Significance
Elevated expression of matrix metalloproteinase 1 (MMP1) is associated with a poor prognosis in a wide variety of advancing tumors, and thus MMPs have emerged as attractive targets for therapeutic strategies of metastatic tumors. However, clinical trials using MMP inhibitors in cancer therapy have proved to be disappointing. These studies support the contention that the use of broad-spectrum inhibitors of MMPs is insufficient for clinical benefit, as new compensatory pathways such as continuous production of exosomes derived from the tumor will be unaffected and will enhance the MMP produced by stromal cells. Therefore, a better understanding of the mechanisms used by tumor-derived exosomes in the induction of MMPs would permit the development of successful clinical strategies for novel MMP inhibitors.
Keywords: ICC, crosstalk, microvesicles, tumor microenvironment
Abstract
During tumor development, constant interplay occurs between tumor cells and surrounding stromal cells. We report evidence that gastrointestinal stromal tumor (GIST) cells invade the interstitial stroma through the release of the oncogenic protein tyrosine kinase (KIT)-containing exosomes, which triggers the phenotypic conversion of progenitor smooth muscle cells to tumor-promoting cells. These recipient cells display morphologic changes and acquire tumor-associated phenotypes, including enhanced adhesion to extracellular matrix proteins, activation of intracellular pathways downstream of KIT, expression of Interstitial Cell of Cajal–like markers, and release of various matrix metalloproteinases (MMPs), particularly MMP1. This report shows stimulation of MMP1 production by stromal cells via uptake of tumor-derived exosomes, which leads to tumor cell invasion. Exosomes derived from GIST patients but not healthy donors show enhanced MMP1 secretion by smooth muscle cells and tumor cell invasion, whereas selective blocking of exosome-mediated MMP1 secretion decreases tumor invasiveness. Our study indicates that exosome release and subsequent MMP1 induction creates a positive feedback mechanism established between tumor and stromal cells that drives GIST development and offers unique insights for potential therapeutic strategies to block GIST progression and metastatic spread.
With the ultimate goal of fulfilling their needs for exogenous supply of growth factors, chemokines, cytokines, and matrix-degrading enzymes, tumor cells communicate with their surrounding stroma using several intercellular communication mechanisms (1, 2). In the last decade, various tumors have been shown to rely on the release of bioactive membrane vesicles known as exosomes (3). Tumor cells of various origins constitutively release these vesicles, which are believed to be important in the processes of malignant transformation and tumor progression. Exosomes share certain common characteristics, including their shape, size, density, and general protein composition (3). Their effects are mediated via transfer of cargo that comprises an array of proteins (4), RNA (5), and mitochondrial DNA (6). Accordingly, their role as mediators in tumor progression has been the focus of several recent studies (7, 8). However, the majority of these studies focused on human malignancies of epithelial origin (i.e., carcinomas), whereas little attention has been given to the tumor microenvironment associated with malignancies of mesenchymal origin (i.e., sarcomas).
Sarcomas represent an interesting and unique tumor type in which cancer cells of mesenchymal origin are surrounded by stromal cells of the same origin. The focus of this study is on the most common mesenchymal tumor of the digestive tract, gastrointestinal stromal tumor (GIST) (9). Approximately 75% of GISTs contain oncogenic mutations in the receptor tyrosine kinase c-KIT [normal cellular homolog of the viral oncoprotein v-Kit (v-Kit, Hardy Zuckerman 4 feline sarcoma viral oncogene homolog)] (10, 11), which plays a central role in the pathogenesis of this disease (10, 12). GISTs are believed to arise from the Interstitial Cells of Cajal (ICCs) (13), the pacemaker cells of the gastrointestinal tract, or interstitial mesenchymal precursor stem cells (14). This similarity between GISTs and ICCs is further borne out by the expression of the KIT protein (also called CD117) in nonneoplastic ICC and most GISTs (11). The identification of a fundamental hallmark of the biology of GISTs (i.e., gain-of-function KIT mutations) resulted in the rapid transformation of the treatment paradigm for this tumor type (15, 16). Small molecule tyrosine kinase inhibitors, most notably imatinib mesylate (also known as Gleevec), have been developed and were found to be effective in the treatment of GISTs (16–18). However, the median time to recurrence for patients with metastatic GIST receiving imatinib is only 2 y. Although much is known about the molecular genetic features of GIST, the importance of their interactions with the stromal microenvironment during metastasis has received little attention. Therefore, we investigated the role of exosomes in mediating the complex interplay between tumor and stroma during progression. We report evidence that (i) GIST cells secrete a high number of oncogenic KIT-containing exosomes, (ii) stromal cell uptake of GIST-derived exosomes promotes the generation of ICC-like cells and induce tumor invasiveness, and (iii) circulating exosomes from GIST patients induce matrix metalloproteinase 1 (MMP1) secretion by host stromal cells.
Results
GIST–T1 Cells Constitutively Shed High Numbers of Exosomes Enriched in Oncogenic KIT.
