK. A unified model for ferritin iron loading by the catalytic center: implications for controlling “free iron” during oxidative stress. ChemBioChem. 2013;14(4):415–419. doi: 10.1002/cbic.201200783. [DOI] [PubMed] [Google Scholar]
- 56.Dhakshinamoorthy A, Navalon S, Alvaro M, Garcia H. Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem. 2012;5(1):46–64. doi: 10.1002/cssc.201100517. [DOI] [PubMed] [Google Scholar]
- 57.Wang JL, Xu LJ. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit Rev Environ Sci Technol. 2012;42(3):251–325. [Google Scholar]
- 58.Enami S, Colussi AJ. Long-range specific ion-ion interactions in hydrogen-bonded liquid films. J Chem Phys. 2013;138(18):184706. doi: 10.1063/1.4803652. [DOI] [PubMed] [Google Scholar]
- 59.Enami S, Mishra H, Hoffmann MR, Colussi AJ. Hofmeister effects in micromolar electrolyte solutions. J Chem Phys. 2012;136(15):154707. doi: 10.1063/1.4704752. [DOI] [PubMed] [Google Scholar]
- 60.Mollah S, Pris AD, Johnson SK, Gwizdala AB, Houk RS. Identification of metal cations, metal complexes, and anions by electrospray mass spectrometry in the negative ion mode. Anal Chem. 2000;72(5):985–991. doi: 10.1021/ac9908647. [DOI] [PubMed] [Google Scholar]
- 61.Enami S, Colussi AJ. Long-range Hofmeister effects of anionic and cationic amphiphiles. J Phys Chem B. 2013;117(20):6276–6281. doi: 10.1021/jp401285f. [DOI] [PubMed] [Google Scholar]
- 62.Enami S, Hoffmann MR, Colussi AJ. Dry deposition of biogenic terpenes via cationic oligomerization on environmental aqueous surfaces. J Phys Chem Lett. 2012;3(21):3102–3108. doi: 10.1021/jz301294q. [DOI] [PubMed] [Google Scholar]
- 63.Mishra H, et al. Brønsted basicity of the air-water interface. Proc Natl Acad Sci USA. 2012;109(46):18679–18683. doi: 10.1073/pnas.1209307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enami S, Stewart LA, Hoffmann MR, Colussi AJ. Superacid chemistry on mildly acidic water. J Phys Chem Lett. 2010;1(24):3488–3493. [Google Scholar]
- 65.Enami S, Hoffmann MR, Colussi AJ. Proton availability at the air/water interface. J Phys Chem Lett. 2010;1(10):1599–1604. [Google Scholar]
- 66.Enami S, Hoffmann MR, Colussi AJ. Acidity enhances the formation of a persistent ozonide at aqueous ascorbate/ozone gas interfaces. Proc Natl Acad Sci USA. 2008;105(21):7365–7369. doi: 10.1073/pnas.0710791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra H, et al. Anions dramatically enhance proton transfer through aqueous interfaces. Proc Natl Acad Sci USA. 2012;109(26):10228–10232. doi: 10.1073/pnas.1200949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zilch LW, Maze JT, Smith JW, Ewing GE, Jarrold MF. Charge separation in the aerodynamic breakup of micrometer-sized water droplets. J Phys Chem A. 2008;112(51):13352–13363. doi: 10.1021/jp806995h. [DOI] [PubMed] [Google Scholar]
- 69.Enami S, Vecitis CD, Cheng J, Hoffmann MR, Colussi AJ. Global inorganic source of atmospheric bromine. J Phys Chem A. 2007;111(36):8749–8752. doi: 10.1021/jp074903r. [DOI] [PubMed] [Google Scholar]
- 70.Vacha R, Slavicek P, Mucha M, Finlayson-Pitts BJ, Jungwirth P. Adsorption of atmospherically relevant gases at the air/water interface: Free energy profiles of aqueous solvation of N2, O2, O3, OH, H2O, HO2, and H2O2. J Phys Chem A. 2004;108(52):11573–11579. [Google Scholar]
- 71.Enami S, Hoffmann MR, Colussi AJ. Prompt formation of organic acids in pulse ozonation of terpenes on aqueous surfaces. J Phys Chem Lett. 2010;1(15):2374–2379. [Google Scholar]
- 72.Finlayson-Pitts BJ. Reactions at surfaces in the atmosphere: Integration of experiments and theory as necessary (but not necessarily sufficient) for predicting the physical chemistry of aerosols. Phys Chem Chem Phys. 2009;11(36):7760–7779. doi: 10.1039/b906540g. [DOI] [PubMed] [Google Scholar]
- 73.Donaldson DJ, Valsaraj KT. Adsorption and reaction of trace gas-phase organic compounds on atmospheric water film surfaces: a critical review. Environ Sci Technol. 2010;44(3):865–873. doi: 10.1021/es902720s. [DOI] [PubMed] [Google Scholar]
- 74.Shiraiwa M, et al. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nat Chem. 2011;3(4):291–295. doi: 10.1038/nchem.988. [DOI] [PubMed] [Google Scholar]
