Significance
Bacteriophytochromes (BphPs) are regulatory proteins that bind a light-absorbing chromophore called biliverdin. Recombinant BphPs show promise for use in regulating neuron function in mammals with light. We explored the possibility that BphPs may sense cues in addition to light. Our motivation was that biliverdin requires oxygen for its synthesis, and some bacteria use BphPs to control photosynthesis in the absence of oxygen. We found that the photosynthetic bacterium Rhodopseudomonas palustris requires two BphP proteins to sense low light when grown in the absence of oxygen; however, the BphPs do not need to have their chromophore to sense low light intensities. BphPs may respond to intracellular signals, such as reducing conditions, in addition to light to regulate downstream functions.
Keywords: purple nonsulfur bacteria, gene regulation, redox control, photoregulation
Abstract
Bacteriophytochromes (BphPs) are light-sensing regulatory proteins encoded by photosynthetic and nonphotosynthetic bacteria. This protein class has been characterized structurally, but its biological activities remain relatively unexplored. Two BphPs in the anoxygenic photosynthetic bacterium Rhodopseudomonas palustris, designated regulatory proteins RpBphP2 and RpBphP3, are configured as light-regulated histidine kinases, which initiate a signal transduction system that controls expression of genes for the low light harvesting 4 (LH4) antenna complex. In vitro, RpBphP2 and RpBphP3 respond to light quality by reversible photoconversion, a property that requires the light-absorbing chromophore biliverdin. In vivo, RpBphP2 and RpBphP3 are both required for the expression of the LH4 antenna complex under anaerobic conditions, but biliverdin requires oxygen for its synthesis by heme oxygenase. On further investigation, we found that the apo-bacteriophytochrome forms of RpBphP2 and RpBphP3 are necessary and sufficient to control LH4 expression in response to light intensity in conjunction with other signal transduction proteins. One possibility is that the system senses a reduced quinone pool generated when light energy is absorbed by bacteriochlorophyll. The biliverdin-bound forms of the BphPs have the additional property of being able to fine-tune LH4 expression in response to light quality. These observations support the concept that some bacteriophytochromes can function with or without a chromophore and may be involved in regulating physiological processes not directly related to light sensing.
Light can be both a beneficial source of energy and a detrimental source of cellular damage. It is not surprising, then, that organisms have evolved mechanisms to sense light. Phytochromes are a major family of photoreceptors found in plants, fungi, and bacteria that help organisms sense and respond to their environment by light-induced isomerization of a chromophore. Phytochromes are promising tools in the field of optogenetics and in the development of near-infrared fluorescent markers, and a large body of biochemical and biophysical analysis exists for this family of photoreceptors (1–3); however, despite advances in elucidating the function of plant phytochromes (3), the function of many phytochromes in bacteria, known as bacteriophytochromes (BphPs), remains unknown (4, 5).
Much of our current knowledge about the physiological role of BphPs comes from work with the anoxygenic photosynthetic bacterium Rhodopseudomonas palustris. R. palustris generates energy from light by cyclic photophosphorylation under semiaerobic and anaerobic growth conditions. Light harvesting complexes consisting of repeating units composed of small alpha and beta proteins that bind bacteriochlorophyll and carotenoids absorb light energy, which is then transferred to reaction centers, where it is converted into a proton gradient used to generate ATP (6). R. palustris has a light harvesting 2 (LH2) antenna complex, which absorbs maximally at ∼800 and 860 nm and predominates under moderate to high light intensities. It also has a light harvesting 4 (LH4) antenna complex with a ∼800-nm absorption maximum. LH4 predominates under low light intensities, where it allows for more efficient light capture.
Two R. palustris BphPs, designated RpBphP2 and RpBphP3, function in a signal transduction system that controls the expression of LH4 antenna complex genes (7, 8). Each of these BphPs has a chromophore-binding domain and a C-terminal histidine kinase domain. The genes RpBphP2 and RpBphP3 are adjacent to rpa3014, rpa3017, and rpa3018, which encode the proteins composing the signal transduction system controlling transcription of the pucBAd LH4 genes (Fig. 1A). In vitro studies have characterized the reversible photoconversion of RpBphP2 and RpBphP3 between a red light (700 nm)-absorbing form, which is also the form that the proteins assume in the dark, and a far-red (750 nm)–absorbing or near-red (650 nm)–absorbing form, respectively (9–12). BphPs bind the chromophore biliverdin IXα (BV), a linear tetrapyrrole that is incorporated autocatalytically into the N-terminal photosensory domain (13, 14). BV is synthesized by heme oxygenase from heme, a reaction that requires oxygen (15).
Fig. 1.
Intact RpBphP2 and RpBphP3 are required for LH4 expression. (A) Arrangement of genes (gray arrows) adjacent to the pucBAd operon (white arrows), which encodes the alpha and beta peptides of LH4. The predicted domain structures for each of the genes shown in gray are shown below the gene arrangement. REC, response receiver domain; HTH, helix-turn-helix domain; PAS, Per Arnt Sim domain; GAF, cGMP-phosphodiesterases, adenyl cyclases, and FhlA domain; PHY, phytochrome domain; HK, histidine kinase domain. CBD, chromophore-binding domain that includes PAS-GAF-PHY domains. (B) Representative whole-cell absorption spectra of intact WT R. palustris, R. palustris ΔbphP2, R. palustris ΔbphP3, and R. palustris ΔHO grown with anoxic conditions under low-intensity white light (4 μmol photons/m2/s).
An increase in LH4 synthesis is seen in R. palustris cells grown in red light under semiaerobic conditions (7, 16). This finding is consistent with the conclusion that under semiaerobic conditions, BV is synthesized and incorporated into RpBphPs, allowing them to respond to red light. R. palustris also increases the synthesis of its LH4 complexes in response to a decrease in white light intensity when grown anaerobically, and both RpBphP2 and RpBphP3 are required for this response (7, 8, 16). In plants and cyanobacteria, phytochromes have been implicated in the sensing of light intensity, for which their ability to bind a chromophore and carry out photoconversion is essential (17, 18).
We wondered how RpBphP2 and RpBphP3 might function to sense light intensity in anaerobically grown R. palustris, a condition in which they are expected to not have a chromophore, because oxygen is required for BV synthesis. Here we investigated this question and were surprised to find that RpBphP2 and RpBphP3 are required for LH4 synthesis, but that the apo-phytochrome forms of these proteins are sufficient for this function, with bound BV not necessary.
Results
Intact RpBphP2 and RpBphP3 Are Required for R. palustris LH4 Expression.