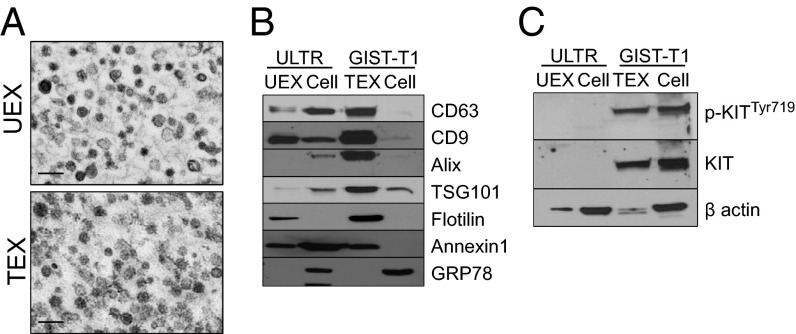
GIST–T1 cells were chosen as the quintessential model of GIST, as they have been well characterized by us and others in vitro and in vivo (15, 19) and express the most common type of mutation found in GISTs (i.e., KIT exon 11), which is involved in the pathogenesis of this disease (19). We investigated whether these GIST cells release exosomes enriched in oncogenic KIT. Specifically, we collected conditioned medium from GIST–T1 cells and ULTR, a retrovirally transformed human uterine leiomyomatous smooth-muscle cell line, and evaluated the basal production of exosomes into the culture medium using methods we have previously published (20, 21). Phase-contrast electron micrographs (Fig. 1A and Fig. S1A) and nanoparticle tracking analysis of the isolated vesicles (n = 3) showed a size distribution consistent with exosomes, with a mean size of ∼133 ± 13 nm for ULTR-derived exosomes (UEXs) and 183 ± 27 nm for GIST–T1−derived exosomes (TEXs), respectively (Fig. S1 B and C). In addition, we found that different numbers of vesicles were obtained from both lines; 1.86 × 108 particles were released by ULTR cells and 2.65 × 108 particles by GIST–T1 cells per 106 cells per 24 h (Fig. S1D). This difference was also reflected at the exosomal protein level, as significantly more protein was found associated with TEX compared with UEX particles (19 fg/108 particles versus 3 fg/108 particles, respectively; n = 19) (Fig. S1E). This result suggested an inherent difference in the basal levels of exosome-associated protein content. Immunoblot analysis of the preparations revealed that the exosomal markers tetraspanins CD63 and CD9 were enriched in exosome fractions purified from both cell lines compared with parental cell lysates (Fig. 1B). We also observed an enriched expression of additional exosome-associated markers such as Alix, TSG101, flotilin, and Annexin 1 in TEXs as well as flotilin and Annexin 1 in UEXs. In contrast, the cellular protein glucose regulated protein 78 kDa (GRP78) was detected exclusively in the cell lysates (Fig. 1B). It is now widely accepted that the molecular composition of exosomes mirrors the specialized functions of the parental cells of origin (22). Thus, we next examined whether TEXs contained oncogenic KIT, a hallmark of ∼75% of GISTs. Immunoblot analyses revealed that TEXs contained high basal levels of phospho-KITTyr719 and total KIT, comparable to levels observed in the parental cellular lysate (Fig. 1C). As expected, UEX and ULTR cellular lysates were negative for p-KIT and KIT (Fig. 1C). In addition, flow cytometry experiments using isolated TEX, ULTR, and GIST–T1 cells confirmed that both TEX and GIST cells express KIT at the exosomal surface and cell membrane, whereas ULTR cells were negative for surface-associated KIT (Fig. S1F). Our results demonstrate that GIST cells secrete exosomes carrying the mutant receptor and represent a unique oncogenic KIT protein delivery system.
Fig. 1.
GIST–T1 cells constitutively release exosomes carrying oncogenic KIT. (A) Screenshots of representative electron microscopy images of purified ULTR (UEX) and GIST–T1-cell–derived exosomes (TEXs). (Scale bar, 200 nm.) (B) Western blot analysis of exosomal markers and (C) p-KIT and KIT expression in purified TEX, UEX, and parental cell lysates.
Uptake of GIST-Derived Exosomes Triggers a Network of Stellar Cells with Enhanced Adhesive Properties.
