Significance
Direct conversion is a recently established method to generate neuronal progenitor cells (NPCs) from skin fibroblasts in a fast and efficient manner. In this study, we show that this method can be used to model neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). Because the origin of ALS is mainly sporadic with unknown cause, methods to model the disease are urgently needed. The produced NPCs are differentiated into astrocytes, which are involved in motor neuron death in ALS. Strikingly, skin-derived astrocytes show similar toxicity toward motor neurons as astrocytes from autopsies of patients. This tool now allows studying ALS while the patient is still alive and can help in testing potential therapeutics for individual patients.
Keywords: neurotoxicity, neurodegeneration, reprogramming
Abstract
Amyotrophic lateral sclerosis (ALS) causes motor neuron degeneration, paralysis, and death. Accurate disease modeling, identifying disease mechanisms, and developing therapeutics is urgently needed. We previously reported motor neuron toxicity through postmortem ALS spinal cord-derived astrocytes. However, these cells can only be harvested after death, and their expansion is limited. We now report a rapid, highly reproducible method to convert adult human fibroblasts from living ALS patients to induced neuronal progenitor cells and subsequent differentiation into astrocytes (i-astrocytes). Non-cell autonomous toxicity to motor neurons is found following coculture of i-astrocytes from familial ALS patients with mutation in superoxide dismutase or hexanucleotide expansion in C9orf72 (ORF 72 on chromosome 9) the two most frequent causes of ALS. Remarkably, i-astrocytes from sporadic ALS patients are as toxic as those with causative mutations, suggesting a common mechanism. Easy production and expansion of i-astrocytes now enables rapid disease modeling and high-throughput drug screening to alleviate astrocyte-derived toxicity.
ALS, or Lou Gehrig disease, is a devastating disorder affecting mainly upper and lower motor neurons (MNs) in the motor cortex, brainstem, and spinal cord (1). Patients typically suffer from muscular atrophy and paralysis, ultimately leading to death within 2–5 y after diagnosis. Although 5–10% of cases follow an autosomal dominant inheritance pattern and are considered familial (fALS), the remaining ∼90% are classified as sporadic (sALS). To date, more than 10 different genes have been identified to cause ALS, with the highest proportion of patients carrying a large hexanucleotide expansion repeat in the ORF 72 on chromosome 9 (C9orf72) (2, 3). Although ALS leads to selective degeneration of MNs, evidence from multiple groups supports the contribution of other cell types of the central nervous system (CNS), including astrocytes, microglia, and oligodendrocytes to disease progression (4–6). A lack in understanding disease origin, along with known interweaving contributions of multiple cell types, hampers studying disease mechanisms and testing potential therapeutic strategies.
To test non-cell autonomous interactions in familial and sporadic ALS, we previously developed a coculture assay enabling the screening for therapeutics on astrocytes, differentiated from spinal cord autopsy-derived neuronal progenitor cells (NPCs) (5). However, the isolation and expansion of these NPCs is difficult and postmortem tissues are of limited availability. In addition, it is unclear how the inflammatory and necrotic environment of an end-stage ALS patient spinal cord might influence the properties of the isolated cells.
Currently, many laboratories use reprogramming techniques to generate induced pluripotent stem cells (iPSCs) from patient fibroblasts that can then be differentiated into various cell types of interest. The process of deriving iPS lines and subsequently inducing differentiation is very time consuming and inefficient. Furthermore, few studies have identified phenotypic markers of ALS in cells differentiated from iPS lines (7). Recent advances have led to the development of more direct approaches to convert fibroblasts into specific cell types of interest. In 2011, Kim et al. (8) reported the production of NPCs from embryonic and adult mouse fibroblasts by direct conversion using four reprogramming factors introduced by viral vectors and subsequent exposure to NPC-stimulating growth factors. Since this discovery, several laboratories reported the generation of neurons or neuronal/oligodendroglial progenitor cells from mouse or human fibroblasts using a combination of transcription factors (9–15). However, the conversion of adult human patient fibroblasts into NPCs has not been reported, nor has the use of these cells for modeling disease phenotypes.