We created in-frame deletion mutants of RpBphP2 or RpBphP3 and assayed LH4 expression in cells by measuring the ratio of whole-cell absorbance at 800 nm to absorbance at 860 nm. The LH4 complex predominates when this ratio is >1, and the LH2 complex predominates when the ratio is <1 (16, 19). We found that deletion of either one of the RpBphP genes disrupted LH4 synthesis in response to low light intensity, similar to previous reports (7, 8) (Fig. 1B and Table 1). The in-frame deletion mutants of RpBphP2 or RpBphP3 were complemented by expression of the WT allele in trans (Table S1). This indicates that both RpBphP2 and RpBphP3 are required for LH4 synthesis, and that the presence of only one of these RpBphPs is insufficient to induce LH4 synthesis in response to low light.
Table 1.
Whole-cell absorption spectra at an 800 nm:860 nm absorption ratio
| Genotype | Growth condition* | Light source | Light intensity, μmol/m2/s | 800:860 absorption ratio (SD)† |
| WT | Anaerobic | 15W halogen | 4 | 1.9 (0.04) |
| ΔbphP2 | Anaerobic | 15W halogen | 4 | 0.7 (0.03) |
| ΔbphP3 | Anaerobic | 15W halogen | 4 | 0.7 (0.01) |
| BphP2R249A | Anaerobic | 15W halogen | 4 | 1.8 (0.07) |
| BphP3R263A | Anaerobic | 15W halogen | 4 | 2.0 (0.06) |
| ΔHO‡ | Anaerobic | 15W halogen | 4 | 1.8 (0.1) |
| WT | Anaerobic | 700 nm LED | 10 | 1.3 (0.03) |
| ΔbphP2 | Anaerobic | 700 nm LED | 10 | 0.7 (0.01) |
| BphP2R249A | Anaerobic | 700 nm LED | 10 | 1.2 (0.04) |
| WT | Semiaerobic | 700 nm LED | 10 | 1.9 (0.04) |
| ΔbphP2 | Semiaerobic | 700 nm LED | 10 | 0.6 (0.1) |
| BphP2R249A | Semiaerobic | 700 nm LED | 10 | 1.2 (0.04) |
| WT | Anaerobic | 750 nm LED | 15 | 0.9 (0.05) |
| ΔbphP2 | Anaerobic | 750 nm LED | 15 | 0.7 (0.01) |
| BphP2R249A | Anaerobic | 750 nm LED | 15 | 0.9 (0.05) |
| WT | Anaerobic | 750 nm LED | 4 | 1.7 (0.03) |
| ΔbphP2 | Anaerobic | 750 nm LED | 4 | 0.7 (0.01) |
| BphP2R249A | Anaerobic | 750 nm LED | 4 | 1.6 (0.1) |
All cultures were transferred into fresh PM medium supplemented with 20 mM acetate under the same growth conditions at least twice before the whole-cell absorption spectra were determined.
Values are the average of at least two or more experiments.
ΔHO has deletions in all four putative heme oxygenases: rpa0359, hmuO, rpa2125, and rpa3279.
RpBphP2 Purified from R. palustris Binds Biliverdin.
Earlier work revealed that BV is covalently attached to RpBphP2 and RpBphP3 when these BphPs are expressed and purified from Escherichia coli expressing Bradyrhizobium japonicum heme oxygenase (7). To determine whether a chromophore is bound to RpBphP2 in R. palustris, we purified His-tagged RpBphP2 from R. palustris cells grown in light under extremely oxygen-limited conditions obtained by boiling and gassing the growth medium and dispensing it in an anaerobic chamber (hereinafter referred to as anoxic growth conditions). The purified protein was capable of photoconversion, and its spectrum was similar to that of RpBphP2 purified from E. coli (Fig. 2A). Zinc fluorescence indicated that RpBphP2 purified from R. palustris had a covalently bound chromophore (Fig. 2B), which could suggest that RpBphP2 binds a unique chromophore that is not dependent on heme oxygenase conversion of heme to BV. Alternatively, R. palustris may have a high-affinity heme oxygenase that can scavenge traces of oxygen from anoxic growth medium to catalyze BV synthesis.
Fig. 2.
BV is the natural chromophore of RpBphP2. His-tagged RpBphP2 was purified from anoxically grown R. palustris extracts using nickel chelate chromatography. (A) Absorption spectra of purified His-tagged proteins that were incubated in the dark (dark-adapted state) included RpBphP2 purified from WT R. palustris (BphP2 dark), R. palustris ΔHO (BphP2 ΔHO dark), RpBphP2R249A purified from WT R. palustris (BphP2R249A dark), and a vector-only control (vector). The absorption spectra for RpBphP2 purified from WT R. palustris after illumination with red light for 5 min (BphP2 light) is shown as well. (B) Purified protein was subjected to SDS/PAGE and assayed for assembly with BV using a zinc-induced fluorescence assay (Zn). This was followed by staining for protein (Prot). Samples include RpBphP2 (+) purified from E. coli with the addition of exogenous BV, WT R. palustris (WT), R. palustris with deletion of one of the four putative heme oxygenases (ΔhmuO, Δrpa0359, Δrpa2125, or Δrpa3279), and R. palustris with deletions in all four heme oxygenases (ΔHO). A vector-only (−) control was used.
To investigate further, we purified RpBphP2 from R. palustris mutants with deletions in each of four putative heme oxygenases encoded in its genome: hmuO (rpa1539), which is encoded next to RpBphP1 (rpa1537) and is homologous to heme oxygenases encoded next to BphPs in other bacterial species; rpa3279, a Pseudomonas aeruginosa pigA homolog; rpa2125, a Staphylococcus aureus isdG homolog; and rpa0359, a Helicobacter pylori hugZ homolog. As shown in Fig. 2B, RpBphP2, isolated from the heme oxygenase hmuO mutant, had a barely detectable amount of zinc-induced fluorescence, indicating that it is responsible for chromophore synthesis. RpBphP2 isolated from a quadruple heme oxygenase deletion mutant (R. palustris ∆HO) also had barely detectable zinc-induced fluorescence, as expected (Fig. 2B). In addition, RpBphP2, purified from the R. palustris ∆HO mutant strain, did not exhibit detectable red or far-red absorption properties (Fig. 2A), indicating that it does not have a bound chromophore. Even though RpBphP2 from the ∆HO mutant strain had no bound chromophore, R. palustris ∆HO mutant cells expressed WT levels of LH4 (Fig. 1B and Table 1).
Identification of RpBphP2 and RpBphP3 Variants Unable to Bind BV in Vitro and in Vivo.