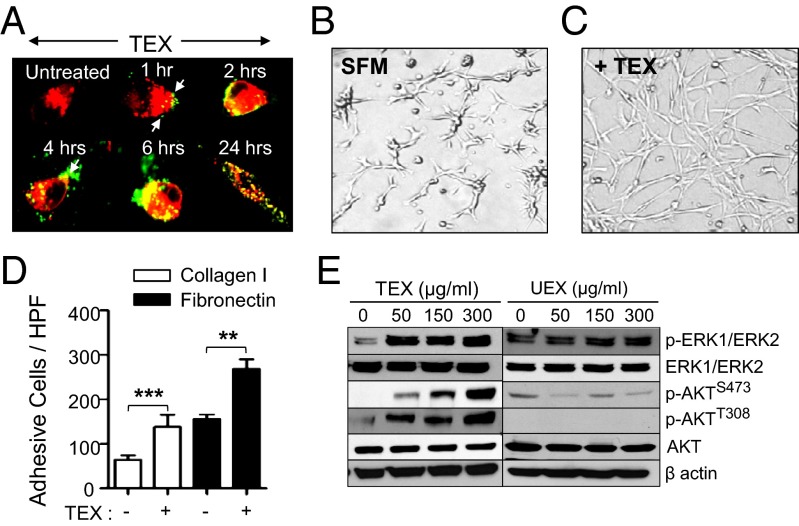
Our findings that KIT is sorted into exosomes motivated further evaluation of the phenotypic and functional impact of GIST-derived exosomes after internalization by progenitor cells—in this case, normal human myometrial smooth muscle cells (ULTR). In an effort to use a physiological concentration of cell-line–derived exosomes for our studies, we purified exosomes from the plasma of patients with GIST and matched control subjects. The number of exosomes as assessed by total protein was substantially elevated in the plasma from patients with GIST (486.2 ± 32.9 µg, n = 7) versus healthy donors (147.1 ± 26.9 µg, n = 7; Fig. S2A). To mimic the effect that exosomes exert in vivo, we used a range of subphysiological concentration, which varied between 50 and 300 µg of exosomal preparation, for subsequent experiments. We then analyzed the possible interaction occurring between GIST-derived exosomes and smooth muscle cells. GIST-derived exosomes were efficiently internalized by ULTR cells cultured in serum-free medium (SFM). As shown in Fig. 2A, 1 h after coincubation, small vesicles were observed docked at the plasma membrane by confocal microscopy with extensive internalization achieved by 24 h. Flow cytometry results also confirmed that ULTR cells efficiently take up TEXs, as we observed a significant increase in the number of PKH67-positive cells (Fig. S2B), as well as mean fluorescence intensity, at 24 h postincubation (Fig. S2C). In addition, a significant increase in surface and intracytosolic KIT receptor acquisition by these cells was observed by flow cytometry, with 10% of the cells displaying a strong membrane staining; and by immunofluorescence, where 100% of the observed cells showed intracellular and membranous KIT staining at 24 h after incubation with increasing amounts of TEXs (Fig. S2 D–F). We found that 24 h after uptake of TEXs by ULTR cells, changes in cellular morphology and intercellular interaction occurred with a marked stellar phenotype, which was reminiscent of the putative cells of origin of GISTs (i.e., ICCs; Fig. 2 B and C and Fig. S2G). The potential effect of exosomes on ULTR cell survival and proliferation was also assessed, and we observed that neither were significantly altered after preconditioning with TEXs or UEXs (Fig. S3 A, Lower and B). Moreover, we show that this phenotypic conversion was induced at concentrations as low as 2.5 µg of TEXs (Fig. S3 A, Upper). We next tested the idea that TEX internalization by ULTR cells might affect their adhesiveness to common ECM proteins naturally present in the tumor microenvironment of GISTs, such as type I collagen and fibronectin (FN). A significant number of ULTR cells adhered and spread on type I collagen- and FN-coated wells after TEX-treated compared with untreated cells (Fig. 2D and Fig. S4 A and B). We then analyzed ULTR expression of various intercellular adhesion molecules (ICAMs), such as integrin receptors, following TEX treatment. We observed a decrease in integrins α4, αv, β4, and FN and an increase in ICAM-1 (Fig. S4 C and D), suggesting that ULTR adhesion to the ECM is mediated by an integrin-independent mechanism upon treatment with TEXs.
Fig. 2.
Uptake of TEXs by smooth muscle cells is sufficient to activate specific phosphokinase signaling pathways downstream of KIT. (A) Confocal microscopy analysis of PKH67-labeled (green) TEX uptake by PKH26-labeled ULTR cells. Arrows indicate exosomes bound to the cellular surface. (B and C) ULTR cells display a highly organized network of star-like cells upon TEX treatment (+TEX) (C) compared with untreated ULTR cells cultured in SFMs (B). (D) TEX enhances the adhesive abilities of ULTR cells to FN-1 (20 µg/mL) and type I collagen (10 µg/mL). Adhesion was conducted for 30 min, and results are shown as means ± SEM (n = 3). **P = 0.0078; ***P = 0.0001 (t test). (E) Immunoblotting analysis of pERK1/2Thr202/Tyr204, pAKTS473, and pAKTT308 levels in ULTR cells treated with purified UEXs or TEXs. β actin was used as a loading control in all cases.
Intracellular Pathways Downstream of KIT Are Activated by Tumor-Released Exosomes in Smooth Muscle Cells After Uptake.
We next examined the activation status of AKT and ERK1/2, known downstream mediators of KIT, in recipient ULTR cells after exposure to TEXs. As shown in Fig. 2E, treatment with TEX led to a dose-dependent enhancement in the phosphorylation levels of ERK1/2, as well as phosphorylated AKTSer473 and AKTThr308, whereas exposure to UEXs had no significant effect on intracellular signaling events (Fig. 2E). We further confirmed that TEXs could lead to similar changes in KIT-negative, short-term cultured primary myometrial cells, Myo1, 2, and 3 (Fig. S4 E and F). Therefore, enhanced uptake of TEXs by smooth muscle cells is sufficient to activate the clinically relevant phosphokinase signaling pathways downstream of KIT.
Tumor-Derived Exosomes Promote the Generation of ICC-Like Cells.
