Significance
The innate immune system plays a key role in host defense that involves the detection of microbial components and a series of signaling events that lead to production of interferons and cytokines. Recently, the identification of mitochondrial antiviral-signaling (MAVS) protein placed mitochondria at the forefront of the innate immune response against virus infection. However, how the MAVS signaling complex is assembled and regulated on the mitochondria outer membrane is only partially understood. Here we show that tripartite motif 14 (TRIM14) facilitates the assembly of the MAVS complex assembly. Upon virus infection, TRIM14 recruits NF-κB essential modulator (NEMO) to the MAVS complex via ubiquitin chains. Knockdown of TRIM14 disrupts the MAVS–NEMO association and attenuates the antiviral response. Our results thus provide a missing link in MAVS-mediated innate immune signaling.
Abstract
Innate immunity provides the first line of host defense against invading microbial pathogens. This defense involves retinoic acid-inducible gene-I–like receptors that detect viral RNA and activate the mitochondrial antiviral-signaling (MAVS) protein, an adaptor protein, leading to activation of the innate antiviral immune response. The mechanisms by which the MAVS signalosome assembles on mitochondria are only partially understood. Here, we identify tripartite motif 14 (TRIM14) as a mediator in the immune response against viral infection. TRIM14 localizes to the outer membrane of mitochondria and interacts with MAVS. Upon viral infection, TRIM14 undergoes Lys-63–linked polyubiquitination at Lys-365 and recruits NF-κB essential modulator to the MAVS signalosome, leading to the activation of both the IFN regulatory factor 3 and NF-κB pathways. Knockdown of TRIM14 disrupts the association between NF-κB essential modulator and MAVS and attenuates the antiviral response. Our results indicate that TRIM14 is a component of the mitochondrial antiviral immunity that facilitates the immune response mediated by retinoic acid-inducible gene-I–like receptors.
Activation of the innate immune response involves the detection of pathogen-associated molecular patterns (PAMPs), such as microbial nucleic acids, proteins, lipids, and carbohydrates. PAMPs are recognized by cellular pattern recognition receptors (PRRs), including Toll-like receptors, retinoic acid-inducible gene-I (RIG-I)–like receptors (RLRs), NOD-like receptors, and C-type lectin receptors. Upon recognition, PRRs trigger a series of signaling events that lead to the induction of type I IFNs and proinflammatory cytokines (1).
RLRs such as RIG-I and melanoma differentiation-associated antigen 5 (MDA5) recognize cytosolic viral RNA (2). Upon binding of RNA to the helicase domain, RIG-I or MDA5 undergoes a conformational change (3) and is recruited to the mitochondrial antiviral signaling (MAVS) adaptor. After binding of RIG-1 or MAD5, MAVS recruits various downstream molecules and further activates two kinase complexes: the noncanonical IκB kinases (IKKs) [TANK-binding kinase 1 (TBK1)/IKKi] and the canonical IKK complexes comprised of IKKα, IKK-β, and NF-κB essential modulator (NEMO) (4, 5). The TBK1/IKKi kinases phosphorylate IFN regulatory factor 3/7 (IRF3/7), which translocates to the nucleus and drives the transcription of IFNs (6). The canonical IKKs phosphorylate IκBα, resulting in the ubiquitination and proteasomal degradation of IκBα. NF-κB then is released to the nucleus and stimulates the expression of proinflammatory genes (7).
MAVS-deficient mice show abolished virus-triggered induction of IFNs and increased susceptibility to viral infection (8), indicating that MAVS is essential for the innate immune response. MAVS consists of an N-terminal caspase activation and recruitment domain, a proline-rich domain, and a C-terminal transmembrane domain that targets it to the mitochondrial outer membrane (9). It has been suggested that the mitochondrial outer membrane provides a physical platform where MAVS acts as a central adaptor and recruits cytosolic proteins to assemble a signaling complex. However, the molecular mechanisms by which the MAVS-associated signaling complex is assembled and how MAVS relays activation signals to IRF3 and NF-κB remain largely unknown.
Recent studies suggest that NEMO is involved in the activation of both IRF3 and NF-κB downstream of MAVS. NEMO mediates TBK1 activation by recruiting TBK1 to MAVS and eventually triggers IRF3 activation (5, 10, 11). In addition, NEMO is critical for activation of the canonical IKK complexes that control NF-κB activity (12). In NEMO-deficient cells, viral infection-induced activation of IRF3 and NF-κB is severely impaired (5). These results suggest that NEMO bridges the IRF3 and NF-κB pathways. However, although it has been suggested that NEMO is recruited to the MAVS complex on mitochondria (13, 14), the specific link that directs NEMO to MAVS remains elusive.
The tripartite motif (TRIM) family of proteins contains more than 60 members, which are thought to mediate a variety of biological processes (15). Recently, some TRIM proteins have been implicated in the regulation of innate immunity (16). In the present study, we identified tripartite motif 14 (TRIM14) as a previously uncharacterized protein involved in RLR-mediated immune response. TRIM14 localizes to mitochondria and facilitates RLR-mediated IRF3 and NF-κB activation. Furthermore, TRIM14 recruits NEMO via K63-linked polyubiquitin chains and links NEMO to the MAVS signalosome. Our findings indicate that TRIM14 is a mediator of mitochondrial antiviral immunity.
Results
Identification of TRIM14 as a Positive Regulator of the Type I IFN Response.
To identify proteins involved in virus-triggered innate immune signaling, we performed a screen using a library of about 300 siRNAs against the genes that are up-regulated during influenza virus infection. Briefly, A549 cells stably expressing an IFN-β promoter–luciferase reporter gene were transfected with individual siRNA oligos from the library, infected with Sendai virus (SeV), and harvested to assess the effect of each siRNA on IFN-β promoter activity. Consistent with previous reports (9, 17), knockdown of MAVS and RIG-I significantly reduced IFN-β activation (Fig. S1A), showing that the screen is functional. Interestingly, knockdown of TRIM14, a member of the TRIM family of genes, remarkably impaired SeV-induced IFN-β activation (Fig. S1A), suggesting that TRIM14 has a role in the innate immune response against viruses.
