Abstract
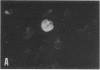
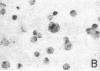
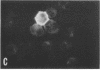
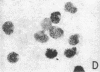
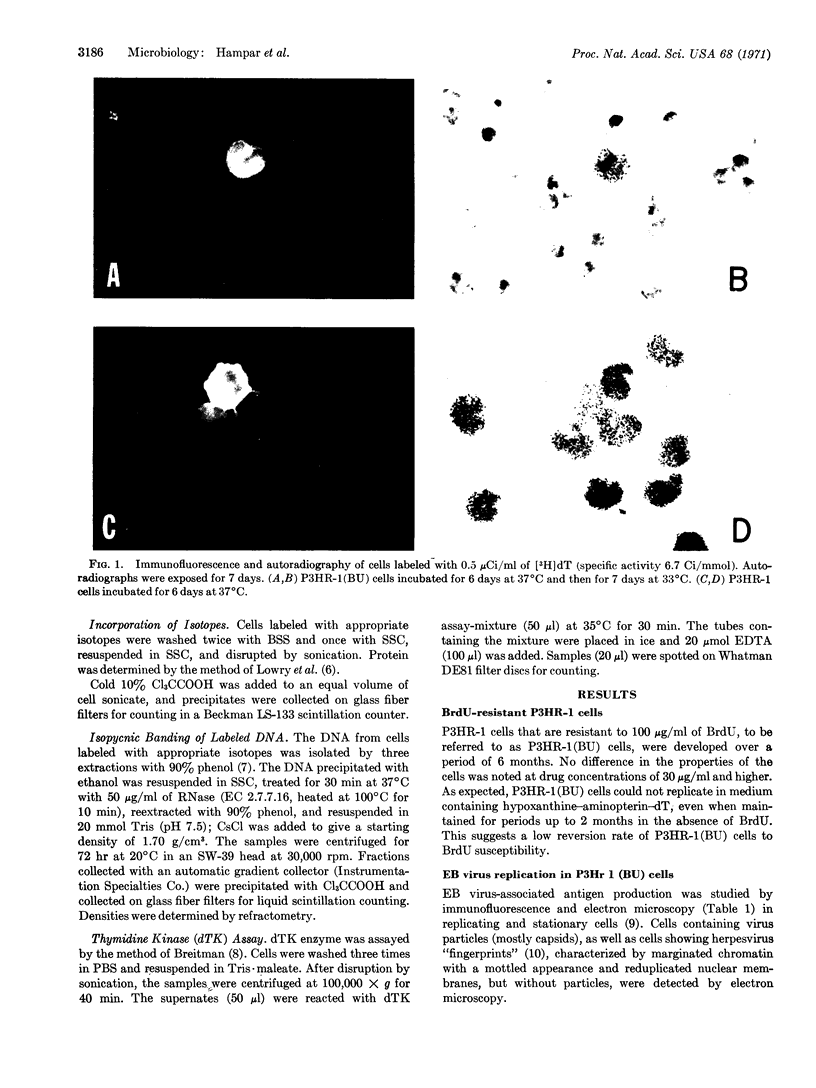
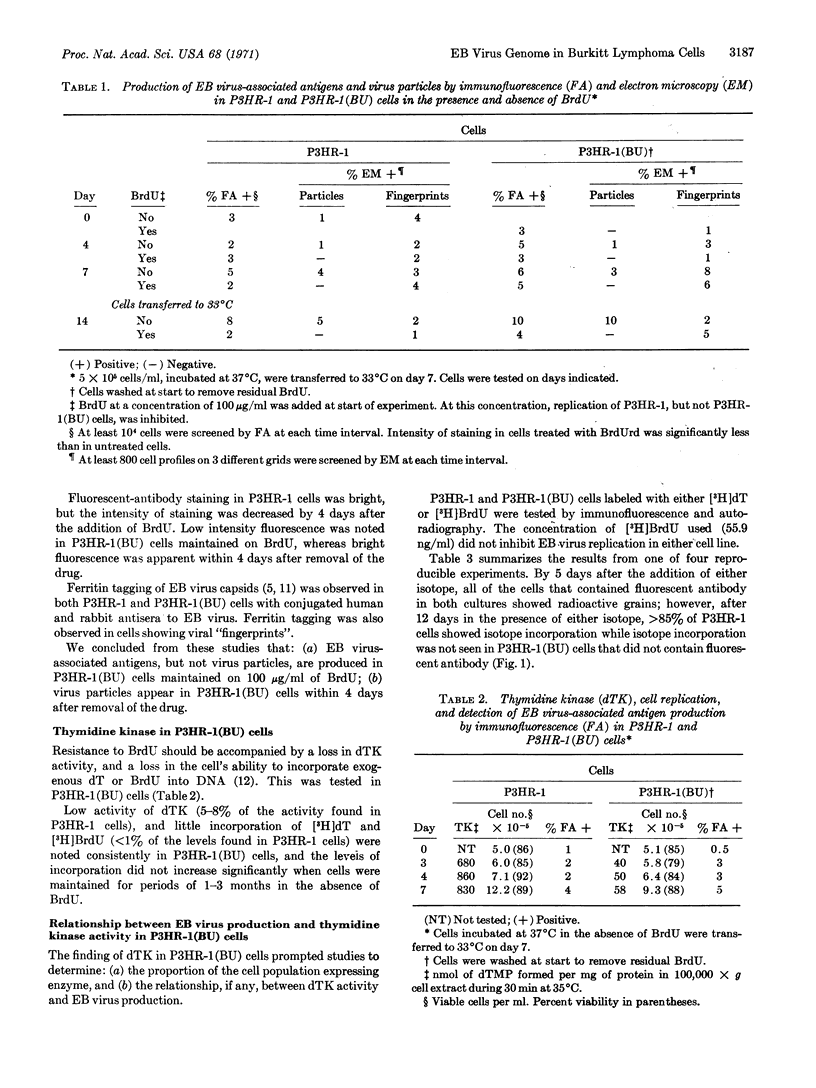
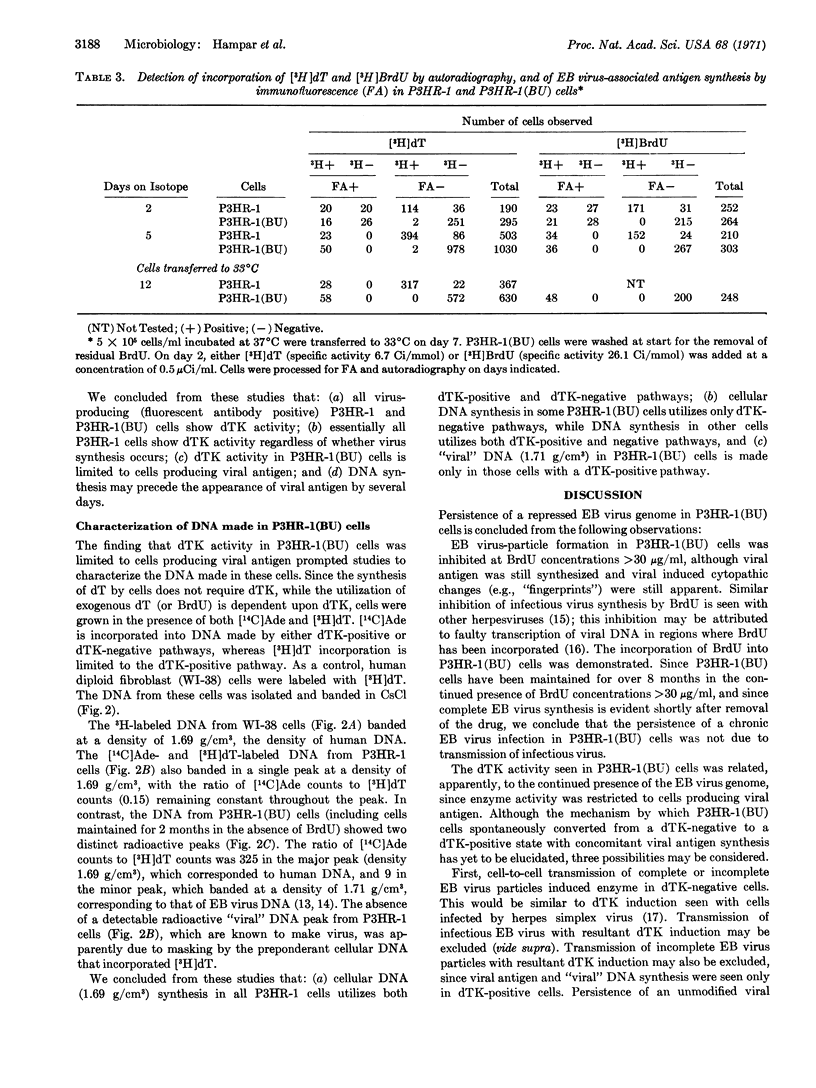
The P3HR-1 line of human lymphoblastoid cells that is Epstein-Barr virus