Recognizing this, we sought to generate induced NPCs (iNPCs) from adult human fibroblasts from patients who had been diagnosed with ALS and from age-matched healthy controls, using an approach similar to that of Kim et al. (8). Using this technique, we were able to produce tripotent iNPCs from patients and controls within 1 mo. These cells could then be differentiated into astrocytes to test the suitability of this technique for modeling ALS and potentially other neurodegenerative diseases. Strikingly, our data demonstrate a very similar toxicity of iNPC derived astrocytes toward MNs as previously shown with autopsy derived ones (5). In addition, we report non-cell autonomous toxicity of astrocytes carrying a C9orf72 expansion mutation. Our findings underline the crucial role of astrocytes in ALS and suggest common underlying mechanisms leading to astrocyte mediated toxicity in sporadic ALS and ALS with known genetic origin.
Results
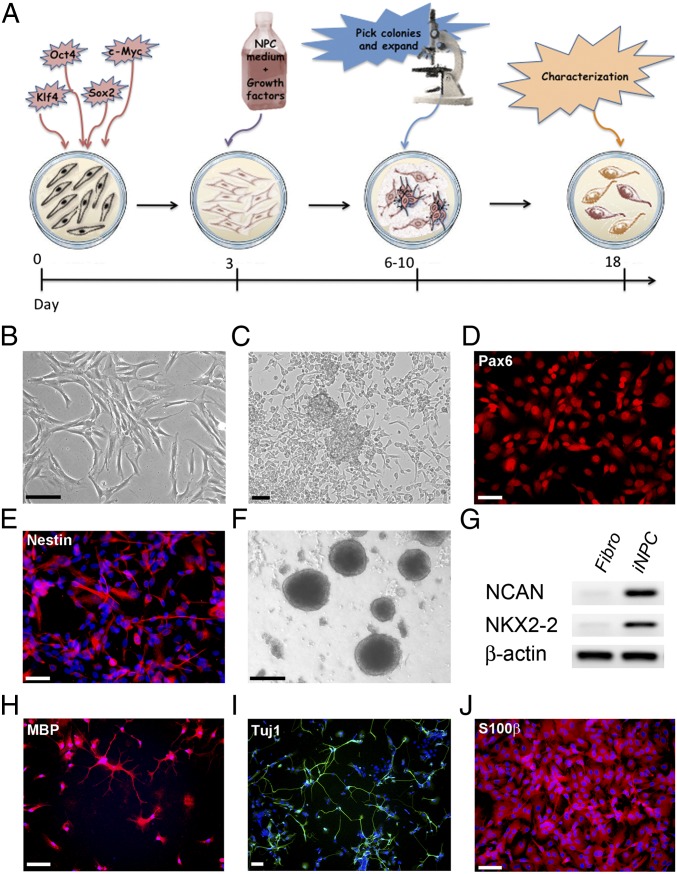
Fibroblast samples from one fALS patient carrying a SOD1A4V mutation, three ALS patients carrying the C9orf72 expansion repeat as well as three sporadic ALS patients ranging in age from 51 to 81 y were either collected by skin biopsy or purchased from established tissue banks (Table S1). Additionally, we included four healthy age-matched controls in this study. To generate iNPCs, skin fibroblasts were infected with a mixture of retroviral vectors expressing Kruppel-like factor 4 (Klf4), POU transcription factor Oct-3/4 (Oct3/4), SRY-related HMG-Box Gene 2 (Sox2), and c-Myc (16). To promote NPC conversion, at 72 h postinfection, the culture medium was switched to medium containing the growth factors fibroblast growth factor 2 (FGF2), epidermal growth factor (EGF), and heparin, and this was continued for 18 d, followed by supplementation with only FGF2 (Fig. 1A). Within 7 d after infection, cells underwent morphological changes from a flat fibroblastic cell shape to become smaller with more distinct extensions. The cells also began to form sphere-like structures that could be picked and dissociated for further growth into monolayers (Fig. 1 B and C). NPC marker expression was evaluated by immunohistochemistry and RNA expression analysis (Tables S2 and S3). We confirmed the expression of NPC markers, such as Pax6 and Nestin, indicating successful conversion to an NPC stage (Fig. 1 D and E). We observed that the conversion efficiency ranges between 60% and 95%, in correlation with the proliferative potential of the initial fibroblast cultures, as well as the quality of the viral vectors. Further characterization revealed that similar to NPCs generated from human fetal fibroblasts or mouse cells by other groups (8, 17, 18), the iNPCs were able to form neurospheres when cultured in uncoated dishes and expressed NPC markers, such as neuronal cell-adhesion molecule (N-CAM) and homeodomain transcription factor NKX2-2 (Fig. 1 F and G).
Fig. 1.