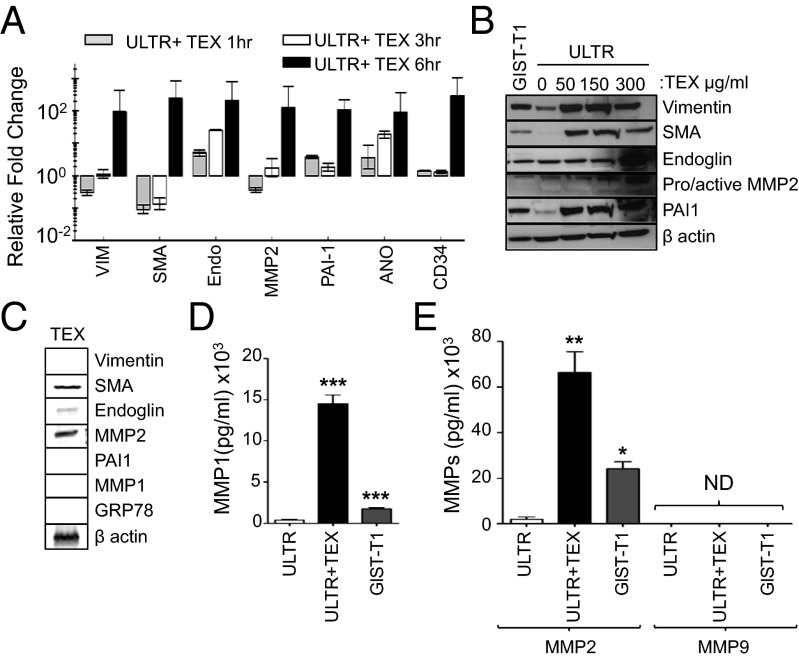
To determine which TEX-dependent transcripts are induced in ULTR cells after uptake, total RNA was extracted from GIST–T1 cells, TEXs, ULTR cells, and ULTR cells exposed to TEXs. The quality and size distribution of total RNA were analyzed using a bioanalyzer. As shown in Fig. S5 A and B, an enrichment of small RNAs and other larger RNAs was observed in TEX-treated ULTR cells. Based on the phenotypic alterations mentioned, we evaluated whether smooth muscle cells in the presence of TEXs could induce an ICC-like phenotype. Indeed, primary and TEX-exposed ULTR cells showed an increased expression of ICC-associated markers, such as vimentin, smooth muscle actin (SMA), and endoglin, as well as tumor-associated biomarkers such as plasminogen activator inhibitor–1 (PAI-1) and MMP2 at mRNA (Fig. 3A) and protein levels (Fig. 3B) in a time- and dose-dependent manner. These results were corroborated by experiments with primary myometrial cells (Fig. S6A). To determine whether the expression of these ICC-associated transcripts and proteins were induced de novo and not via transfer of TEX-associated exosomal mRNA or proteins into recipient cells, we evaluated these transcripts and proteins in purified TEX preparations by quantitative RT-PCR analysis, using similar input of RNA. As shown in Fig. S6B, negligible amounts of these specific transcripts were present in TEXs, suggesting that these transcripts were mainly induced in ULTR cells rather than transferred via exosomes. Similar analyses using 30 µg of cellular or exosomal proteins showed that exosome preparations failed to express any detectable levels of vimentin and PAI-1, whereas low amounts of SMA, endoglin, and MMP2 were associated with TEXs (Fig. 3 B and C), and all were induced in ULTR cells upon TEX uptake. In addition, SMA, endoglin, and MMP2 proteins’ expression levels were lower in the exosome preparation versus that of TEX-treated recipient cells, suggesting that these proteins were induced and not transferred by exosomes to recipient cells.
Fig. 3.
Uptake of TEXs induces unique transcriptomic/proteomic/secretomic profiles in ULTR cells. (A) Endogenous and TEX-induced expression of ICC- and tumor-associated transcripts in ULTR cells at the mRNA level, expressed as fold change ± SEM of each target transcript relative to untreated cells. (B) ULTR cells cocultured with TEX induce vimentin, SMA, endoglin, MMP2, and PAI-1 protein expression, and basal level of expression in untreated GIST–T1 cells (30 µg). (C) Analysis of endogenous ICC and tumor marker expression on purified TEXs by Western blot (30 µg). (D and E) Comparative basal and TEX-induced (D) MMP1, (E) MMP2, or MMP9 activity in ULTR-cell–derived conditioned medium by Luminex ELISA. Compared with untreated ULTR cells. *P = 0.01; **P < 0.01; ***P < 0.001 (t test).
TEX Stimulates ICC-Like Cells to Secrete Active Tumor-Associated MMPs.
Because production of MMPs has been shown to be critical in the process of tumor invasion and metastasis, gelatin zymography was used to evaluate MMP production by ULTR and three independent primary smooth muscle cells (Myo1–3) following TEX exposure. The basal levels of MMP activity in the conditioned medium from untreated ULTR cells and Myo2 were almost undetectable compared with GIST–T1 cells cultured under serum-free conditions (Fig. S6 C–E). In contrast, Myo1 and Myo3 exhibited both basal MMP activity in their respective conditioned medium, whereas purified TEXs exhibited high gelatinase activity (Fig. S6E). In all cases, the enzymatic activities were dramatically increased in the conditioned medium after TEX exposure (Fig. S6 D and E). Together, these observations demonstrate that TEXs are efficient modifiers of the enzymatic activity profiles of smooth muscle cells. These data further imply that up-regulation of these enzymatic activities represents a previously undescribed mechanism by which exosomes may increase tumor invasion, angiogenic switch, and subsequently local tumor spread.