To obtain additional evidence that R. palustris apo-bacteriophytochromes can direct LH4 synthesis, we used site-directed mutagenesis to generate RpBphP2 and RpBphP3 variants that were unable to bind BV. Previous work has shown that covalent attachment of BV to BphPs is mediated by a thioether linkage formed with a conserved cysteine in the N-terminal photosensory core domain (20). Disrupting the thioether linkage using an alanine substitution at this conserved cysteine is a common strategy used to abrogate covalent attachment of BV to BphP. However, in Deinococcus radiodurans BphP (DrBphP), although alanine substitutions at this position disrupted covalent attachment of BV, DrBphP was still able to interact with BV and maintain a relatively normal absorbance spectrum (21). Similar results have been reported for other BphPs and phytochromes as well (22, 23).
To identify mutations that would disrupt both covalent attachment of BV and noncovalent association of BV with the BphP, we subjected all of the RpBphP2 and RpBphP3 variants that we generated to the zinc-induced fluorescence assay to look for covalent attachment of BV. We also ascertained the spectral properties of each variant to detect noncovalent associations with BV.
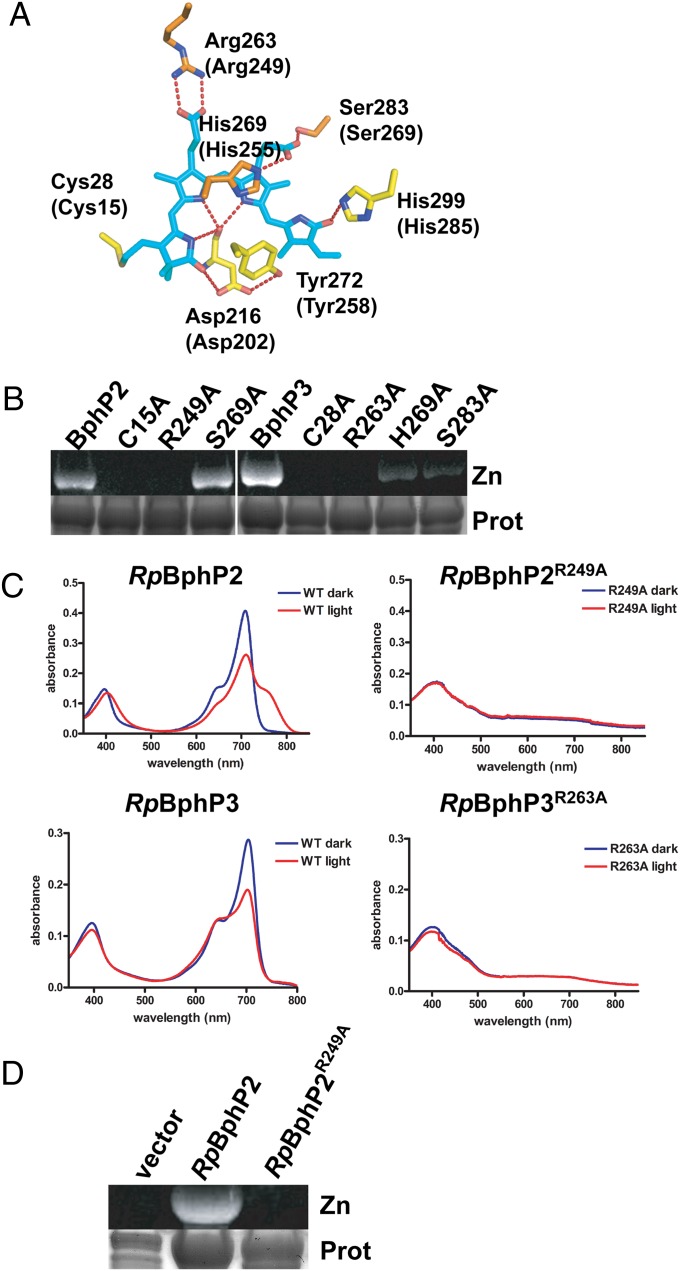
In addition to alanine substitutions at the conserved cysteine (C15 for RpBphP2 and C28 for RpBphP3), we constructed variants with alanine substitutions at R249, H255, and S269 in RpBphP2 and the corresponding residues in RpBphP3 (R263, H269, and S283) (Fig. 3A). We chose these residues because they are conserved in most BphPs, and because crystal structures of the chromophore-binding pocket of RpBphP2 and RpBphP3 show that these residues interact with the propionate side chains of BV (9, 24). We did not investigate residues that interact with the D pyrrole ring of BV, because these have been shown to be involved in photoconversion (9, 12). All RpBphP variants were purified from E. coli expressing a heme oxygenase, and cell extracts were incubated with exogenously added BV before protein purification.
Fig. 3.
Variants of RpBphP2 and RpBphP3 defective in binding BV. (A) Structure showing the residues of RpBphP3 that interact with BV. The corresponding residues in RpBphP2 are given in parentheses. BV is in cyan, and oxygen and nitrogen atoms are in red and blue, respectively. (B–D) His-tagged recombinant proteins were purified by nickel chelate chromatography. (B) Purified protein was subjected to SDS/PAGE and assayed for assembly with BV using a zinc-induced fluorescence assay (Zn), followed by staining for protein (Prot). (C) Absorption spectra of purified RpBphP2, RpBphP2R249A, RpBphP3, and RpBphP3R263A. Spectra were determined for protein incubated in the dark (blue line) and after illumination with red light for 5 min (red line). (D) Protein purified from R. palustris was subjected to SDS/PAGE and assayed for assembly with BV using a zinc-induced fluorescence assay (Zn), followed by staining for protein (Prot). Samples include a vector-only control, RpBphP2, and RpBphP2R249A from WT R. palustris.
Like other BphPs, RpBphP2 and RpBphP3 with alanine substitutions at the conserved cysteines were unable to covalently bind BV (Fig. 3B); however, even though the amount of associated BV was reduced, these variants of RpBphP2 and RpBphP3 still had detectable absorption at 700 nm (Fig. S1). An alanine substitution at H255 in RpBphP2 resulted in an unstable protein and was not characterized further. An alanine substitution at S269 in RpBphP2 demonstrated no effect on BV binding or photoconversion (Fig. 3B and Fig. S1). Unlike in RpBphP2, alanine substitutions at H269 or S283 in RpBphP3 resulted in variants that could still covalently bind BV, but with reduced BV binding and abnormal absorption spectra (Fig. 3B and Fig. S1). The differing properties observed for these variants of RpBphP2 and RpBphP3 could be related to the increased number of hydrogen bonds in the BV-binding pocket of RpBphP3, which is thought to make the BV environment more rigid (24). This could be why changes in the BV-binding pocket have a greater effect on BV binding in RpBphP3 than in RpBphP2.