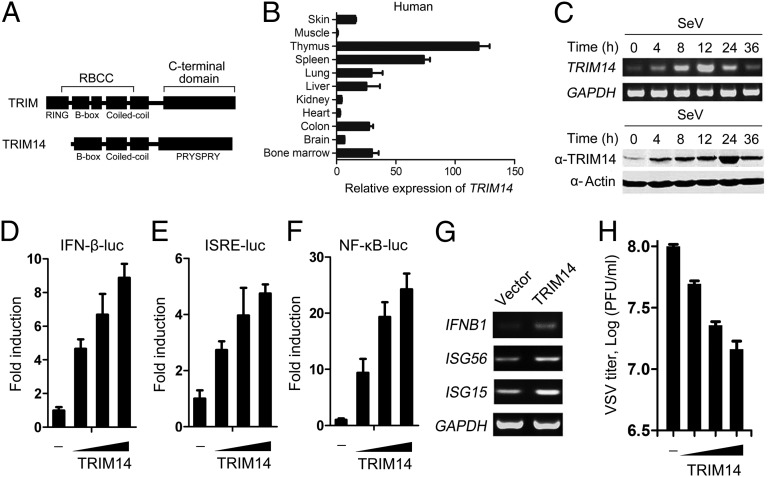
TRIM14 contains a B-box, a coiled-coil, and a C-terminal PRYSPRY domain but lacks the N-terminal RING domain which exists in most TRIM family proteins (Fig. 1A). The biological function of TRIM14 is unknown. Human TRIM14 protein shares 78% homology with the mouse counterpart (Fig. S1B). Real-time quantitative PCR analysis revealed that TRIM14 is expressed in various tissues and that TRIM14 is transcribed in a similar pattern in human and mouse tissues (Fig. 1B and Fig. S1C). We further found that mRNA and protein levels of TRIM14 increased rapidly upon SeV infection (Fig. 1C). Similarly, IFN-β also up-regulated TRIM14 (Fig. S1D), as is consistent with a previous finding that TRIM14 is an IFN-inducible gene (18).
Fig. 1.
TRIM14 facilitates the type-I IFN response. (A) Schematic diagram of TRIM proteins containing a RING domain (Upper) and the TRIM14 protein (Lower). (B) Distribution of TRIM14 in various human tissues was determined using real-time quantitative PCR analyses. Data are mean values ± SD (n = 3). (C) A549 cells were treated with SeV (∼50 HA U/mL) for the indicated time. (Upper) mRNA levels were analyzed by RT-PCR. (Lower) Protein levels were analyzed by Western blot. (D–F) HEK293T cells were transfected with an IFN-β (D), ISRE (E), or NF-κB (F) reporter plasmid, along with a control plasmid or with increasing amounts of plasmids expressing TRIM14 (50 ng, 100 ng, or 200 ng; wedges). Cells were analyzed 24 h after transfection for (D) IFN-β–, (E) ISRE-, or (F) NF-κB–dependent promoter activity. (G) HeLa cells were transfected with a control plasmid or a TRIM14 plasmid (200 ng). After 24 h, total RNA was analyzed for expression of IFNB1, ISG56, ISG15, or GAPDH (control) by RT-PCR. (H) HEK293T cells were transfected with a control plasmid or increasing amounts of TRIM14 plasmids (50 ng, 100 ng, or 200 ng; wedges) for 24 h and then were infected with VSV at an MOI of 0.1. Supernatants were harvested at 16 hpi. The virus titers were determined using standard plaque assays. Data are mean values ± SD (n = 3).
To investigate the role of TRIM14 in the IFN response, cells were transiently transfected with increasing amounts of TRIM14 expression plasmids. We found that TRIM14 activated the IFN-β–responsive promoter in a dose-dependent manner (Fig. 1D). We further investigated whether TRIM14 activates IRF3 and NF-κB, which coordinately regulate the expression of IFN-β. TRIM14 stimulated the activity of IFN-sensitive response element (ISRE), which is an IRF3-dependent promoter (Fig. 1E), and NF-κB (Fig. 1F) in a dose-dependent manner. Consistent with the results of the reporter assays, TRIM14 increased IRF3 phosphorylation and NF-κB DNA-binding activity, which are hallmarks of IRF3 and NF-κB activation, respectively (Fig. S2 A and B). These results indicate that TRIM14 facilitates type I IFN responses.
Next, we cotransfected TRIM14 with signaling proteins involved in innate antiviral response and determined the activation of ISRE or NF-κB promoters. The overexpression of TRIM14 with the RLR signaling proteins, including RIG-I, MDA5, or MAVS, synergistically activated both ISRE and NF-κB promoters, whereas overexpression of TRIM14 with TIR domain-containing adaptor-inducing interferon-β (TRIF), a critical adaptor in TLR signaling, did not produce the synergistic effect (Fig. S2 C and D). These results suggest that TRIM14 is involved in RLR-mediated signaling.
We further determined whether TRIM14 can antagonize viral infection. First, overexpression of TRIM14 increased the mRNA levels of IFNB1, IFN-stimulated gene 56 (ISG56), and IFN-stimulated gene 15 (ISG15) (Fig. 1G), indicating that TRIM14 activates the IFN antiviral response. The overexpression of TRIM14 in HEK293T cells rendered them resistant to infection of vesicular stomatitis virus (VSV) (Fig. 1H) and Newcastle disease virus (NDV) (Fig. S2E). Collectively, these results suggest that TRIM14 plays a positive role in the cellular antiviral IFN response.
TRIM14 Knockdown Inhibits the Cellular Antiviral Response.
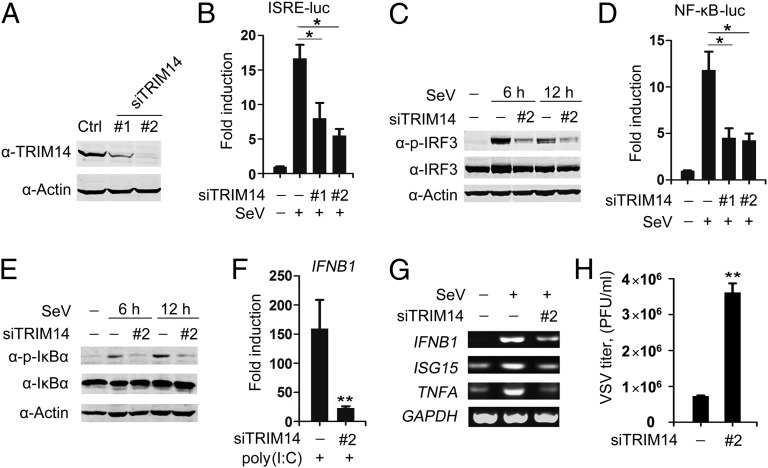
To analyze further the functional significance of TRIM14, we used two independent siRNAs to knock down endogenous TRIM14 (Fig. 2A). We found that down-regulation of TRIM14 decreased SeV-induced activation of the ISRE promoter (Fig. 2B) and IRF3 phosphorylation (Fig. 2C). TRIM14 knockdown also inhibited SeV-induced NF-κB promoter activity (Fig. 2D) and IκBα phosphorylation (Fig. 2E), a critical step in the NF-κB pathway. These results suggest that TRIM14 is required for the activation of both IRF3 and NF-κB following SeV infection.