positive was made resistant to 5-bromodeoxyuridine. Epstein-Barr virus-associated antigens, but not virus particles, were produced in P3HR-1(BU) cells maintained on 5-bromodeoxyuridine. However, virus particles did appear within 4 days after removal of the drug. Thymidine kinase activity was limited to P3HR-1(BU) cells producing viral antigen, whereas all control P3HR-1 cells showed thymidine kinase activity regardless of viral antigen synthesis.
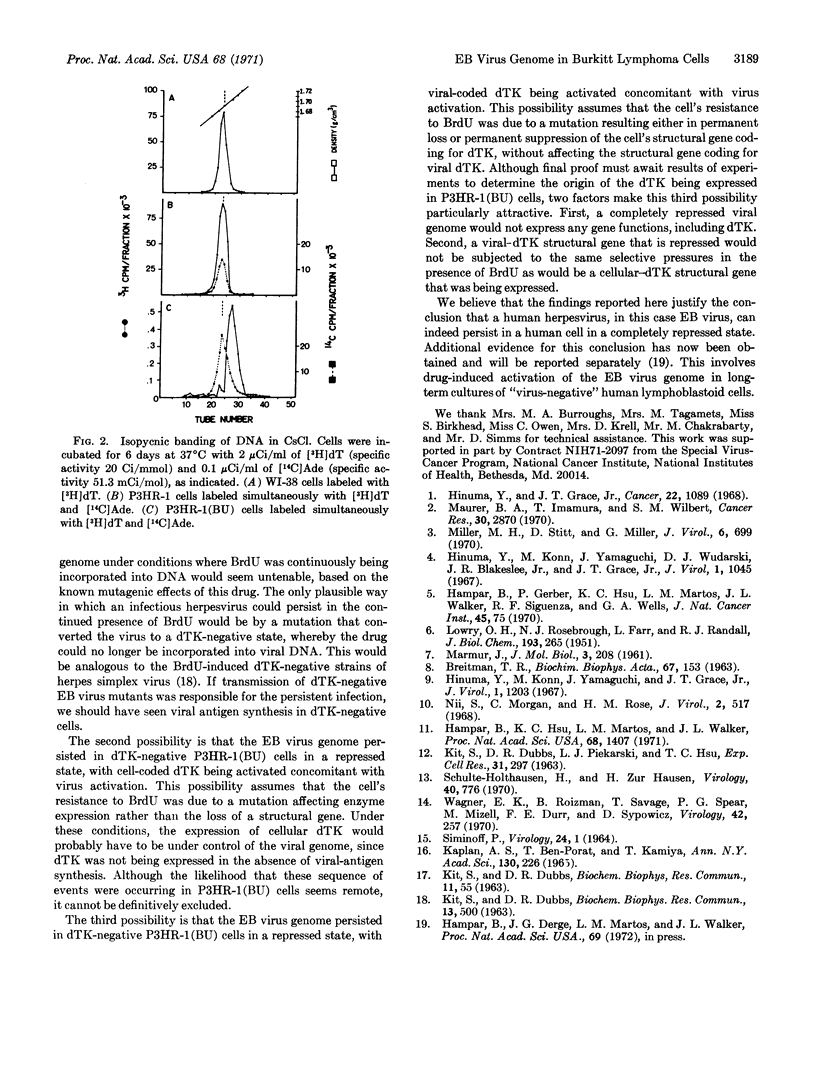
Cellular DNA in most P3HR-1(BU) cells was made via pathways that did not involve thymidine kinase. In cells having a pathway that involved thymidine kinase, a second DNA of density 1.71 g/cm3, corresponding to Epstein-Barr virus, was detected.