Of all of the variants tested, only RpBphP2R249A and RpBphP3R263A were unable to associate with BV. These variants were unable to covalently bind BV and also exhibited little or no red, far-red, or near-red absorption, indicating that unlike RpBphP2C15A and RpBphP3C28A, these variants are not able to associate with BV (Fig. 3 B and C). This finding indicates that substitution of the arginine with alanine, which abolishes two salt bridges that form with the propionate side chain of the B pyrrole ring, is sufficient to prevent both covalent and noncovalent association of BV with either of these RpBphPs. Thus, the conserved arginine in these two RpBphPs may be required for autocatalytic incorporation of BV. This is in contrast to DrBphP, where an alanine substitution at this conserved arginine is unable to disrupt BV binding, but a more disruptive glutamine substitution blocks BV binding (21).
We purified the RpBphP2R249A variant from R. palustris to confirm that it does not bind BV in this background. As shown in Fig. 2A and Fig. 3D, no chromophore was associated with RpBphP2R249A. This finding indicates that amino acid substitutions in RpBphP2 that disrupt BV binding in vitro also disrupt BV binding in R. palustris in vivo, and that RpBphP2R249A can be used to determine whether a BphP can function in the absence of BV.
Biliverdin Is Required to Sense Light Quality Under Semiaerobic Conditions.
Under semiaerobic conditions, LH4 synthesis occurs in response to red light irrespective of light intensity (7). Because BV is required for activation of purified RpBphP2 and RpBphP3 by red light, BV should be required in vivo for RpBphP2/P3 to up-regulate LH4 expression in response to red light under semiaerobic conditions. We tested this idea for RpBphP2, using chromosomal allelic exchange to create a RpBphP2R249A mutant strain of R. palustris and assaying LH4 expression by measuring whole-cell absorbance.
As shown in Fig. 4A and Table 1, semiaerobically grown cells of the RpBphP2R249A mutant were defective in LH4 synthesis, indicating that BV is required to increase LH4 synthesis in response to red light. The RpBphP2R249A mutant synthesized more LH4 compared with a RpBphP2 deletion mutant, however, indicating that BV is not required for RpBphP2 activity, but does modulate RpBphP2 activity in response to changes in light quality under semiaerobic conditions.
Fig. 4.
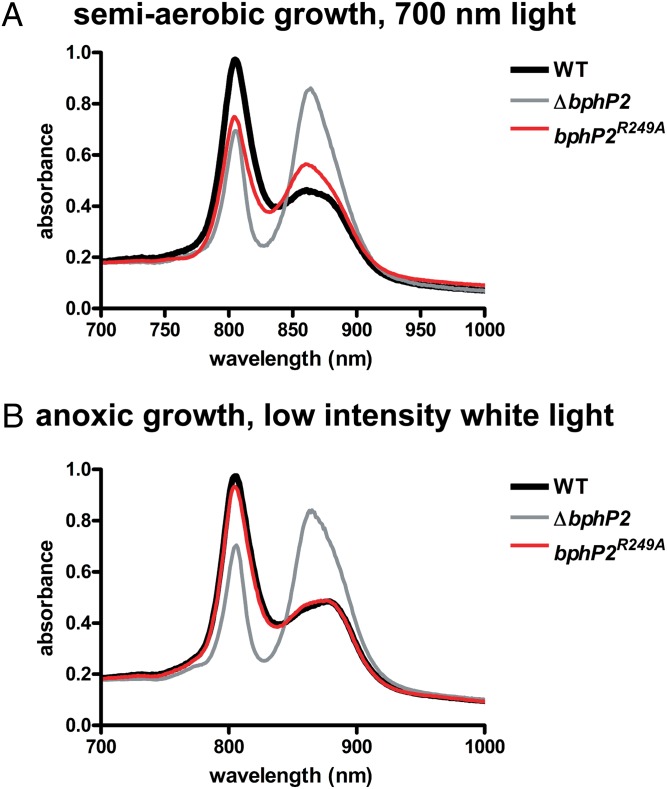
BV is not required for RpBphP2 to function. Representative whole-cell absorption spectra of intact WT R. palustris, R. palustris ΔbphP2, and R. palustris bphP2R249A grown with semiaerobic cultivation under 700-nm light (10 μmol photons/m2/s) (A) or with anoxic cultivation under low light intensity (4 μmol photons/m2/s) (B).
Apo-Phytochrome RpBphP2 and RpBphP3 Are Required to Sense Light Intensity.
Previous work has shown that enhancement of LH4 synthesis occurs in response to decreasing intensity of white light under anaerobic conditions (8). Along with its role in sensing light quality, BV also may play a role in sensing light intensity. To determine whether BV is required for this, we measured whole-cell absorption spectra of RpBphP2R249A and RpBphP3R263A mutant cells grown in anoxic medium in low-intensity white light. As shown in Fig. S2, Fig. 4B, and Table 1, the mutant strains demonstrated normal LH4 expression.
Although RpBphP2 and RpBphP3 do not require a chromophore to function under low-intensity white light, variants that are not bound to a chromophore may be less proficient in responding to activating red (700 nm) or deactivating far-red (750 nm) light. In our evaluation of the ability of RpBphP2R249A mutant cells to up-regulate LH4 synthesis in response to red and far-red light under anoxic conditions, they behaved as WT cells (Fig. 5 and Table 1), suggesting that cells respond to the intensity rather than the wavelength of the red and far-red light. We tested this idea by decreasing the intensity of the far-red light. Under these conditions, both RpBphP2 and RpBphP3 should be in their inactive, dark-adapted state, yet high levels of LH4 synthesis were seen (Fig. 5B and Table 1). This finding indicates that LH4 gene expression responds to light intensity rather than to light quality under anoxic conditions. It also rules out the possibility that another photosensory protein that responds to different light wavelengths, such as a blue light photosensor, is involved in regulating LH4 gene expression.
Fig. 5.
Light quality does not modulate LH4 synthesis under anoxic conditions. Representative whole-cell absorption spectra of intact WT R. palustris, R. palustris ΔbphP2, and R. palustris bphP2R249A grown with anoxic cultivation under 700-nm light (10 μmol photons/m2/s) (A) or with anoxic cultivation under 15 μmol photons/m2/s (moderate intensity) or 4 μmol photons/m2/s (low intensity) 750-nm light (B).
RpBphP1 Can Also Function Without BV.