Fig. 2.
Knockdown of TRIM14 impairs the cellular antiviral response. (A) A549 human lung epithelial cells were transfected with control or TRIM14-specific siRNA oligos as indicated. Forty-eight hours posttransfection, Western blot analysis was used to determine the expression of TRIM14 or actin. (B) A549 cells were transfected with indicated siRNA oligos and an ISRE reporter plasmid. After 48 h, cells were left uninfected or were infected with SeV. Cells were harvested at 12 hpi and analyzed for luciferase activity to determine ISRE activity. Data are mean values ± SD (n = 3). *P < 0.05. (C) A549 cells were transfected with indicated siRNA oligos. After 48 h, cells were left uninfected or were infected with SeV. Cells were harvested at 6 or 12 hpi for Western blot analysis. (D) A549 cells were transfected with indicated siRNA oligos and an NF-κB reporter plasmid. After 48 h, cells left uninfected or infected with SeV for 12 h were analyzed for NF-κB activity. Data are mean values ± SD (n = 3). *P < 0.05. (E) A549 cells were treated as indicated in C and were analyzed by Western blot with indicated antibodies. (F) A549 cells were transfected with indicated siRNA oligos for 48 h. Cells then were transfected with poly(I:C) (500 ng/mL). After 24 h, total RNA was analyzed using real-time quantitative PCR to determine IFNB1 expression. Data are mean values ± SD (n = 3). **P < 0.01. (G) A549 cells were transfected with indicated siRNA oligos for 48 h. Then cells were left uninfected or were infected with SeV. After 16 h, total RNA was analyzed using RT-PCR to determine expression levels of IFNB1, ISG15, TNFA, or GAPDH (control). (H) HEK293T cells were transfected with indicated siRNA oligos. After 48 h, cells were infected with VSV at an MOI of 0.01. Supernatants were harvested at 16 hpi. The virus titers were measured using standard plaque assays. Data are mean values ± SD (n = 3). **P < 0.01.
Next, we observed that TRIM14 knockdown significantly decreased IFNB1 expression induced by transfection of poly(I:C), a synthetic dsRNA that activates RLR-mediated signaling (Fig. 2F). Furthermore, TRIM14 knockdown decreased SeV-induced transcription of IFNB1, ISG15, and TNFA (Fig. 2G), indicating that TRIM14 is critical for IRF3- and/or NF-κB–dependent gene expression following viral infection. In line with this observation, TRIM14 knockdown allowed more robust VSV replication in plaque assays (Fig. 2H), suggesting that TRIM14 is necessary for an efficient cellular antiviral response.
TRIM14 Localizes to Mitochondria.
It has been suggested that the signaling proteins such as MAVS and stimulator of interferon genes, which are involved in the immune response, are localized to specific organelles to exert their functions (9, 19). Using confocal microscopy, we found that endogenous TRIM14 was localized predominantly to mitochondria (Fig. 3A, Upper) but not to the endoplasmic reticulum (ER) (Fig. 3A, Lower Left) or the Golgi apparatus (Fig. 3A, Lower Right). Confocal microscopy and subcellular fraction analysis showed that SeV infection increased mitochondrial distribution of TRIM14 (Fig. 3 B and C). In addition, using a trypsin-protection assay on purified mitochondria, we found that both TRIM14 and MAVS were sensitive to trypsin digestion (Fig. 3D). In contrast, cytochrome c oxidase subunit IV (COXIV), a mitochondria inner membrane protein, and voltage-dependent anion channel 1 (VDAC1), an ion channel extensively inserted in the outer membrane of the mitochondria, were not sensitive to trypsin digestion (Fig. 3D). Overall, these results suggest that TRIM14 are constitutively localized to the outer mitochondrial membrane.
Fig. 3.
TRIM14 is localized to the mitochondria. (A) A549 cells were colabeled using anti-TRIM14 (green) and the mitochondria marker MitoTracker (red) (Upper), anti-TRIM14 (green) and the ER marker anti-calreticulin (red) (Lower Left), or anti-TRIM14 (green) and the Golgi marker anti-GM130 (red) (Lower Right). (Scale bar: 10 μm.) (B) A549 cells were mock infected or were infected with SeV. After 16 h, cells were labeled using anti-TRIM14 (green) and MitoTracker (red). (Scale bar: 10 μm.) (C) A549 cells were mock infected or were infected with SeV. After 16 h, cell fractions were subjected to Western blot analysis using the indicated antibodies. VDAC1 marks mitochondria, Calnexin marks ER, and GAPDH was used as a cytosolic marker. (D) The purified mitochondria were left untreated or were treated with 5 μM of trypsin followed by Western blotting with indicated antibodies. (E) HeLa cells were transfected with the indicated Flag-tagged TRIM14 mutation constructs. Twenty-four hours later, cells were labeled using anti-Flag and MitoTracker. (Scale bar: 10 μm.) (F and G) HEK293T cells were transfected with an IFN-β (F) or an NF-κB (G) reporter plasmid as well as a control plasmid or the indicated Flag-tagged TRIM14 constructs. Luciferase activity was analyzed 24 h later. Data are mean values ± SD (n = 3).
The classic mitochondrial targeting sequence is an N-terminal motif that forms an amphipathic helix with a high proportion of positively charged basic residues (20, 21). Secondary structure prediction and helical wheel representation suggest that the region of TRIM14 from amino acids 29–46 may form such an amphipathic α-helix (Fig. S3A). Consistent with this prediction, deletion of amino acids 30–50 disrupted mitochondrial localization of TRIM14 (Fig. S3 B and C). Arginine, serine, and leucine residues are known to be critical for mitochondrial targeting (20). Thus, we mutated these residues to alanine within residues 29–46 of TRIM14. Mutation of arginine 30, leucine 34, or leucine 46 disrupted the mitochondrial localization of TRIM14 (Fig. 3E). Importantly, overexpression of TRIM14 mutants R30A, L34A, and L46A showed substantial reduction in stimulating IFN-β and NF-κB promoter activities. In contrast, TRIM14 mutations that did not alter its mitochondrial targeting, such as R37A, R38A, R40A, or R41A, were able to stimulate IFN-β and NF-κB promoter activation (Fig. 3 F and G). These results suggest that mitochondrial localization is required for TRIM14 activity.
TRIM14 Interacts with MAVS.