Direct conversion of human skin fibroblasts to tripotent iNPCs. (A) Schematic of the conversion process from fibroblasts to induced neuronal progenitor cells (iNPCs). Fibroblasts were transduced with retroviral vectors containing four reprogramming factors (Sox2, KLF4, Oct3/4, c-Myc). (B and C) Within 6–10 d, cells underwent marked morphological changes from a fibroblastic spindle like shape (B) to a sphere-like form commonly seen with NPCs (C). (D and E) Immunofluorescence of cultures at day 12 reveals expression of the NPC markers Pax6 and Nestin, as shown in red. DAPI staining (blue) was used to visualize nuclei. (F) iNPCs can form and grow as neurospheres when plated in uncoated dishes. (G) RT-PCR analysis demonstrates a strong up-regulation of the prototypic NPC markers NCAN and NKX2-2 in iNPCs. β-Actin was used as loading control. (H–J) iNPCs are tripotent and upon differentiation they can give rise to oligodendrocytes (H), neurons (I), and astrocytes (J). (Scale bars: black, 100 µm; white, 50 μm.) Fibro, fibroblast.
We next determined the differentiation potential of iNPCs and found that they were tripotent, capable of differentiating into oligodendrocytes, neurons, and astrocytes (Fig. 1 H–J). Addition of insulin-like growth factor 1 (IGF-1) and PDGF receptor α (PDGF-α) to the medium in absence of FGF-2 resulted in cells demonstrating the typical ramified oligodendritic shape that expressed myelin-binding protein (MBP), a marker for mature oligodendrocytes (Fig. 1H). The differentiation efficiency toward neurons varied between cell lines and according to the protocol used. Approximately 50% of cells surviving after differentiation with retinoic acid/forskolin were positive for the pan-neuronal marker neuronal class III β-tubulin (TUJ1). When using the protocol developed for the generation of MNs previously published by our laboratory (19), ∼10–30% of the surviving cells expressed the MN markers HB9 homeobox transcription factor (HB9) and choline acetyltransferase (ChAT), along with Tuj1 (Fig. S1 A and B). This finding highlights that the produced iNPCs have the potential to generate MNs, thereby providing a model to study ALS in several affected cell types.