- 75.Meng S, Chakarov DV, Kasemo B, Gao SW. Two-dimensional hydration shells of alkali metal ions at a hydrophobic surface. J Chem Phys. 2004;121(24):12572–12576. doi: 10.1063/1.1827215. [DOI] [PubMed] [Google Scholar]
- 76.Roeselova M, Jungwirth P, Tobias DJ, Gerber RB. Impact, trapping, and accommodation of hydroxyl radical and ozone at aqueous salt aerosol surfaces. A molecular dynamics study. J Phys Chem B. 2003;107(46):12690–12699. [Google Scholar]
- 77.Hug SJ, Leupin O. Iron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction. Environ Sci Technol. 2003;37(12):2734–2742. doi: 10.1021/es026208x. [DOI] [PubMed] [Google Scholar]
- 78.Saykally RJ. Air/water interface: Two sides of the acid-base story. Nat Chem. 2013;5(2):82–84. doi: 10.1038/nchem.1556. [DOI] [PubMed] [Google Scholar]
- 79.Staehelin J, Hoigne J. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ Sci Technol. 1985;19(12):1206–1213. doi: 10.1021/es00142a012. [DOI] [PubMed] [Google Scholar]
- 80.De Laat J, Gallard H. Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: Mechanism and kinetic modeling. Environ Sci Technol. 1999;33(16):2726–2732. [Google Scholar]
- 81.Finlayson-Pitts BJ, Pitts JN. Chemistry of the Upper and Lower Atmosphere. San Diego, CA: Academic; 2000. [Google Scholar]
- 82.Louwerse MJ, Vassilev P, Baerends EJ. Oxidation of methanol by FeO2+ in water: DFT calculations in the gas phase and ab initio MD simulations in water solution. J Phys Chem A. 2008;112(5):1000–1012. doi: 10.1021/jp075914n. [DOI] [PubMed] [Google Scholar]
- 83.Callahan KM, et al. Effect of magnesium cation on the interfacial properties of aqueous salt solutions. J Phys Chem A. 2010;114(32):8359–8368. doi: 10.1021/jp103485t. [DOI] [PubMed] [Google Scholar]
- 84.Luo G, et al. X-ray reflectivity reveals a nonmonotonic ion-density profile perpendicular to the surface of ErCl3 aqueous solutions. J Phys Chem C. 2013;117(37):19082–19090. [Google Scholar]
- 85.Vaida V. Perspective: Water cluster mediated atmospheric chemistry. J Chem Phys. 2011;135(2):020901. doi: 10.1063/1.3608919. [DOI] [PubMed] [Google Scholar]
- 86.Buszek RJ, Francisco JS, Anglada JM. Water effects on atmospheric reactions. Int Rev Phys Chem. 2011;30(3):335–369. [Google Scholar]
- 87.Kerisit S, Rosso KM. Transition path sampling of water exchange rates and mechanisms around aqueous ions. J Chem Phys. 2009;131(11):114512. doi: 10.1063/1.3224737. [DOI] [PubMed] [Google Scholar]
- 88.Rotzinger FP. Mechanism of water exchange for the di- and trivalent metal hexaaqua ions of the first transition series. J Am Chem Soc. 1997;119(22):5230–5238. [Google Scholar]
- 89.Cheng YK, Rossky PJ. Surface topography dependence of biomolecular hydrophobic hydration. Nature. 1998;392(6677):696–699. doi: 10.1038/33653. [DOI] [PubMed] [Google Scholar]
- 90.Enami S, Mishra H, Hoffmann MR, Colussi AJ. Protonation and oligomerization of gaseous isoprene on mildly acidic surfaces: Implications for atmospheric chemistry. J Phys Chem A. 2012;116(24):6027–6032. doi: 10.1021/jp2110133. [DOI] [PubMed] [Google Scholar]
- 91.Cheng J, Hoffmann MR, Colussi AJ. Anion fractionation and reactivity at air/water:methanol interfaces. Implications for the origin of hofmeister effects. J Phys Chem B. 2008;112(24):7157–7161. doi: 10.1021/jp803184r. [DOI] [PubMed] [Google Scholar]
- 92.Cheng J, Vecitis CD, Hoffmann MR, Colussi AJ. Experimental anion affinities for the air/water interface. J Phys Chem B. 2006;110(51):25598–25602. doi: 10.1021/jp066197k. [DOI] [PubMed] [Google Scholar]
- 93.Hwang H, Dasgupta PK. Thermodynamics of the hydrogen peroxide-water system. Environ Sci Technol. 1985;19(3):255–258. doi: 10.1021/es00133a006. [DOI] [PubMed] [Google Scholar]
- 94.Dempsey LP, Brastad SM, Nathanson GM. Interfacial acid dissociation and proton exchange following collisions of DCl with salty glycerol and salty water. J Phys Chem Lett. 2011;2(6):622–627. [Google Scholar]
- 95.Davidovits P, Kolb CE, Williams LR, Jayne JT, Worsnop DR. Mass accommodation and chemical reactions at gas-liquid interfaces. Chem Rev. 2006;106(4):1323–1354. doi: 10.1021/cr040366k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.