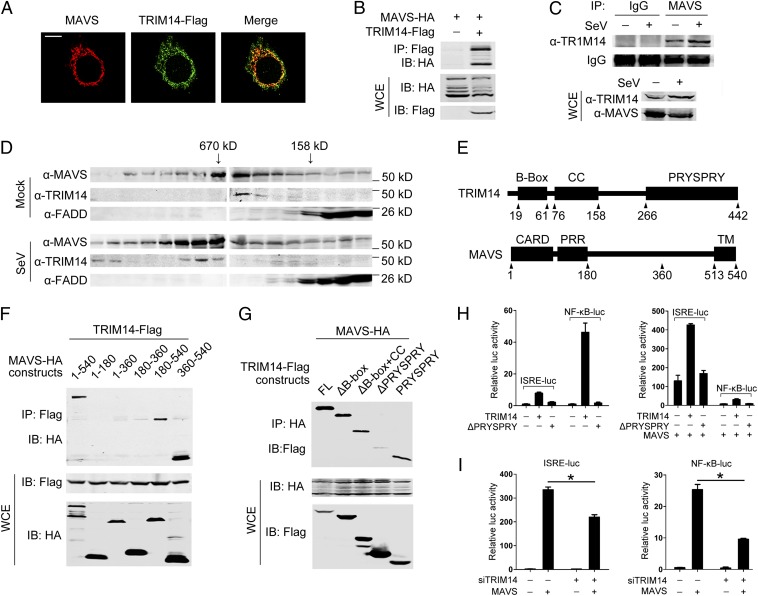
Because MAVS is a critical mitochondrial adaptor protein involved in the innate antiviral response, we next examined if TRIM14 associates with MAVS. Colocalization of TRIM14 and MAVS was observed (Fig. 4A), and a coimmunoprecipitation experiment showed that Flag-tagged TRIM14 interacted with HA-tagged MAVS (Fig. 4B). This interaction also was detected between endogenous TRIM14 and MAVS, and the association was enhanced upon viral infection (Fig. 4C).
Fig. 4.
TRIM14 interacts with MAVS. (A) HeLa cells were transfected with Flag-tagged TRIM14 plasmid. After 24 h, cells were labeled using anti-Flag (green) and anti-MAVS (red). (Scale bar: 10 μm.) (B) HEK293T cells were transfected with HA-tagged MAVS and Flag-tagged TRIM14 plasmids. After 24 h, cell lysates were immunoprecipitated using anti-Flag, followed by Western blot analysis using indicated antibodies. WCE, whole-cell extract. (C) A549 cells were mock infected or were infected with SeV for 12 h. Cell lysates were immunoprecipitated using anti-MAVS or control IgG, followed by Western blot analysis using the indicated antibodies. (D) HEK293T cells were mock infected or were infected with SeV for 12 h. Cell lysates were fractionated on a size-exclusion column. The positions corresponding to the elution of the standard markers of molecular weight are indicated. Cell fractions were analyzed by Western blotting using the indicated antibodies. Anti-FADD was used as a control for nonspecific aggregation. (E) Schematic representations of TRIM14 and MAVS. CC, coiled-coil domain; PRR, proline-rich domain; TM, transmembrane domain. (F and G) HEK293T cells were transfected with the indicated plasmids. After 24 h, cell lysates were immunoprecipitated using anti-Flag (F) or anti-HA (G). The precipitates were analyzed by Western blotting using the indicated antibodies. (H) HEK293T cells were transfected with an ISRE or an NF-κB reporter plasmid as well as a control, TRIM14, or TRM14ΔPRYSPRY plasmid (Left) or together with a MAVS plasmid (Right). Cells were harvested 24 h later, and luciferase activity was analyzed. (I) HEK 293T cells were transfected with control or TRIM14-specific siRNA oligos, along with an ISRE (Left) or NF-κB (Right) reporter plasmid. Forty-eight hours later, cells were transfected with a control plasmid or an MAVS plasmid. Cells were harvested 24 h later, and luciferase activity was analyzed. All data are mean values ± SD (n = 3). *P < 0.05.
Because MAVS is known to assemble into a large signaling complex on mitochondria (22), we examined if TRIM14 is associated with the complex. Size-exclusion chromatography revealed that TRIM14 is coeluted with MAVS in a complex with a molecular mass of ∼600 kDa (Fig. 4D). Upon SeV infection, MAVS and TRIM14 comigrated into higher molecular weight fractions (Fig. 4D). These results indicate that viral infection promotes the assembly of a MAVS–TRIM14 signaling complex.
We subsequently determined the domains that mediate MAVS and TRIM14 association and found that the C-terminal domain of MAVS (residues 360–540) and the PRYSPRY domain of TRIM14 are critical for their interaction (Fig. 4 E–G). Consistently, TRIM14 lacking the PRYSPRY domain (TRIM14ΔPRYSPRY) failed to stimulate the promoter activity of ISRE and NF-κB (Fig. 4H, Left). Furthermore, TRIM14ΔPRYSPRY failed to synergize MAVS-triggered ISRE and NF-κB activation (Fig. 4H, Right), suggesting that PRYSPRY-mediated association with MAVS is required for TRIM14 activity. Additionally, we found that knockdown of TRIM14 impaired MAVS-stimulated activity of the ISRE and NF-κB promoters (Fig. 4I), indicating that TRIM14 exerts its function downstream of MAVS.
Lys-63–Linked Polyubiquitination of TRIM14 Recruits NEMO.
To investigate the mechanism by which TRIM14 regulates MAVS-mediated signaling, we examined the interaction of TRIM14 with proteins involved in the RLR-mediated pathway, including RIG-I, MDA5, TNF receptor-associated factor 3 (TRAF3), TNF receptor-associated factor 6 (TRAF6), NEMO, TBK1, and IRF3. Coimmunoprecipitation experiments revealed that HA-tagged TRIM14 pulled down Flag-tagged TRAF3, NEMO, and TBK1 but not RIG-I, MDA5, TRAF6, or IRF3 (Fig. 5A). Next, we used purified TRAF3, NEMO, and TBK1 to determine the direct binding partner(s) of TRIM14. Although bacterially expressed TRIM14 bound efficiently to purified NEMO, it bound comparatively much less efficiently to TRAF3 and TBK1 (Fig. 5B and Fig. S4). Furthermore, we found that the TRIM14–NEMO binding was further enhanced by SeV infection (Fig. 5C).
Fig. 5.