Enhanced Tumor Cell Invasiveness Is Dependent on MMP1 Secretion by ICC-Like Cells.
Most tumor cells have the ability to produce various proteases involved in the remodeling of the ECM, either by themselves or via tumor−stromal cell interactions (2). To ascertain the identity of the MMPs secreted in the conditioned medium of TEX-treated cells, as well as their endogenous levels in the conditioned medium of untreated primary smooth muscle cells, ULTR cells, and untreated GIST–T1 cells, we used a multiplexed MAP Luminex ELISA. We found that MMP1 and MMP2 were significantly increased in the conditioned medium after TEX treatment of ULTR cells (38- and 33-fold, respectively; Fig. 3 D and E). However, ULTR cells secreted significantly more MMP1 than MMP2 (P < 0.001 for MMP1 versus P < 0.01 for MMP2) in response to TEX challenge compared with untreated ULTR cells (Fig. 3 D and E). We obtained similar results from conditioned medium collected after TEX conditioning of primary smooth muscle cells compared with untreated cells (Fig. S6 F and G). In contrast, MMP9, another crucial gelatinase associated with tumor invasion, was not detectable in any of our preparations (Fig. 3E and Fig. S6G).
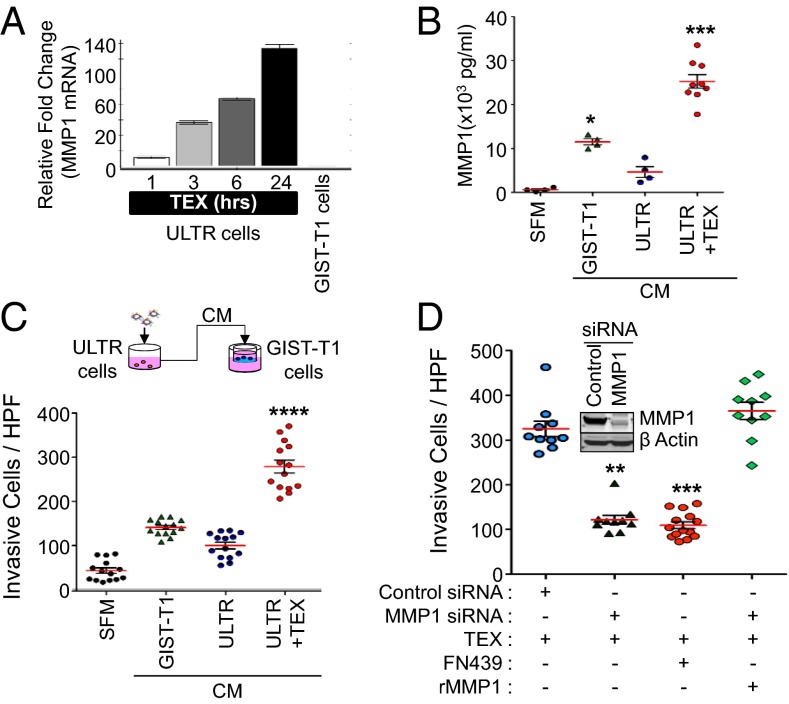
As previously mentioned, GISTs are known to grow in an endophytic manner parallel to the bowel lumen. Therefore, these tumors must constantly invade the interstitial stroma, which is composed of type I collagen (23). Because degradation of interstitial collagen is an essential step for GIST growth and invasion in vivo, we choose to focus our investigation on MMP1, the prototypical interstitial collagenase. We first ensured that MMP1 was a transcriptional target induced by TEXs in ULTR cells, as shown in Fig. 4A. A significant increase in mRNA coding for MMP1 was observed compared with untreated cells. Because the multiplex ELISA we used in our early studies quantified total secreted proteases (both inactive and active forms) and only activated MMPs are capable of proteolytically degrading ECM components, we next used the Sensolyte Plus 520 MMP1 assay to specifically analyze MMP1 with an anti-MMP1 antibody and measure specifically MMP1 activity after TEX conditioning. We found that basal MMP1 activity in GIST–T1−conditioned medium was significantly elevated (19-fold) compared with SFM, whereas MMP1 activity was only eightfold greater than SFM in ULTR cell-derived conditioned medium (Fig. 4B), suggesting that smooth muscle cells secrete less MMP1 in resting conditions compared with GIST–T1 cells. In contrast, MMP1 activity detected in the conditioned medium of ULTR cells was induced 42-fold after TEX treatment (Fig. 4B) compared with SFM. Overall, TEX-challenged ULTR cells increased MMP1 release into the conditioned medium by fivefold compared with untreated cells (Fig. 4B). Together, these findings further support the premise that uptake of GIST-derived exosomes by stromal cells increases MMP1 secretion in the recipient cells.
Fig. 4.