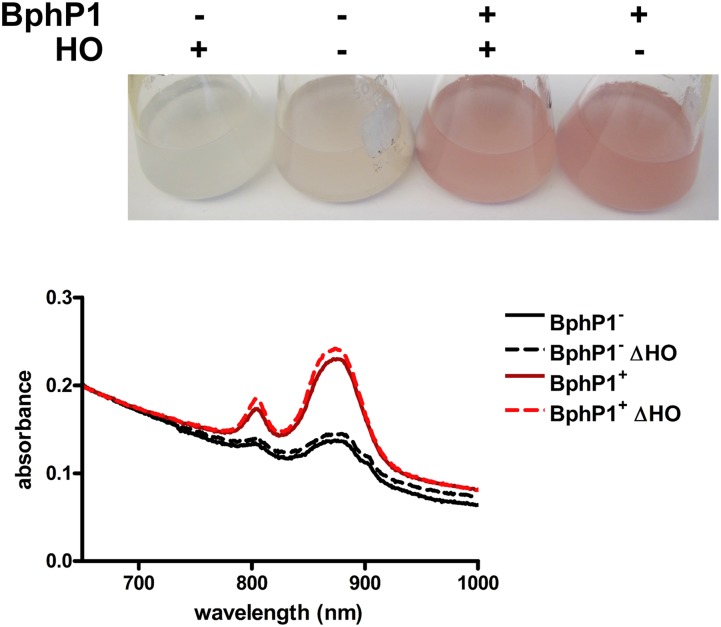
Our data indicate that RpBphP2 and RpBphP3 do not require BV to function in vivo in sensing light intensity. This also seems to be true for another RpBphP in R. palustris, RpBphP1 (Rpa1537). Unlike RpBphP2 and RpBphP3, the RpBphP1 output domain is composed of a Per Arnt Sim (PAS) domain and a two-helix output sensor (HOS) domain, which allows it to interact with its cognate repressor, PpsR2 (25). PpsR2 is an oxygen-responsive repressor that controls expression of a large number of core photosynthesis genes (26). Under high aeration, synthesis of the photosynthetic apparatus is repressed, resulting in reduced pigmentation and a decrease in the photosystem as determined by absorption spectra of intact cells (27, 28). In R. palustris CGA009, RpBphP1 contains a frameshift mutation. When this frameshift mutation is repaired, an increase in pigmentation and photosystem synthesis is seen even under high aeration, supporting the role of RpBphP1 as an antirepressor of PpsR2 (Fig. 6) (28).
Fig. 6.
BV is not required for RpBphP1 to function. (Upper) Cultures of R. palustris with (+) or without (−) an intact RpBphP1 gene (BphP1) or all four heme oxygenases (ΔHO) grown aerobically in white light (30 μmol photons/m2/s). (Lower) Whole-cell absorption spectra of intact cells taken from the cultures.
To determine whether RpBphP1 requires a chromophore to function in vivo, we repaired the frameshift mutation in RpBphP1 in the R. palustris strain CGA009 ∆HO strain. As shown in Fig. 6, deletion of the heme oxygenases in the repaired strain did not affect the ability of RpBphP1 to antagonize PpsR2 repression, and increased pigmentation and photosystem synthesis was seen even under high aeration in light. This finding indicates that RpBphP1, like RpBphP2 and RpBphP3, does not require a chromophore to function in vivo. In addition, because RpBphP1 has a different output domain than RpBphP2 and RpBphP3, this finding suggests that the type of BphP output domain is not a determining factor in whether or not the BphP can function without BV.
Discussion
Our results show that the apo-phytochrome forms of RpBphP2 and RpBphP3 are absolutely required to sense light intensity and play a central role in signal transduction leading to LH4 expression in the photosynthetic bacterium R. palustris. These proteins also bind a BV chromophore under aerobic and low-oxygen growth conditions, and the chromophore senses light quality to fine-tune the activities of RpBphP2 and RpBphP3.
Extensive in vitro work has shown that RpBphP2 and RpBphP3 can assemble with BV and function as light-regulated histidine kinases (7); however, because BV synthesis requires oxygen, it was thought that these RpBphPs might not always be associated with BV in vivo, particularly under anaerobic photosynthetic growth conditions (8, 15, 20, 29). We found that RpBphP2 is bound to BV produced by HmuO in vivo in cells grown under extremely oxygen-limited conditions. This indicates that HmuO most likely has a high affinity for oxygen, allowing it to scavenge trace amounts of oxygen to synthesize BV. In support of this conclusion, other work has shown that HmuO from Corynebacterium diphtheriae has a 20-fold greater oxygen affinity than mammalian myoglobins, and heme oxygenases have been found in strict anaerobes, including Clostridium tetani and Clostridium perfringens, where they may function in maintaining an anoxic environment (30–32). HmuO was identified in the proteome of anaerobically grown R. palustris, which suggests that HmuO functions under culture conditions considered anaerobic (33).
Although it is conceivable that under much stricter anaerobic conditions, RpBphP2 and RpBphP3 are not associated with BV and do not function, our findings indicate otherwise. LH4 regulation was normal when we disrupted BV binding in vivo either by introducing amino acid substitutions in RpBphP that abrogated BV binding in vitro or by deleting the heme oxygenase so that both RpBphP2 and RpBphP3 were not associated with BV.
Under anoxic conditions, the RpBphP2/P3-initiated regulatory cascade that controls LH4 synthesis responds to a decreasing intensity of white light (19). Phytochromes and cyanobacteriochromes have been implicated in sensing not only light quality, but also light intensity (17, 34). We were surprised to find that that the RpBphP2/P3 chromophore BV is not required to sense light intensity under anoxic conditions. Involvement of another photosensory protein, such as a blue light photosensor, or another chromophore bound to RpBphPs is unlikely, given that lowering the intensity of 750-nm light is sufficient for synthesis of LH4. This indicates that light intensity is more important than wavelength under these conditions, and that light intensity is not sensed as a change in light quality.
Even though light intensity is not being sensed as a change in light quality, there must be involvement of a light-absorbing chromophore that can transmit this signal. We believe that this chromophore is bacteriochlorophyll bound by the LH peptides, which absorbs light and transmits it to the reaction center, where it is used to reduce quinones in the membrane. In other systems, the redox status of the quinone pool changes under different light intensities, and light intensity is sensed as a change in the redox state of the quinone pool (35–40). We hypothesize that light intensity is sensed as a redox signal by R. palustris, and that this redox signal combined with the pool of active BphPs (even in the absence of BV) is sufficient for LH4 synthesis. We also altered the redox state of the cell by growing R. palustris under nitrogen-fixing conditions, and observed even more LH4 synthesis under low light intensity, suggesting that LH4 synthesis is responding to a redox signal.