Lys63-linked ubiquitination of TRIM14 recruits NEMO. (A) HEK293T cells were transfected with the indicated plasmids. After 24 h, cell lysates were immunoprecipitated using anti-Flag, followed by Western blot analysis using the indicated antibodies. (B) Purified GST or GST-TRIM14 proteins were incubated with purified NEMO-Flag protein and subjected to GST pull-down assays. The proteins bound to glutathione-Sepharose beads were analyzed by Western blotting with the indicated antibodies. (C) HEK293T cells were transfected with the indicated plasmids. After 24 h, cells were mock infected or were infected with SeV for 2 h, 4 h, 6 h, or 8 h. Cell lysates were immunoprecipitated using anti-HA, followed by Western blot analysis using the indicated antibodies. (D) Schematic representation of the structure of NEMO and its mutants. CC1 and CC2, coiled-coil domains 1 and 2; LZ, leucine zipper region; NUB, NEMO-ubiquitin binding; ZF, zinc finger domain. (E and F) HEK293T cells were transfected with the indicated plasmids. After 24 h, cell lysates were immunoprecipitated using anti-HA, followed by Western blot analysis using the indicated antibodies. (G) NEMO-deficient MEFs were transfected with an IFN-β reporter plasmid along with Flag-tagged wild-type NEMO or the indicated NEMO mutants. After 16 h, cells were infected with SeV for 18 h. Cells then were analyzed for IFN-β–dependent promoter activity. Data are mean values ± SD from one experiment representative of three independent experiments. (H and I) HEK293T cells were cotransfected with Flag-tagged TRIM14 plasmids and HA-tagged wild-type ubiquitin (Ub) plasmid (H) or with only the K63 ubiquitin mutant plasmid (I). After 24 h, cells were infected with SeV for 2 or 4 h. Cell lysates then were immunoprecipitated using anti-Flag, followed by Western blot analysis using the indicated antibodies. (J) HEK293T cells were transfected with the indicated plasmids. After 24 h, cells were mock infected or were infected with SeV for 4 h. Cell lysates were subjected to Ni-NTA pull-down assay, followed by Western blot analysis using the indicated antibodies. (K) HEK293T cells were transfected with the indicated plasmids. After 24 h, cells were mock infected or were infected with SeV for 4 h. Cell lysates were immunoprecipitated with anti-Flag, followed by Western blot analysis using the indicated antibodies. (L and M) HEK293T cells were transfected with an IFN-β reporter plasmid along with a control, with increasing amounts of TRIM14 plasmids, or with increasing amounts of TRIM14 K365R plasmids (L) or together with an MAVS plasmid (M). Cells were harvested 24 h later, and IFN-β–dependent promoter activity was analyzed. Data are mean values ± SD (n = 3).
To define the region of NEMO that mediates its interaction with TRIM14, we generated a series of NEMO-deletion mutants (Fig. 5D). We found that the regions in the CC1 domain (residues 93–151) and in the NEMO ubiquitin-binding (NUB) domain (residues 306–320) were critical for binding to TRIM14 (Fig. 5E). Because NEMO contains two ubiquitin-binding domains (UBDs)—the NUB domain and the zinc finger (ZF) domain (23), both of which are required for IRF3 activation (10)—we examined if the ZF domain also was required for interaction with TRIM14. Deletion of the NUB domain (residues 290–320) or the ZF domain alone did not abolish binding of NEMO to TRIM14, but deletion of both the NUB and ZF domains abrogated binding of NEMO to TRIM14 (Fig. 5F), suggesting that both UBDs contribute to this interaction. Moreover, the wild-type NEMO restored SeV-induced IFN promoter activity in NEMO-deficient mouse embryonic fibroblasts (MEFs), whereas NEMO mutants lacking both UBDs (ΔNUB + ΔZF) failed to do so (Fig. 5G). The activation of IFN promoter was partially rescued in the NEMO mutants lacking either UBD (ΔNUB or ΔZF) (Fig. 5G). Together, these results suggest that the UBD-mediated association between TRIM14 and NEMO is involved in viral-triggered IFN activation.
It has been reported that, in response to viral infection, NEMO binds to K63-linked polyubiquitinated proteins through UBD domains to mediate antiviral signaling, whereas the polyubiquitinated target protein remains elusive (10). Because viral infection potentiates the interaction of TRIM14 with NEMO through the UBD domains, we reasoned that TRIM14 could be a target of NEMO. This notion would suggest that TRIM14 undergoes K63-linked ubiquitination. To test this possibility, we examined if TRIM14 is ubiquitinated upon viral infection. Indeed, SeV infection substantially increased the ubiquitination of TRIM14 (Fig. 5H). To determine if TRIM14 undergoes K63-linked ubiquitination, we expressed a mutant ubiquitin construct that forms polyubiquitin chains only at K63 (Ub-K63). We found that SeV infection promoted K63-linked ubiquitination of TRIM14 (Fig. 5I).
We next mapped the K63 ubiquitination site(s) of TRIM14. Sequence alignment of human and mouse TRIM14 showed 16 conserved lysine residues (Fig. S5A). We mutated each conserved lysine residue of TRIM14 to arginine and analyzed the K63 ubiquitination upon SeV infection. Using Ni-NTA pull-down of His-tagged TRIM14, we found that the point mutation at Lys-365 (K365R) significantly reduced TRIM14 K63 ubiquitination, whereas other mutations had little effect (Fig. 5J and Fig. S5B), indicating that K365 is a key K63 ubiquitination site of TRIM14.
We subsequently examined if K365 is involved in the NEMO–TRIM14 association. Coimmunoprecipitation experiments showed that the TRIM14 K365R mutant bound less NEMO than wild-type TRIM14 (Fig. 5K), suggesting that ubiquitination at K365 participates in viral-induced recruitment of NEMO. We next determined whether K365 is required for IFN induction. The K365R mutant had considerably less ability than wild-type TRIM14 to stimulate IFN promoter activity (Fig. 5L). Moreover, the K365R mutant failed to synergize MAVS-induced IFN activation (Fig. 5M). Collectively, these results suggest that K63 ubiquitination of TRIM14 at K365 recruits NEMO and mediates IFN activation in MAVS-mediated signaling.
TRIM14 Bridges NEMO to MAVS.
NEMO is recruited to the MAVS following poly(I:C)-triggered RLR activation (14); however, the underlying mechanism remains unresolved. Because TRIM14 associates with MAVS on mitochondria and recruits NEMO upon viral infection, we speculated that TRIM14 might serve as a link between NEMO and MAVS association. To test this notion, we examined the effect of TRIM14 overexpression on the MAVS–NEMO association. Coimmunoprecipitation revealed that SeV infection induced association between Flag-tagged NEMO and HA-tagged MAVS, with a peak binding activity at 6 h postinfection (hpi) (Fig. 6A, lanes 1, 3, 5 and 7; also see Fig. 6B). Notably, overexpression of TRIM14 increased the MAVS–NEMO association in the absence or presence of viral infection (Fig. 6A, compare lanes 2 and 1, lanes 4 and 3, lanes 6 and 5, and lanes 8 and 7; also see Fig. 6B). We further investigated if ablation of TRIM14 affected the recruitment of NEMO to MAVS. To do so, we generated THP-1 monocytes stably expressing control or TRIM14 shRNA and found that knockdown of TRIM14 severely impaired the viral-induced endogenous association between MAVS and NEMO (Fig. 6C). In contrast, binding of IRF3 to MAVS was not substantially affected (Fig. 6C). These results strongly suggest that TRIM14 mediates the association between NEMO and MAVS.