Stromal-cell–derived MMP1 produced in response to TEX challenge is required to enhance the invasiveness of GIST–T1 cells. (A) MMP1 transcript in GIST–T1 cells and in TEX-treated ULTR cells. Mean fold change relative to untreated ULTR cells ± SEM is indicated (n = 3). (B) Endogenous concentration of active MMP1 in pg/mL detected in SFM, in conditioned medium derived from GIST–T1 cells (CM GIST–T1), ULTR cells (CM ULTR), and ULTR cells treated with TEX for 24 h (CM ULTR + TEX), measured with SensoLyte Plus 520 MMP1 Assay Kit. *P < 0.05; ***P < 0.001 (Kruskal–Wallis test and Mann–Whitney U posttest). Red line indicates mean, and black brackets indicate ± SEM. (C) Schematic representation of invasion assays. CM ULTR + TEX enhances GIST–T1 cell invasiveness after 48 h in the collagen type I invasion assay. Mean number of invasive cells per HPF. Data are presented as means ± SEM (n = 3). Compared with GIST–T1 CM, ***P < 0.0001 (t test). (D) Knockdown or chemical inhibition of MMP1 in ULTR cells before TEX challenge inhibits invasion of GIST–T1 cells in a collagen I invasion system. Compared with control siRNA + TEX, **P < 0.01; ***P < 0.001 (n = 3; t test). (D, Inset) Efficient and specific knockdown of MMP1 in ULTR cells occurred at the protein levels.
After establishing that MMP1 production and secretion is dramatically induced in TEX-treated smooth muscle cells, we investigated whether conditioned medium from these cells would enhance GIST–T1 invasion in vitro using a type I collagen invasion assay. GIST–T1 cells were mixed with exosome-free conditioned medium derived from GIST–T1 cells, ULTR cells, or ULTR cells treated with TEXs. Invasiveness was evaluated after 48 h in a type I collagen-coated invasion chamber. The levels of MMP1 in aliquots of conditioned medium were determined before use in the invasion assay (Fig. 4B). Addition of conditioned medium derived from TEX-treated ULTR cells to the upper compartment of the chamber significantly enhanced invasion of GIST–T1 cells (sevenfold difference; Fig. 4C and Fig. S7A) compared with SFM. Notably, the amount of MMP1 in the conditioned medium from GIST–T1 cells was sufficient to increase the invasion of these cells by fourfold compared with SFM-mediated invasion. In addition, we found that untreated ULTR cells released some MMP1 in resting conditions (Fig. 4C), which enhanced invasion of GIST–T1 cells by twofold compared with SFM. However, the level of induction was lower than that of GIST–T1 CM-derived MMP1 (fourfold difference). We also found that the most significant enhancement of GIST invasion occurred using conditioned medium derived from TEX-treated ULTR cells (Fig. 4C and Fig. S7A). Finally, we assessed the potential effect of conditioned medium derived from untreated GIST–T1 cells, ULTR cells, or ULTR cells plus TEX on GIST–T1 cell proliferation and observed that none of the treatments significantly altered proliferation rates (Fig. S7B).
To further validate our observations and demonstrate the functional relevance of TEX-treated ULTR cell-derived MMP1 on invasion of GIST–T1 cells, we performed inhibition studies using an MMP1-specific siRNA approach and the well-characterized MMP1 inhibitor FN439 (24). We observed that MMP1 levels were depleted by ∼70% with small interfering RNAs to MMP1 (MMP1 siRNAs) compared with control siRNA-transfected cells (Fig. 4D, Inset and Fig. S8 A–D). We also observed that MMP2 protein levels mimicked the MMP1 expression pattern in these cells, indicated by a decrease in MMP2 in MMP1 siRNA-treated cells (Fig. S8C). This suggests that MMP1 may participate in the activation of other MMPs. In addition, conditioned medium collected from control cells or MMP1 siRNA-transfected ULTR cells without any TEX conditioning did not cause changes in GIST–T1 cell invasion (Fig. S8D), suggesting that TEX challenge is required for invasion. Although invasive ability through collagen matrix of GIST–T1 cells coincubated with conditioned medium derived from TEX-challenged MMP1 knocked-down ULTR cells (MMP1 siRNA + TEX) was significantly reduced to a similar extent to that observed in FN439–treated conditioned medium from TEX challenged untransfected ULTR cells (CM ULTR + TEX + FN439) (Fig. 4D and Fig. S8E), these levels were significantly reduced compared with invasive ability observed with TEX-challenged control siRNA transfected cells (control siRNA + TEX). In addition, exogenous replenishment of MMP1 levels by addition of recombinant MMP1 in the CM of MMP1 siRNA-transfected ULTR cells treated with TEXs restored their invasiveness, suggesting that MMP1 expression induced by TEXs directly contributes to GIST cell invasion. MMP1 levels secreted in the conditioned medium displayed a significantly positive correlation with the invasiveness of GIST–T1 cells, further supporting our findings (Pearson’s value, R = 0.9928; P = 0.007; Fig. S9A). Together, these results strongly indicate that MMP1 secreted after TEX uptake by ULTR cells enhances the invasion of GIST cells.
In Vivo–Derived GIST Exosomes Enhance the Invasiveness of Tumor Cells via Secretion of MMP1 by Smooth Muscle Cells.