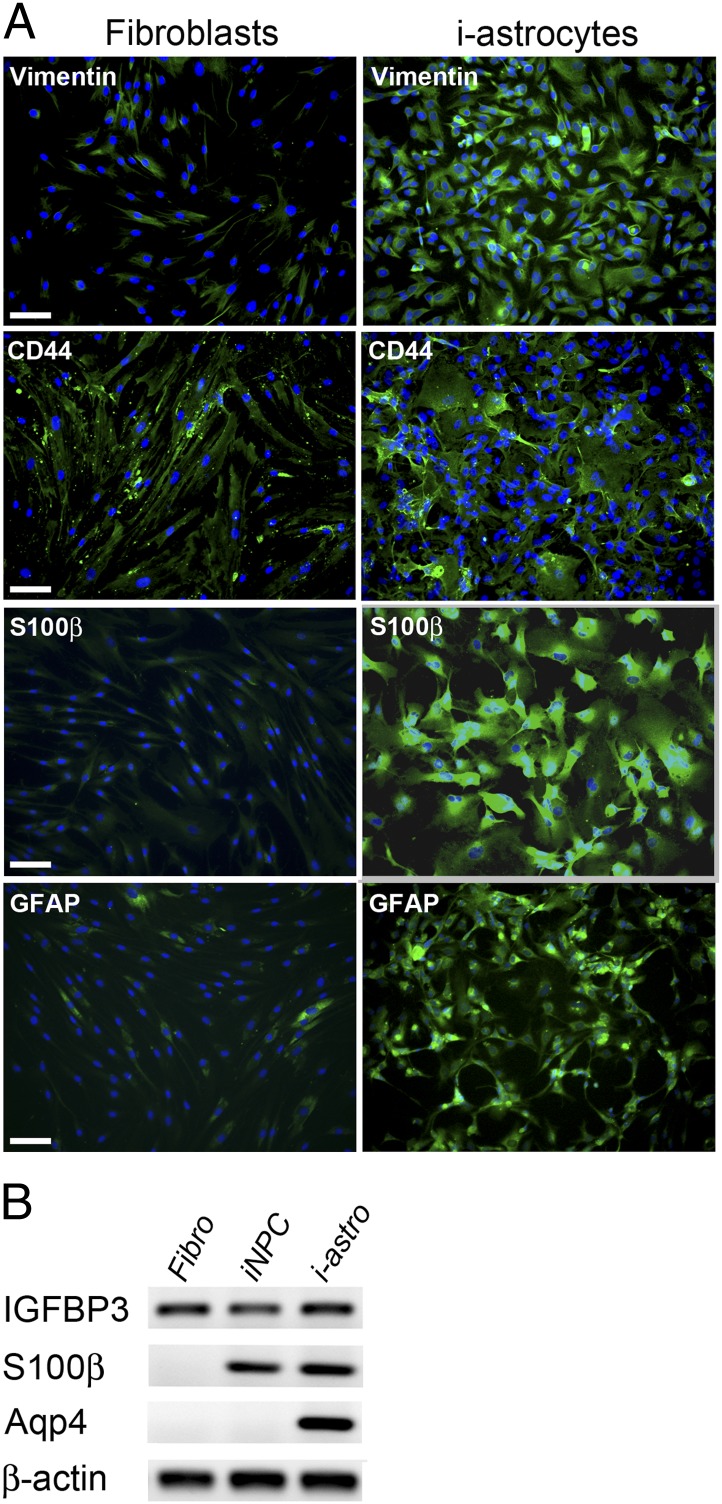
We characterized the derived astrocytes [differentiated from induced neuronal progenitor cells (i-astrocytes)] more thoroughly to create a cell culture model for studying astrocyte–MN interactions in ALS. Compared with the initial fibroblast lines, the differentiated i-astrocytes expressed higher levels of several astrocytic markers, including vimentin, CD44 antigen (CD44), as well as markers for mature astrocytes including s100 calcium binding protein B (S100β) and glial fibrillary acidic protein (GFAP) (Fig. 2A). Analysis of mRNA expression by RT-PCR showed that, similar to a previous report of direct conversion of mouse embryonic fibroblasts, a strong up-regulation of s100β in both iNPCs and i-astrocytes as well as aquaporin 4 (Aqp4) in i-astrocytes was observed, while the levels of the additional marker insulin-like growth factor binding protein (IGFBP3) remained similar between all three cell types (Fig. 2B). These results further indicate that our conversion protocol generates astrocyte-like cells with similar properties to previous studies (17). Furthermore, typical fibroblast genes were expressed at a markedly reduced level in i-astrocytes (Fig. S2 A and B). Taken together, our data suggest a strong enrichment toward astrocyte-like cells without a further purification or selection step. Since purification or clonal selection is not required, we were able to generate i-astrocytes from controls and patients in less than four weeks. These cells maintained highly consistent and reproducible characteristics and provided a virtually unlimited source of human astrocytes.
Fig. 2.
I-astrocytes express prototypic astrocyte markers. (A) Immunofluorescence analysis reveals strong up-regulation of astrocytic markers such as Vimentin, CD44, S100β, and GFAP in i-astrocytes compared with the initial fibroblasts. DAPI (blue) was used to visualize nuclei. (B) RT-PCR analysis revealed expression of IGFB3 in fibroblasts, iNPCs, and i-astrocytes, whereas expression of S100β and Aqp4 was detected in iNPCs and i-astrocytes or i-astrocytes only, respectively. (Scale bar: 100 μm.) Fibro, fibroblast. i-Astro, i-astrocytes.