Fig. 6.
TRIM14 recruits NEMO to MAVS. (A) HEK293T cells were transfected with the indicated plasmids. After 24 h, cells were mock infected or were infected with SeV for 6 h, 10 h, or 14 h. Cell lysates were immunoprecipitated using anti-HA, followed by Western blot analysis using the indicated antibodies. (B) Quantitative analysis of results from A. Data are mean values ± SD (n = 3). (C) Control or TRIM14-knockdown THP-1 cells were mock infected or were infected with SeV for 4 or 8 h. Cell lysates were immunoprecipitated using anti-MAVS, followed by Western blot analysis using the indicated antibodies. (D) A549 cells were transfected with the indicated siRNA oligos. After 48 h, cells were mock infected or were infected with SeV for 4 h. Cells then were labeled using anti-NEMO (green) and MitoTracker (red). (Scale bar: 10 μm.) (E) Quantitative analysis of translocation of NEMO to mitochondria in control or TRIM14-knockdown cells. Data are mean values ± SD (n = 3), *P < 0.05. (F) TRIM14-knockdown A549 cells were transfected with shRNA-resistant wild-type TRIM14 (WT-Flag) or the TRIM14 K365R mutant (K365R-Flag) plasmid. After 24 h, cells were infected with SeV for 4 h, followed by immunostaining using anti-Flag (red) or anti-NEMO (green). (Scale bar: 10 μm.) (G) Quantitative analysis of colocalization of TRIM14 and NEMO. Data are mean values ± SD (n = 4). *P < 0.05. (H) A Proposed model illustrating how TRIM14 functions in RLR-mediated signaling.
Because the MAVS signalosome assembles on mitochondria after viral infection, we examined if TRIM14 is involved in the translocation of NEMO to mitochondria. We found that after SeV infection NEMO is translocated to punctate structures that colocalize with mitochondria (Fig. 6D). Such translocation was substantially reduced in the TRIM14 knockdown cells, (Fig. 6 D and E). Furthermore, we found that mutation of the key ubiquitin site (K365) on TRIM14 significantly impaired colocalization of TRIM14 and NEMO (Fig. 6 F and G). These results, in line with the observations that TRIM14 links NEMO to MAVS, indicate that TRIM14 is a critical adaptor for recruitment of NEMO to the mitochondrial antiviral platform where the MAVS signalosome assembles.
Discussion
Upon detection of viral RNAs, RIG-I–like receptors such as RIG-I and MDA5 are recruited to the mitochondrial adaptor MAVS. Subsequently, MAVS engages various downstream signaling proteins to form a mitochondrial signalosome (24). It has been demonstrated that, through its TRAF-interaction motifs, MAVS interacts directly with TRAF family proteins, such as TRAF3 (25). However, although downstream effectors such as NEMO and TBK1 also are linked to the MAVS signalosome (26), the direct interaction between MAVS and these proteins is not detected (13, 27). Thus, an additional mitochondrial adaptor(s) may act as a cofactor to assemble the MAVS signalosome. In this study, we have demonstrated TRIM14 is such a protein. Similar to MAVS, TRIM14 is localized to the outer membrane of mitochondria. Biochemical analysis suggested that TRIM14 is associated with MAVS, and viral infection promotes the assembly of a MAVS–TRIM14 associated complex. We further demonstrated that TRIM14 links NEMO to MAVS because overexpression of TRIM14 facilitates the association of NEMO with MAVS, whereas knockdown of TRIM14 disrupts this interaction. Thus, our results strongly suggest that TRIM14 acts as an integral component of the mitochondrial signalosome that couples MAVS to facilitate antiviral signaling.
The TRIM family is one of the largest subfamilies of putative ubiquitin E3 ligases. Most TRIM proteins contain an N-terminal RING domain that specifies their E3 ligase activities (15). Accumulating evidence suggests that TRIMs regulate the innate immune response by conjugating ubiquitin to target proteins (16). TRIM25 promotes K63-linked ubiquitination of RIG-I to initiate antiviral response (28), whereas TRIM21 negatively regulates the immune response to dsDNA by inducing K48-linked ubiquitination and degradation of DDX41 (29). Recently, a systematic analysis of all 75 known human TRIMs revealed that nearly half of these TRIMs could enhance the innate immune response. Moreover, mutational analysis demonstrated that all of the six tested TRIMs required an intact RING domain for their activity (30), suggesting that the RING domain may serve as a prevailing machinery for TRIMs. Intriguingly, TRIM14 does not contain an N-terminal RING domain but still potently enhances the innate immune response. We observed that the N-terminal sequence of TRIM14 directs it to the mitochondrial outer membrane, and mutation of this region disrupts the mitochondrial localization and activity of TRIM14, indicating that the N-terminal sequence of TRIM14 plays a unique role. Coimmunoprecipitation assays revealed that the C-terminal PRYSPRY domain of TRIM14 mediates its association with MAVS. Moreover, a lysine residue in the PRYSPRY domain (K365) is critical for NEMO association. Thus, we propose that the N terminus of TRIM14 directs its localization, whereas the C-terminal PRYSPRY domain serves as an interface for protein–protein interaction for assembly of the MAVS signalosome. We speculate that TRIM14 acts as a mitochondrial scaffold protein rather than an E3 ligase. Therefore, our findings provide insights in the mechanisms by which TRIM family proteins regulate the innate immune response.
NEMO is a component of the canonical IKK complex and controls NF-κB activation by binding to ubiquitin chains in the TNF-R1 pathways (31, 32). Recently, it has been suggested that, similar to its role in the canonical IKK pathway, NEMO functions as a sensor of K63 ubiquitin chains to mediate IRF3 activation (10). However, the target protein recognized by NEMO in antiviral signaling remains elusive. TRAF3 has been suggested as a candidate, because it is required for MAVS-mediated signaling and undergoes autoubiquitination following viral infection (25). However, TRAF3-deficient MEFs still retain the capacity to produce IFN-β and support IRF3 activation (10). Additionally, it has been demonstrated that TRAF3 is not required for NF-κB activation (33). These studies suggest that an additional unknown component is involved also. The results presented here suggest that TRIM14 is such a target protein. This notion is supported by several lines of evidence. First, viral infection promoted K63 ubiquitination of TRIM14 and potentiated TRIM14–NEMO association. Second, both UBDs of NEMO are required for TRIM14 binding and IFN-β activation. Third, mutation of the key K63 ubiquitination site (K365) in TRIM14 substantially reduced its binding to NEMO as well as its ability to activate IFN-β. Finally, knockdown of TRIM14 impaired both IRF3 and NF-κB activation triggered by viral infection. Thus, it is highly likely that TRIM14 is a target protein that is recognized by NEMO in the antiviral signaling pathway. It is noteworthy that the TRIM14 K365R mutation did not completely abrogate NEMO binding. Also, the K365R mutant could stimulate IFN-β, albeit at a reduced level. Therefore, in addition to K365, another site(s) on TRIM14 may contribute to NEMO binding. Additionally, because TRIM14 is unlikely to undergo autoubiquitination because it lacks the RING domain, the E3 ubiquitin ligase for TRIM14 remains to be identified.