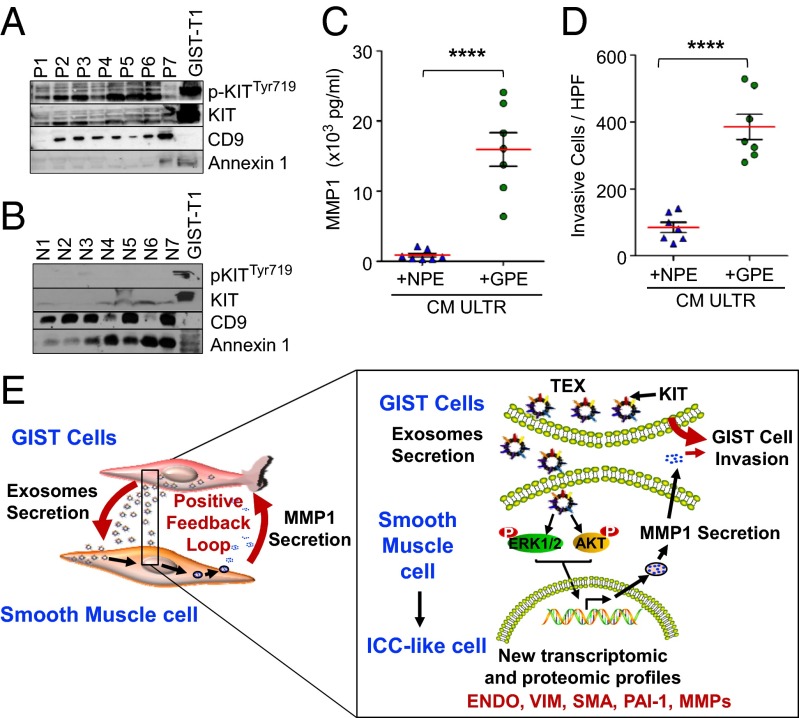
Based on several reports that exosomes are present in different biological fluids from both tumor patients and healthy subjects (25, 26), we purified exosomes from the plasma of patients with GIST (n = 7) and healthy donors (n = 7). Electron microscopic analysis confirmed the presence of vesicles the size of exosomes (Fig. S9B). Western blotting analysis showed that exosomes from patients with GIST expressed various molecular forms of phosphorylated KIT and total KIT, as well as the previously defined exosomal markers CD9 and Annexin 1 (Fig. 5A). Conversely, we found that, although a low level of total KIT was present in exosomes derived from healthy donors, active KIT was undetectable (Fig. 5B). This result confirms that active KIT is only found in tumor-derived exosomes from the plasma of GIST patients. To further support our findings, ULTR cells were treated with exosomes derived from patients or healthy donors (n = 7) for 24 h. ULTR cells produced significantly more MMP1 when treated with GIST-patient–derived exosomes compared with those from healthy donors (Fig. 5C). We found that conditioned medium derived from ULTR cells treated with GIST-patient–derived exosomes significantly enhanced the invasion of GIST–T1 cells compared with healthy-donor–derived exosomes (4.5-fold increase; Fig. 5D and Fig. S9C). To further support our findings, we analyzed the correlative value observed between MMP1 amounts present in the conditioned medium and the number of invasive cells in a collagen type I invasion assay after mixture with conditioned medium of GIST–T1 cells with healthy-donor–derived exosome-treated (n = 7) and GIST-patients–derived exosome-treated (n = 7) ULTR cells. We found a strong positive correlation between MMP1 production after culture with GIST-patient–derived exosomes (R = 0.9532; P = 0.0009) or healthy-donor–derived exosomes (R = 0.9449; P = 0.0013) and the number of invasive cells per high powerfield (HPF) (Fig. S9 D and E). However, the most significant positive correlation was obtained with GIST-patient–derived exosome-treated ULTR cells (Fig. S9D) compared with healthy-donor–derived exosomes (Fig. S9E), strongly suggesting that exosomes from patients with GIST have unique inherent properties that allow tumor cell invasion via interaction with stromal cells.
Fig. 5.
pKIT is highly expressed in GIST-patient–derived exosomes and stimulates invasion of GIST–T1 cells. (A) High amount of pKITTyr719 and KIT protein is detected on exosomes purified from plasma samples from GIST patients (GIST patients derived exosomes, GPE, n = 7), but not (B) healthy donors (noncancer-bearing patients-derived exosomes, NPE, n = 7). GIST–T1 cells were used as positive control. (C) Enhanced active MMP1 protein (in pg/mL) is detected in the CM of ULTR cells after coculture with purified NPE or GPE for 24 h (n = 7 each) by SensoLyte Plus MMP1 Assay. (D) CMs from ULTR cells cultured with GPE exosomes (n = 7 each) enhance invasion on type I collagen of GIST–T1 cells. Data represented as mean number of invasive cells per HPF (n = 3, five random fields were analyzed for each plasma-derived exosomes). Compared with the parental control, ****P < 0.0001 (t test). (E) A schematic model of the TEX–MMP1-positive feedback loop in tumor invasion.