How the RpBphP2/P3-initiated regulatory cascade might sense a redox signal remains unclear. Another RpBphP from R. palustris (RpBphP4; rpa1490) has been characterized as existing naturally in an apo-phytochrome form. This protein has redox-sensitive cysteines that respond to low oxygen tension to control the synthesis of LH2 genes (41). RpBphP2/P3 do not have similarly placed cysteines, but these RpBphPs do have a PAS domain, and other PAS domains have been implicated in sensing redox potential (42). Moreover, Rpa3018, a putative response regulator encoded downstream of RpBphP2 and RpBphP3, has been proposed to act as a redox sensor for incorporating a redox signal into the RpBphP2/P3-initiated regulatory cascade (20).
It is possible that functioning without BV is a unique characteristic of BphPs in anaerobic bacteria, because they are more likely to encounter conditions in which BV might not be synthesized. It is also possible that this is an intrinsic property of many BphPs. If so, this may confound in vivo studies of BphPs. If BV is not necessary for BphPs to function, then looking for a phenotype in response to changes in light quality might not be sufficient to identify a role for a BphP, and actually might contribute to the poor understanding of the function of many BphPs in nonphotosynthetic bacteria. This factor also should be taken into consideration when using BphPs in optogenetics; careful engineering of BphPs may be needed to tightly regulate their activity by light.
Methods
Bacterial Strains and Culture Conditions.
All R. palustris strains were grown aerobically during manipulation on defined mineral medium (PM)-agar supplemented with 10 mM succinate at 30 °C (43). All aerobic and semiaerobic cultures were grown as described previously (26) in PM supplemented with 20 mM acetate. All R. palustris strains grown were grown anoxically in PM supplemented with 20 mM acetate in light at 30 °C. The growth medium was deaerated by heating, followed by extensive bubbling with argon gas. The medium was dispensed into culture tubes in an anaerobic glove box, and the tubes were sealed with rubber stoppers. All cultures were initially grown anaerobically with 30 μmol photons/m2/s from a 60W halogen light bulb (General Electric) and then diluted at least twice into fresh PM medium supplemented with 20 mM acetate after exposure to either low light (4 μmol photons/m2/s) from a 15W halogen light bulb (General Electric) or 700-nm light (10 μmol photons/m2/s), 750-nm light (15 μmol photons/m2/s), or 750-nm light (4 μmol photons/m2/s) from a series of LEDs with a bandwidth <30 nm (Roithner Lasertechnik; LED700-02AU and LED750-03AU). E. coli strains Rosetta 2(DE3)pLysS (EMD Bioscience) and S17-1 (44) were grown in LB medium at 37 °C. When appropriate, R. palustris was grown with gentamicin at 100 μg/mL. E. coli cultures were supplemented with ampicillin 100 μg/mL, kanamycin 50 μg/mL, or chloramphenicol 25 μg/mL.
Genetic Manipulation of R. palustris.
All strains and plasmids used are listed in Table S2. To create a deletion of hmuO, PCR was performed using Platinum Taq High-Fidelity DNA polymerase (Invitrogen) to amplify 1 kb upstream of hmuO plus 200 bp of the 5′ end of hmuO using primers incorporating a XbaI restriction site at the 5′ end and a SpeI restriction site at the 3′ end of the amplified region. The 1-kb sequence downstream of hmuO plus 200 bp of the 3′ end of hmuO was also amplified using primers incorporating a SpeI restriction site at the 5′ end and a BamHI restriction site at the 3′ end of the amplified region. These fragments were then ligated into pBluescript II SK+ vector (Agilent Technologies). This construct was then digested with XbaI and BamHI and ligated into the pJQ200SK suicide vector (45).
In-frame deletions of RpBphP2, RpBphP3, rpa0359, rpa2125, and rpa3279 were created by PCR using Phusion High-Fidelity DNA polymerase (New England Biolabs) to amplify 1 kb upstream of the second codon in the coding region, as well as the 1-kb sequence downstream of the stop codon, for each of these genes. These fragments were then incorporated into PstI-digested (RpBphP2, RpBphP3, and rpa0359) or NotI/PstI-digested (rpa2125 and rpa3279) pJQ200SK suicide vector using the In-Fusion PCR cloning system (Clontech).
Complementing vectors p-bphP2 and p-bphP3 were constructed using PCR amplification with Phusion High-Fidelity DNA polymerase (New England Biolabs). For p-bphP2, the RpBphP2 coding sequence plus 381 bp upstream of the translation start site was amplified and incorporated into EcoRI- and XbaI-digested pBB1RMCS-5 using the In-Fusion PCR cloning system (Clontech). For p-bphP3, the RpBphP3 coding sequence was amplified with primers incorporating a ribosomal binding site and then introduced into EcoR1- and XbaI-digested pBBPgdh.
The vectors used for allelic exchange of WT RpBphP2 for RpBphP2R249A or WT RpBphP3 for RpBphP3R263A were constructed as follows. PCR amplification of RpBphP2 or RpBphP3 was carried out using Phusion High-Fidelity DNA polymerase (New England Biolabs). The resulting 2.3-kb fragment was incorporated into PstI-digested pJQ200SK using the In-Fusion PCR cloning system (Clontech). Site-directed mutagenesis of the resulting plasmid using the PCR-based QuikChange method (Agilent Technologies) was carried out to introduce the R249A substitution into the RpBphP2 coding sequence or the R263A substitution into the RpBphP3 coding sequence.
All plasmids were mobilized into R. palustris by conjugation with E. coli S17-1, and double-crossover events for deletions or allelic exchange was achieved using a selection and screening strategy as described previously (46). All deletions were verified by PCR, and allelic exchange was verified using PCR and sequencing of the resulting PCR product.
A plasmid that would allow expression of His-tagged RpBphP2 was constructed by PCR from an previously described expression vector (9) that encoded RpBphP2 residues 1–505. The region amplified from this vector included the ribosomal-binding site to the 3′ end of the coding sequence, and the primers used incorporated an EcoRI restriction site at the 5′ end and an XbaI restriction site at the 3′ end. This fragment was then digested with EcoRI and XbaI and ligated into the plasmid pBBPgdh (47). This construct was mobilized into WT R. palustris or R. palustris ΔHO by conjugation with E. coli S17-1.
Protein Purification from E. coli.
Site-directed mutagenesis of the previously described pBAD::rpa3015 (RpBphP2) and pBAD::rpa3016 (RpBphP3) was carried out using the PCR-based QuikChange method (Agilent Technologies) (7). After mutations were confirmed by sequencing, plasmids encoding variants of RpBphP2 and RpBphP3 were transformed and expressed in E. coli Rosetta 2(DE3)pLysS (EMD Bioscience). Cells were grown to an OD600 of 0.4–0.5, and protein expression was induced by the addition of l-arabinose to a final concentration of 1 mM, followed by growth for 6 h at 37 °C with shaking. Cells were then harvested by centrifugation at 4,000 × g for 15 min at 4 °C.