It was concluded that: (a) a repressed Epstein-Barr virus genome persists in P3HR-1(BU) cells that do not contain thymidine kinase, with activation of the viral genome being accompanied by productive infection and the appearance of enzyme, and (b) thymidine kinase activity in P3HR-1(BU) cells could be used as a marker for viral genome expression.
Keywords: thymidine kinase, viral antigen, immunofluorescence, electron microscopy
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREITMAN T. R. The feedback inhibition of thymidine kinase. Biochim Biophys Acta. 1963 Jan 8;67:153–155. doi: 10.1016/0006-3002(63)91807-9. [DOI] [PubMed] [Google Scholar]
- Hampar B., Gerber P., Hsu K. C., Martos L. M., Walker J. L., Sigüenza R. F., Wells G. A. Immunoferritin and immunofluorescent studies with Epstein-Barr virus and herpes simplex virus by use of human sera and hyperimmune rabbit sera. J Natl Cancer Inst. 1970 Jul;45(1):75–85. [PubMed] [Google Scholar]
- Hampar B., Hsu K. C., Martos L. M., Walker J. L. Serologic evidence that a herpes-type virus is the etiologic agent of heterophile-positive infectious mononucleosis. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1407–1411. doi: 10.1073/pnas.68.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of Burkitt lymphoma cells cultured in vitro. Cancer. 1968 Dec;22(6):1089–1095. doi: 10.1002/1097-0142(196811)22:6<1089::aid-cncr2820220603>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Grace J. T., Jr Replication of herpes-type virus in a Burkitt lymphoma cell line. J Virol. 1967 Dec;1(6):1203–1206. doi: 10.1128/jvi.1.6.1203-1206.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Benporat T., Kamiya T. Incorporation of 5-bromodeoxyuridine and 5-iododeoxyuridine into viral DNA and its effect on the infective process. Ann N Y Acad Sci. 1965 Jul 30;130(1):226–239. doi: 10.1111/j.1749-6632.1965.tb12556.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maurer B. A., Wilbert S. M., Imamura T. Incidence of EB virus-containing cells in primary and secondary clones of several Burkitt lymphoma cell lines. Cancer Res. 1970 Dec;30(12):2870–2875. [PubMed] [Google Scholar]
- Miller M. H., Stitt D., Miller G. Epstein-Barr viral antigen in single cell clones of two human leukocytic lines. J Virol. 1970 Nov;6(5):699–701. doi: 10.1128/jvi.6.5.699-701.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMINOFF P. THE EFFECT OF 5-BROMODEOXYURIDINE ON HERPES SIMPLEX INFECTION OF HELA CELLS. Virology. 1964 Sep;24:1–12. doi: 10.1016/0042-6822(64)90141-2. [DOI] [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B., Savage T., Spear P. G., Mizell M., Durr F. E., Sypowicz D. Characterization of the DNA of herpesviruses associated with Lucké adenocarcinoma of the frog and Burkitt lymphoma of man. Virology. 1970 Sep;42(1):257–261. doi: 10.1016/0042-6822(70)90265-5. [DOI] [PubMed] [Google Scholar]