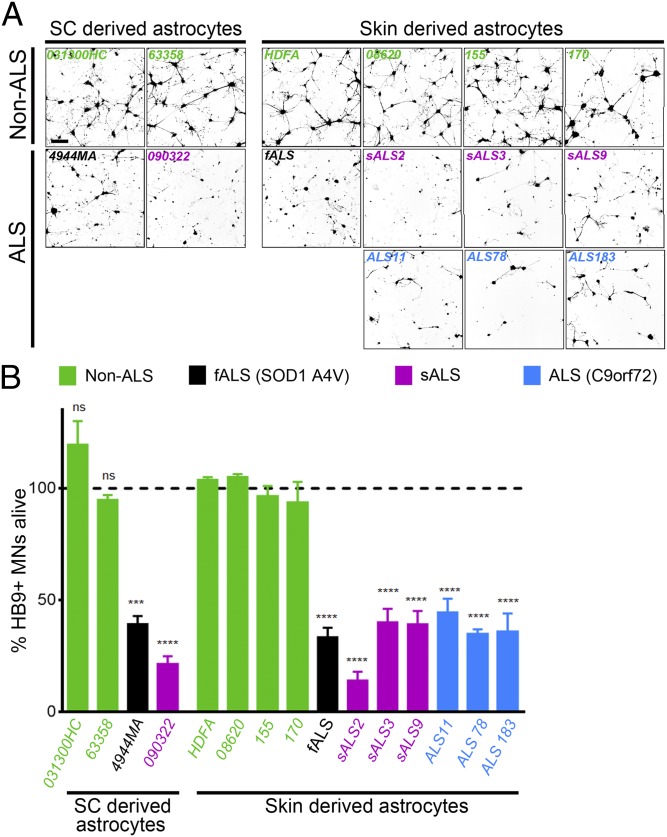
I-astrocytes from control or ALS patients were used in a coculture assay to determine their effect on MN survival. As previously described (5), mouse embryonic stem cell-derived MNs expressing green fluorescence protein (GFP) under the control of the HB9 promoter were sorted and added to i-astrocytes from patients and controls. Their survival was monitored in a blinded manner with daily confocal image acquisition. One day after plating, the MNs had settled down equally between all groups and started to extend neurites (Fig. S3A). On day 2, no difference was observed between MNs cultured on control i-astrocytes versus ALS i-astrocytes, whereas fewer cells remained on ALS i-astrocytes on day 3 and remaining cells exhibited shorter neurites. After 4–5 d, MN survival was clearly reduced in cultures with i-astrocytes from ALS patient samples, with 60–80% of the cells dying and the surviving cells containing fewer and shorter neurites (Fig. 3 A and B). Strikingly, i-astrocytes from the three patients carrying the C9orf72 mutation demonstrated similar toxicity to MNs compared with i-astrocytes derived from other ALS subtypes. As a control, we also plated MNs in a monoculture and followed their survival over the same time course. MN monocultures survived over the course of the experiment in normal media compared with the ALS i-astrocyte cocultures, indicating that the reduced viability is caused by toxic mechanisms rather than a lack of support from astrocytes (Fig. S3B). To further rule out that the ALS i-astrocytes were less supportive compared with controls, we supplemented the ALS cocultures with either 30% or 60% conditioned medium harvested from two different control i-astrocyte cocultures and monitored the MN survival over the same time period. We did not observe any significant difference between supplemented and nonsupplemented cocultures (Fig. S4). Staining of coculture plates after the survival assay demonstrated that all i-astrocyte lines expressed similarly high levels of the astrocytic markers s100β, vimentin and CD44, whereas the microglial markers Iba1 and CD11b were completely absent (Fig. S5). In addition, we also tested MN survival in combination with various ALS and control fibroblast lines and found no difference in MN survival between groups (Fig. S6). These experiments clearly demonstrate that the observed toxicity toward MNs is caused by i-astrocytes and is likely not due insufficient production of (a) trophic factor(s).
Fig. 3.
I-astrocytes from fALS and sALS patients display toxicity toward MNs. (A) Representative images after 96 h of coculture of HB9-GFP expressing MNs (shown in black) with astrocytes from spinal cord (sc) or skin of ALS patients and controls. A marked loss of MN viability was observed in the presence of ALS astrocytes irrespective of their origin (spinal cord or skin). (B) Relative percentage of MN survival after 96 h of coculture with ALS astrocytes derived from spinal cord or skin and their respective controls. ***P < 0.001; ****P < 0.0001 [compared with the average taken from of all converted control lines (HDFA, 8620, 155, 170)]. Error bars represent SEM. Quantification was performed in triplicate wells of a 96-well plate, and data are representative of n = 5.
Remarkably, the difference in survival of MNs in coculture with ALS i-astrocytes was very similar to our previous report using spinal cord-derived astrocytes (5) (Fig. 3, left images). Taken together, these findings indicate that astrocytes from both fALS and sALS cases— including C9orf72 mutations—convey toxicity toward MNs independent of their origin (spinal cord or skin). In addition, the toxicity seems to be repressed in fibroblasts but becomes active upon conversion to astrocytes.