Purification was carried out as described previously (9) with the following modification. After sonication and centrifugation, cell extracts were incubated with exogenous BV for 5 min at room temperature. Purified protein was then subjected to zinc-induced fluorescence after SDS/PAGE to determine whether covalent attachment of BV to the apoprotein had occurred (48). The protein gel was then stained for protein using Gelcode Blue Safe protein stain (Fisher Scientific).
Protein Purification from R. palustris.
Cells were grown in PM medium with 20 mM acetate and 1% yeast extract. The medium was sparged with argon gas for 30 min before sterilization. After sterilization, the medium was sparged with nitrogen gas for 30 min and then inoculated 1:200 with cells that had been grown anaerobically in PM with 20 mM acetate. The inoculated culture was then sparged with nitrogen gas for an additional 5 min. The culture was then placed in an anaerobic jar with a GasPak EZ satchet (BD Biosciences) and grown in light using a 60W halogen light bulb with slow stirring. The culture was allowed to grow to an OD660 of 4.0. The culture was kept anaerobic until centrifugation at 4,000 × g for 15 min at 4 °C. Purification was carried out as described previously (9), with the following modifications: Cells were disrupted using a French press, and no exogenous BV was added to the cell extracts.
Spectrophotometry Analyses.
All spectroscopy was carried out using a Beckman Coulter DU 800 spectrophotometer. All purified BphPs were incubated overnight in the dark at 4 °C for dark-adapted samples. Equal amounts of protein were then diluted in water, and the spectra were recorded either in the dark or after 5 min of illumination with light passed through a 700-nm OD 2 short-pass filter (Edmunds Optics). Whole-cell absorption spectra of R. palustris were measured as described previously (49).
Supplementary Material
Acknowledgments
We thank Dr. Eric Giraud for providing pBAD::rpa3015 and pBAD::rpa3016. We also thank Dr. William Parson and Dr. Stephen Hawley for helpful discussions, and Ken Schaefer for assistance with assembling the light sources used in this work. This work was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, US Department of Energy (Grant DEFG02-05ER15707, to C.S.H.), and a grant from the National Sciences and Engineering Research Council of Canada (to J.T.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322410111/-/DCSupplemental.
References
- 1.Möglich A, Moffat K. Engineered photoreceptors as novel optogenetic tools. Photochem Photobiol Sci. 2010;9(10):1286–1300. doi: 10.1039/c0pp00167h. [DOI] [PubMed] [Google Scholar]
- 2.Shu X, et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science. 2009;324(5928):804–807. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulijasz AT, Vierstra RD. Phytochrome structure and photochemistry: Recent advances toward a complete molecular picture. Curr Opin Plant Biol. 2011;14(5):498–506. doi: 10.1016/j.pbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61(1):11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auldridge ME, Forest KT. Bacterial phytochromes: More than meets the light. Crit Rev Biochem Mol Biol. 2011;46(1):67–88. doi: 10.3109/10409238.2010.546389. [DOI] [PubMed] [Google Scholar]
- 6.Cogdell RJ, et al. The structural basis of light-harvesting in purple bacteria. FEBS Lett. 2003;555(1):35–39. doi: 10.1016/s0014-5793(03)01102-5. [DOI] [PubMed] [Google Scholar]
- 7.Giraud E, et al. A new type of bacteriophytochrome acts in tandem with a classical bacteriophytochrome to control the antennae synthesis in Rhodopseudomonas palustris. J Biol Chem. 2005;280(37):32389–32397. doi: 10.1074/jbc.M506890200. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Noll S, Beatty JT. Bacteriophytochrome-dependent regulation of light-harvesting complexes in Rhodopseudomonas palustris anaerobic cultures. Curr Microbiol. 2010;61(5):429–434. doi: 10.1007/s00284-010-9634-1. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Stojkovic EA, Kuk J, Moffat K. Crystal structure of the chromophore binding domain of an unusual bacteriophytochrome, RpBphP3, reveals residues that modulate photoconversion. Proc Natl Acad Sci USA. 2007;104(30):12571–12576. doi: 10.1073/pnas.0701737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toh KC, Stojković EA, van Stokkum IH, Moffat K, Kennis JT. Fluorescence quantum yield and photochemistry of bacteriophytochrome constructs. Phys Chem Chem Phys. 2011;13(25):11985–11997. doi: 10.1039/c1cp00050k. [DOI] [PubMed] [Google Scholar]
- 11.Toh KC, Stojkovic EA, van Stokkum IH, Moffat K, Kennis JT. Proton-transfer and hydrogen-bond interactions determine fluorescence quantum yield and photochemical efficiency of bacteriophytochrome. Proc Natl Acad Sci USA. 2010;107(20):9170–9175. doi: 10.1073/pnas.0911535107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh KC, et al. Primary reactions of bacteriophytochrome observed with ultrafast mid-infrared spectroscopy. J Phys Chem A. 2011;115(16):3778–3786. doi: 10.1021/jp106891x. [DOI] [PubMed] [Google Scholar]
- 13.Bhoo SH, Davis SJ, Walker J, Karniol B, Vierstra RD. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature. 2001;414(6865):776–779. doi: 10.1038/414776a. [DOI] [PubMed] [Google Scholar]
- 14.Lamparter T, et al. The biliverdin chromophore binds covalently to a conserved cysteine residue in the N-terminus of Agrobacterium phytochrome Agp1. Biochemistry. 2004;43(12):3659–3669. doi: 10.1021/bi035693l. [DOI] [PubMed] [Google Scholar]
- 15.Dammeyer T, Frankenberg-Dinkel N. Function and distribution of bilin biosynthesis enzymes in photosynthetic organisms. Photochem Photobiol Sci. 2008;7(10):1121–1130. doi: 10.1039/b807209b. [DOI] [PubMed] [Google Scholar]
- 16.Evans K, et al. A bacteriophytochrome regulates the synthesis of LH4 complexes in Rhodopseudomonas palustris. Photosynth Res. 2005;85(2):169–180. doi: 10.1007/s11120-005-1369-7. [DOI] [PubMed] [Google Scholar]
- 17.Rockwell NC, Martin SS, Lagarias JC. Red/green cyanobacteriochromes: Sensors of color and power. Biochemistry. 2012;51(48):9667–9677. doi: 10.1021/bi3013565. [DOI] [PubMed] [Google Scholar]