Of note, binding of NEMO to K63 ubiquitin chains is a conserved mechanism for activation of both the RLR and TNF-R1 pathways. Moreover, these pathways share multiple proteins, such as TRAFs and Death domain-containing proteins (34). Thus, these proteins must be tightly regulated to prevent cross-talk, which may result in aberrant immunological consequences. It is speculated that the distinct sites of signalosome assembly may account for specific activation of both pathways. The TNF-R1 complex is assembled at the plasma membrane, whereas the MAVS signalosome forms on mitochondria. Our results show that a mitochondrial-resident protein, TRIM14, rather than a cytosolic factor, recruits NEMO via K63 ubiquitin chains following viral infection. This mitochondrial location likely assures the correct recruitment of NEMO complexes to the mitochondrial environment in response to infection. This notion is supported by the inability of TRIM14 mutants in which the association with mitochondria is disrupted to activate the IFN response. Therefore, we hypothesize that MAVS and TRIM14 together dictate the specificity of RLR-mediated signaling.
Based on these findings, we propose a working model for TRIM14 in mediating virus-triggered innate immune signaling (Fig. 6H). Upon viral infection, a signalosome containing MAVS and TRIM14 is assembled on the outer membrane of mitochondria. This signalosome concomitantly promotes K63-linked polyubiquitination of TRIM14, which provides a platform for NEMO binding. Thus, in cooperation with TRAF3, TRIM14 links NEMO to the mitochondrial antiviral signalosome and further activates two NEMO-containing protein kinase complexes, the NEMO–TBK1–IKKi complex and the NEMO–IKKα–IKKβ complex, which subsequently activate IRF3/7 and NF-κB, respectively. These two pathways converge on the induction of type I IFN. As a positive-feedback mechanism, type I IFN promotes the expression of TRIM14, thus amplifying the innate immune response to combat viral infection.
Materials and Methods
Viruses and Cells.
SeV, VSV, and GFP-tagged NDV have been described previously (35). The A549, HEK293T, and HeLa cell lines (American Type Culture Collection, ATCC) and NEMO-deficient MEFs (provided by M. Schmidt-Supprian, Harvard University, Cambridge, MA) were maintained in DMEM (HyClone) supplemented with 10% (vol/vol) FBS and antibiotics. The THP-1 cells (ATCC) were maintained in RPMI1640 (HyClone) supplemented with 10% (vol/vol) FBS and antibiotics.
Plasmids, Antibodies, and Proteins.
TRIM14, TRAF3, TRAF6, TBK1, and IRF3 expression plasmids were purchased from Origene. The Flag-tagged RIG-I, MAVS, MDA5, and TRIF constructs have been described elsewhere (36). The Flag-tagged full-length NEMO, NEMO (1–395), NEMO (1–306), NEMO (93–419), NEMO (151–419), NEMO (Δ196–250), and NEMO (Δ262–290) plasmids were gifts from Rongtuan Lin (McGill University, Montreal). The HA-tagged MAVS constructs, pGL3-IFNβ-Luc, pISRE-Luc, pGL3-NF-κB-Luc, and pRL-SV40 plasmids were described previously (35). The Flag-tagged NEMO mutation plasmids, including 1–375, 1–355, 1–335, 1–290, ΔNUB, ΔNUB+Δleucine zipper region (LZ), and the TRIM14 mutation plasmids were generated using a QuikChange Site-Directed Mutagenesis kit (Stratagene).
Rabbit anti-HA (H6908), rabbit anti-GAPDH (G9545), mouse anti-Flag (F3165), rabbit anti-GST (G7781), mouse anti-actin (A2228), and rabbit anti-Calnexin (C4731) antibodies were purchased from Sigma. Rabbit anti–Fas-associated protein with Death Domain (FADD) (sc-5559), mouse anti-VDAC1 (sc-58649), goat anti-COXIV (sc-69360), and rabbit anti-NEMO (sc-8330) antibodies were purchased from Santa Cruz Biotechnology. Mouse anti-GM130 (615823) and mouse anti-NEMO (559675) antibodies were purchased from BD Biosciences. Rabbit anti–phospho-IκBα (2859s), rabbit anti-IκBα (9242), and rabbit anti-ubiquitin (K63) (5621s) antibodies were purchased from Cell Signaling Technology. Rabbit anti–phospho-IRF3 (2562-1) and rabbit anti-IRF3 (2241-1) antibodies were purchased from Epitomics. Rabbit anti-MAVS (210-929) antibody was purchased from Enzo Life Sciences. Rabbit anti-TRIM14 (APR34737) was purchased from Aviva Systems Biology Corporation.
Purified Flag-tagged NEMO, TRAF3, and TBK1 proteins were purchased from Origene.
Reporter Assay.
Cells cultured in 24-well plates were transfected with expression plasmids or siRNA oligos, along with luciferase reporter plasmids. Cells were lysed, and luciferase activities were determined using a Dual-Luciferase Reporter Assay system (Promega). The firefly luciferase activities were normalized to Renilla luciferase activities.
Real-Time PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen) and reverse-transcribed using the SuperScript cDNA synthesis kit (Invitrogen). Real-time quantitative PCR was performed using SYBR green kit (Takara Bio). The primer sequences specific for TRIM14 were 5′-AGGCTTCAGGCATACACGG-3′ and 5′-CTTGACGGGCTCAAAGGAG-3′. The tissue distribution of TRIM14 was determined using TissueScan Normal Tissue cDNA Arrays (HMRT102, MNRT101; Origene) by real-time PCR.
RNAi.
For knockdown of TRIM14, cells were transfected with 50 nM of TRIM14-specific siRNA oligos for 48 h using DharmaFECT 1 (Dharmacon). The sequences targeting human TRIM14 were 5′-GCUAAUGCAGAGUCAAGUA-3′ (#1) and 5′-CAGAUUACUACUUGACGAA-3′ (#2).