Discussion
In summary, we report rapid, reproducible direct conversion of adult human patient fibroblasts into tripotent iNPCs. We establish that i-astrocytes from both familial and sporadic ALS patients are toxic to cocultured MNs in a similar manner as spinal cord-derived astrocytes. Although at this point, we cannot completely rule out that ALS i-astrocytes are less supportive compared with controls, our data from fibroblast cocultures and supplementation assays, as well as monocultures, strongly support a gain of toxic function model. Excitingly, we demonstrate that astrocytes carrying the recently discovered C9orf72 expansion mutation also display toxicity toward MNs, thereby corroborating a crucial role of this cell type in ALS pathogenesis. Furthermore, these findings demonstrate that the toxicity is an intrinsic property of ALS patient-derived astrocytes that is independent of the neuroinflammatory environment of the end-stage ALS spinal cord. Because patient fibroblasts do not exert a notable toxic effect on MNs, the increase in cell death observed in the astrocyte cocultures is likely attributable to cell type-specific toxic properties. The underlying mechanism behind astrocyte toxicity is currently unknown, but there is mounting evidence for the involvement of misfolded SOD1 in sporadic ALS (20–25). Although further studies are needed to address these questions, SOD1 might be a promising target for a large ALS patient population. Recent advances in vector-based gene delivery for efficient targeting of astrocytes led to an exciting expansion of the lifespan of G93A and G37R ALS mice (26). No evidence to date has implicated the involvement of SOD1 in ALS cases linked to C9orf72 repeat expansions; however, several other mechanisms have been described. Despite a potential lack of a C9orf72 protein isoform, the hexanucleotide repeat RNA could lead to the sequestration of RNA-binding proteins, such as Purα, or the translation of aberrant repeat peptides (27–30). Use of i-astrocytes and MN coculture now provides a tool for testing these hypotheses.
Finally, we note that these cultures of i-astrocytes and MNs can be set up as high-throughput model systems and that potential therapeutics can now easily be tested on a variety of ALS backgrounds, including sporadic conditions in which the cause of disease is completely unknown. This approach could also help to improve the classification of patient subpopulations in sporadic cases based on their responsiveness to different drugs. Thus, direct conversion may be sufficiently fast to determine potential therapies that would be most promising for an individual patient with ALS, thereby opening the door to personalized modeling of toxicity in ALS.
Methods
Human skin fibroblast samples were obtained from Stephen J. Kolb (ALS/MND Clinic, Department of Neurology, The Ohio State University, Wexner Medical Center, Columbus, OH), as well as John Ravits (University of California, San Diego, School of Medicine) and P.J.S. and from established tissue banks as shown in Table S1 (Gibco and Coriell Institute). Informed consent was obtained from all subjects before sample collection. Receipt of human tissues was granted through Nationwide Children's Hospital and Ohio State Institutional Review Boards. For direct conversion, 104 fibroblasts were seeded in a well of a six-well plate and treated with retroviral vectors for OCT3, Sox2, KLF4, and C-MYC for 12 h. The medium was switched to NPC medium containing FGF2 and EGF after 48 h posttransduction. Detailed descriptions of all methods, reagents, and information about the cell lines, as well as analysis, are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank Che Best for expert technical assistance and all patients for donating critical samples for this work. This work was funded by US National Institutes of Health Grants R01 NS644912-4 and RC2 NS69476-01 (to A.L.S.), and Packard Center for ALS Research Grant P2ALS and the Helping Link Foundation. K.M. is supported by a fellowship from the Swiss National Science Foundation, and L.F. is supported by a Marie Curie Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314085111/-/DCSupplemental.
References
- 1.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 2.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renton AE, et al. ITALSGEN Consortium A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Haidet-Phillips AM, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29(9):824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187(6):761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egawa N, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4(145):ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011;108(19):7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambasudhan R, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9(2):113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corti S, et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res. 2012;318(13):1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Ambasudhan R, Ding S. Direct lineage reprogramming to neural cells. Curr Opin Neurobiol. 2012;22(5):778–784. doi: 10.1016/j.conb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najm FJ, et al. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31(5):426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ring KL, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11(1):100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son EY, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang N, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31(5):434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109(7):2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thier M, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10(4):473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Hester ME, et al. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol Ther. 2011;19(10):1905–1912. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosco DA, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13(11):1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsberg K, et al. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS ONE. 2010;5(7):e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grad LI, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci USA. 2011;108(39):16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruzman A, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2007;104(30):12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guareschi S, et al. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci USA. 2012;109(13):5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polymenidou M, Cleveland DW. The seeds of neurodegeneration: Prion-like spreading in ALS. Cell. 2011;147(3):498–508. doi: 10.1016/j.cell.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foust KD, et al. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol Ther. 2013 doi: 10.1038/mt.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ash PE, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori K, et al. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013;125(3):413–423. doi: 10.1007/s00401-013-1088-7. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339(6125):1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci USA. 2013;110(19):7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.