- 18.Casal JJ. Shade avoidance. Arabidopsis Book. 2012;10:e0157–e0157. doi: 10.1199/tab.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartigan N, Tharia HA, Sweeney F, Lawless AM, Papiz MZ. The 7.5-A electron density and spectroscopic properties of a novel low-light B800 LH2 from Rhodopseudomonas palustris. Biophys J. 2002;82(2):963–977. doi: 10.1016/S0006-3495(02)75456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giraud E, Verméglio A. Bacteriophytochromes in anoxygenic photosynthetic bacteria. Photosynth Res. 2008;97(2):141–153. doi: 10.1007/s11120-008-9323-0. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JR, et al. Mutational analysis of Deinococcus radiodurans bacteriophytochrome reveals key amino acids necessary for the photochromicity and proton exchange cycle of phytochromes. J Biol Chem. 2008;283(18):12212–12226. doi: 10.1074/jbc.M709355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamparter T, Michael N, Mittmann F, Esteban B. Phytochrome from Agrobacterium tumefaciens has unusual spectral properties and reveals an N-terminal chromophore attachment site. Proc Natl Acad Sci USA. 2002;99(18):11628–11633. doi: 10.1073/pnas.152263999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorissen HJ, Quest B, Lindner I, Tandeau de Marsac N, Gärtner W. Phytochromes with noncovalently bound chromophores: The ability of apophytochromes to direct tetrapyrrole photoisomerization. Photochem Photobiol. 2002;75(5):554–559. doi: 10.1562/0031-8655(2002)075<0554:pwnbct>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Bellini D, Papiz MZ. Dimerization properties of the RpBphP2 chromophore-binding domain crystallized by homologue-directed mutagenesis. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 8):1058–1066. doi: 10.1107/S0907444912020537. [DOI] [PubMed] [Google Scholar]
- 25.Bellini D, Papiz MZ. Structure of a bacteriophytochrome and light-stimulated protomer swapping with a gene repressor. Structure. 2012;20(8):1436–1446. doi: 10.1016/j.str.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Braatsch S, et al. Rhodopseudomonas palustris CGA009 has two functional ppsR genes, each of which encodes a repressor of photosynthesis gene expression. Biochemistry. 2006;45(48):14441–14451. doi: 10.1021/bi061074b. [DOI] [PubMed] [Google Scholar]
- 27.Giraud E, et al. Bacteriophytochrome and regulation of the synthesis of the photosynthetic apparatus in Rhodopseudomonas palustris: Pitfalls of using laboratory strains. Photochem Photobiol Sci. 2004;3(6):587–591. doi: 10.1039/b315770a. [DOI] [PubMed] [Google Scholar]
- 28.Braatsch S, Johnson JA, Noll K, Beatty JT. The O2-responsive repressor PpsR2 but not PpsR1 transduces a light signal sensed by the BphP1 phytochrome in Rhodopseudomonas palustris CGA009. FEMS Microbiol Lett. 2007;272(1):60–64. doi: 10.1111/j.1574-6968.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 29.Rey FE, Harwood CS. FixK, a global regulator of microaerobic growth, controls photosynthesis in Rhodopseudomonas palustris. Mol Microbiol. 2010;75(4):1007–1020. doi: 10.1111/j.1365-2958.2009.07037.x. [DOI] [PubMed] [Google Scholar]
- 30.Brüggemann H, Bauer R, Raffestin S, Gottschalk G. Characterization of a heme oxygenase of Clostridium tetani and its possible role in oxygen tolerance. Arch Microbiol. 2004;182(2-3):259–263. doi: 10.1007/s00203-004-0721-1. [DOI] [PubMed] [Google Scholar]
- 31.Hassan S, et al. Transcriptional regulation of hemO encoding heme oxygenase in Clostridium perfringens. J Microbiol. 2010;48(1):96–101. doi: 10.1007/s12275-009-0384-3. [DOI] [PubMed] [Google Scholar]
- 32.Unno MM, et al. Crystal structure of the dioxygen-bound heme oxygenase from Corynebacterium diphtheriae: Implications for heme oxygenase function. J Biol Chem. 2004;279(20):21055–21061. doi: 10.1074/jbc.M400491200. [DOI] [PubMed] [Google Scholar]
- 33.VerBerkmoes NC, et al. Determination and comparison of the baseline proteomes of the versatile microbe Rhodopseudomonas palustris under its major metabolic states. J Proteome Res. 2006;5(2):287–298. doi: 10.1021/pr0503230. [DOI] [PubMed] [Google Scholar]
- 34.Casal JJ. Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem Photobiol. 2000;71(1):1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Swem LR, Gong X, Yu C-A, Bauer CE. Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB. J Biol Chem. 2006;281(10):6768–6775. doi: 10.1074/jbc.M509687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Bauer CE. RegB kinase activity is controlled in part by monitoring the ratio of oxidized to reduced ubiquinones in the ubiquinone pool. MBio. 2010;1(5):e00272-10. doi: 10.1128/mBio.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamiya K, Takamiya A. Light-induced reactions of ubiquinone in photosynthetic bacterium, Chromatium D, II: Effects of inhibitors and other experimental conditions. Plant Cell Physiol. 1969;10:113–127. [Google Scholar]
- 38.Maxwell DP, Laudenbach DE, Huner N. Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol. 1995;109(3):787–795. doi: 10.1104/pp.109.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escoubas JM, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92(22):10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivleva NB, Gao T, LiWang AC, Golden SS. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc Natl Acad Sci USA. 2006;103(46):17468–17473. doi: 10.1073/pnas.0606639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuillet L, et al. Evolution of a bacteriophytochrome from light to redox sensor. EMBO J. 2007;26(14):3322–3331. doi: 10.1038/sj.emboj.7601770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor BL, Zhulin IB. PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63(2):479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M-K, Harwood CS. Regulation of benzoate-CoA ligase in Rhodopseudomonas palustris. FEMS Microbiol Lett. 2006;83:199–203. [Google Scholar]
- 44.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram-negative bacteria. Nat Biotechnol. 1983;1:784–791. [Google Scholar]
- 45.Quandt J, Hynes MF. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127(1):15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 46.Rey FE, Heiniger EK, Harwood CS. Redirection of metabolism for biological hydrogen production. Appl Environ Microbiol. 2007;73(5):1665–1671. doi: 10.1128/AEM.02565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKinlay JB, Harwood CS. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci USA. 2010;107(26):11669–11675. doi: 10.1073/pnas.1006175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berkelman TR, Lagarias JC. Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Anal Biochem. 1986;156(1):194–201. doi: 10.1016/0003-2697(86)90173-9. [DOI] [PubMed] [Google Scholar]
- 49.Oda Y, et al. Multiple genome sequences reveal adaptations of a phototrophic bacterium to sediment microenvironments. Proc Natl Acad Sci USA. 2008;105(47):18543–18548. doi: 10.1073/pnas.0809160105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.