For generation of control or TRIM14-knockdown cell lines, THP-1 cells were seeded onto 24-well plates. The next day, cells were infected with lentiviruses expressing scrambled or TRIM14-specific shRNA (GenePharma) at a multiplicity of infection (MOI) of 100 in the presence of 8 μg/mL Polybrene. After 72 h, cells were selected by 1 μg/mL puromycin for 3 wk.
Immunofluorescence Assay and Confocal Microscopy.
Immunofluorescence assays were done as described (36). For mitochondria staining, cells were incubated for 10 min at 37 °C in growth medium containing 200 nM MitoTracker Red CMXRos (Invitrogen), followed by washes with prewarmed PBS buffer. Fluorescence images were obtained and analyzed using a laser scanning confocal microscope (Leica TCS SP5).
Analysis of Protein–Protein Interaction.
For immunoprecipitation, cells were harvested 24 h posttransfection and were lysed with RIPA buffer. Lysates were incubated overnight at 4 °C with the indicated antibodies, followed by incubation with protein A/G agarose beads (Santa Cruz Biotechnology) for 2 h at 4 °C. After four washes with RIPA buffer, immunoprecipitates were subjected to Western blot analysis as described (36).
For GST pull-down assays, purified GST-TRIM14 protein or GST protein bound to glutathione-Sepharose beads was incubated with the indicated purified Flag-tagged proteins in GST pull-down buffer [20 mM Tris⋅Cl (pH 7.4), 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.01 mM ZnSO4, 0.1 mM PMSF, 1% Nonidet P-40] for 2 h. After four washes with GST pull-down buffer, the precipitates were subjected to Western blot analysis.
For Ni-NTA pull-down, cells transfected with His-tagged TRIM14 plasmids and ubiquitin expression plasmids were harvested, resuspended in 1 mL of lysis buffer (6 M guanidine-HCl, 0.1 M Na2HPO4/NaH2PO4, 10 mM imidazole, pH 8.0), and subjected to sonication for a total 30 s. Cell lysates were incubated with 30 μL of pre-equilibrated Ni Sepharose for 4 h at room temperature. The Sepharose was washed three times with wash buffer [10 mM Tris⋅Cl (pH 8.0), 8 M urea, 0.1 M NaH2PO4] and three times with 1:4 diluted wash buffer. The precipitates were boiled in SDS loading buffer with 200 mM imidazole and were subjected to Western blot analysis.
Subcellular Fractionation.
Mitochondria and ER were purified on discontinuous sucrose gradients as described (37).
Size-Exclusion Chromatography.
For size-exclusion chromatography, 5 × 107 HEK293T cells were infected with SeV or were mock-infected for 12 h. Cells were harvested and lysed in buffer comprised of 14 mM CHAPS, 150 mM NaCl, and 20 mM Tris⋅HCl (pH 7.4). Cell lysates were loaded onto a Superose 6 10/300 GL column (GE Healthcare, Life Sciences) and eluted at a rate of 0.5 mL/min. Each fraction was precipitated using the chloroform/methanol precipitation method. Molecular weight was determined using standard proteins (151-1901; Bio-Rad).
VSV Plaque Assay.
Cells were transfected with expression plasmids for 24 h or with siRNA oligos for 48 h. Cells then were infected with VSV for 16 h. Supernatants were harvested, serially diluted, and used to infect BHK21 cells. One hour later, supernatants were removed, and medium containing low melting temperature agarose (1%) and FBS (1%) was added to the cells. After 60 h, cells were fixed using paraformaldehyde [4% (wt/vol)] and were stained with crystal violet (1%). Plaques were counted and multiplied by the dilution factor to determine the pfu.
Statistics.
The Student t test was used for two-group comparisons. The values P < 0.05 and P < 0.01 were considered significant.
Supplementary Material
Acknowledgments
We thank Rongtuan Lin (McGill University) for providing reagents; Lijun Rong (University of Illinois), Tao Deng, and Xiaobo Lei for critically reading the manuscript; and Yaowu Yang, Jing Fu, and Chao Wu for technical assistance. This work was supported by Grant 81225014 from the National Science Fund for Distinguished Young Scholars, Grant for New Century Talents NCET-07-0506 from the Chinese Ministry of Education, and Grant 2011CB504903 from the National Basic Research Program of China (973 Project).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316941111/-/DCSupplemental.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29(2):178–181. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199(12):1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao T, et al. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8(6):592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 7.Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18(5-6):483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Zeng W, Xu M, Liu S, Sun L, Chen ZJ. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol Cell. 2009;36(2):315–325. doi: 10.1016/j.molcel.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Li S, Dorf ME. NEMO binds ubiquitinated TANK-binding kinase 1 (TBK1) to regulate innate immune responses to RNA viruses. PLoS ONE. 2012;7(9):e43756. doi: 10.1371/journal.pone.0043756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaoka S, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93(7):1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 13.Castanier C, et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 2012;10(44):44. doi: 10.1186/1741-7007-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Wang L, Berman M, Kong YY, Dorf ME. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35(3):426–440. doi: 10.1016/j.immuni.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8(11):849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol Med. 2011;3(9):513–527. doi: 10.1002/emmm.201100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 18.Carthagena L, et al. Human TRIM gene expression in response to interferons. PLoS ONE. 2009;4(3):e4894. doi: 10.1371/journal.pone.0004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep. 2008;9(1):42–49. doi: 10.1038/sj.embor.7401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasukawa K, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2(84):ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 23.Cordier F, et al. The zinc finger of NEMO is a functional ubiquitin-binding domain. J Biol Chem. 2009;284(5):2902–2907. doi: 10.1074/jbc.M806655200. [DOI] [PubMed] [Google Scholar]
- 24.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha SK, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25(14):3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belgnaoui SM, Paz S, Hiscott J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr Opin Immunol. 2011;23(5):564–572. doi: 10.1016/j.coi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Paz S, et al. Ubiquitin-regulated recruitment of IkappaB kinase epsilon to the MAVS interferon signaling adapter. Mol Cell Biol. 2009;29(12):3401–3412. doi: 10.1128/MCB.00880-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, et al. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat Immunol. 2013;14(2):172–178. doi: 10.1038/ni.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versteeg GA, et al. The E3-ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity. 2013;38(2):384–398. doi: 10.1016/j.immuni.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8(4):398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 33.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439(7073):208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 34.Michallet MC, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28(5):651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 35.You F, et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat Immunol. 2009;10(12):1300–1308. doi: 10.1038/ni.1815. [DOI] [PubMed] [Google Scholar]
- 36.Lei X, et al. The 3C protein of enterovirus 71 inhibits retinoid acid-inducible gene I-mediated interferon regulatory factor 3 activation and type I interferon responses. J Virol. 2010;84(16):8051–8061. doi: 10.1128/JVI.02491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun W, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA. 2009;106(21):8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.