Finally, these in vitro results were further supported by immunofluorescence staining of MMP1 in GIST-patient–derived tumors (n = 5) and adjacent normal gastric (n = 4) tissue. In the noninvolved gastric tissue adjacent to the tumor, MMP1 immunostaining was undetectable (Fig. S10, Left), whereas GIST samples showed strong MMP1 staining in the tumor-associated stroma (Fig. S10, Right). We further analyzed the identity of the stromal cells secreting MMP1 using the smooth-muscle-cell–specific marker, SMA (Fig. S10, Bottom). Our data show that, in the tumor adjacent normal gastric tissue sections, immunoreactivity for KIT was confined to fusiform cells with similar morphology to ICCs (Fig. S10, Left) and that most of the KIT-immunoreactive cells were located in lamina propria of the gastric tissue and were also positive for SMA (Fig. S10, Bottom). On the basis of all of these findings, a model is proposed for the function of the TEX–MMP1 axis in GIST invasion (Fig. 5E).
Discussion
The development of most carcinomas depends on the establishment of intricate communication networks within and between the surrounding stroma (1). However, the critical molecular changes that occur in the stroma of mesenchymal tumors, such as GISTs, remain relatively undefined. Here we provide evidence that significant numbers of oncogenic KIT-bearing exosomes are released by GIST cells in vitro and in vivo and represent potent phenotypic modifiers of their microenvironment. Our data further suggest that one mechanism by which KIT-carrying exosomes promotes tumor progression is through the regulation of downstream KIT-signaling pathways in stromal cells, which differentiate into ICC-like cells (Fig. 5E). In addition, uptake of TEXs by ULTR cells, as well as primary smooth muscle cells, modulates their transcriptomic/proteomic/secretomic profiles and leads to enhanced secretion of the interstitial collagenase MMP1, which is required for tumor cell invasion. To determine the direct contribution of oncogenic KIT in this process, we performed both KIT-silencing and -inhibition experiments via siRNA and imatinib, respectively, before exosome isolation. However, neither experiment proved feasible as we were unable to deplete or inhibit KIT in GIST–T1 cells without affecting cell viability and thus altering the composition of the population of vesicles released by these cells (data not included).
It is now recognized that MMP1 and other MMPs contribute actively to the elaboration of the stromal microenvironment during early and late stages of carcinoma development (27, 28). In the context of sarcomas, MMP1 gene expression has been shown to be a prognostic factor for local recurrence and metastasis in human chondrosarcoma (29). In our study, we found that challenging ULTR cells with GIST-patient–derived exosomes (but not exosomes from healthy donors) significantly increased MMP1 production, which in turn enhanced GIST cell invasion (Fig. 5 C and D). When expression of MMP1 in ULTR recipient cells was knocked down using siRNA, the invasive propensity of tumor cells exposed to ULTR-conditioned medium was comparable to that of untreated cells (Fig. S8D). However, if the same cells were challenged with TEXs, tumor cell invasion was almost completely abrogated (Fig. 4D and Fig. S8E). These data support the hypothesis that a feedback loop exists between exosome-mediated signaling and MMP expression in stromal cells and provide strong evidence for a stroma-modifying role of exosomes. Future mechanistic studies are focused to further refine tumor-derived exosome-mediated signal(s) responsible for altering the phenotype of stromal cells.
The present study focuses on GISTs with oncogenic KIT; however, exosome-mediated transformation in other tumors may not solely be driven by oncogenes. In fact, we know that exosomes are far more complex in composition, comprising other possible exosomal components such as proteins, lipids, and RNAs (Fig. S5), which contribute or enhance the observed transformation. However, in this study we show that ICC- and tumor-associated transcripts as well as proteins, although found in negligible amount in purified TEXs, are primarly induced upon TEX uptake in ULTR cells (Fig. 3 A and B and Fig. S6B). Furthermore, a recent study by Peinado et al. (30) showed that melanoma-associated exosomes could promote metastasis through crosstalk between exosomes and bone marrow progenitor cells (30). Here we demonstrate that tumor cells can use exosomes to modify their local microenvironment by reprogramming normal surrounding stromal cells and, in turn, enhancing release of molecules that can further promote cell growth and invasion.
Many cellular and molecular components of the tumor microenvironment have emerged as attractive targets for therapeutic strategies among carcinomas. MMPs have been implicated in many processes involved in tumor progression, such as antiangiogenesis (31, 32). Despite the wealth of preclinical studies using MMP1 as a therapeutic target, clinical trials of MMP inhibitors in cancer therapy have proved to be disappointing (33). Several studies support the contention that broad-spectrum inhibitors of MMPs are responsible for failed previous clinical trials (34). Additional pathways, such as continuous production and secretion of oncogene-containing exosomes from the tumor, are likely to compensate for such broad-spectrum MMP inhibition (35). We present evidence for a previously unknown tumor−stromal communication loop involving exosome-mediated signaling, increased stromal MMP1 secretion, and enhanced tumor cell invasion: the tumor-derived exosome–MMP1 axis (Fig. 5E). Although further studies are needed to identify the factors that regulate the production of GIST-derived exosomes and their uptake by surrounding stromal cells, these findings provide important and unique insights into the pathogenesis of GISTs and underscore the need to explore alternative therapeutic approaches to impair MMP-driven mechanisms of tumor invasion and metastasis.
Materials and Methods
Detailed descriptions of the cell lines, primary cell culture conditions, exosome purifications, Western blot, zymography, qRT-PCR, adhesion and invasion assays, measurement of MMP levels, viability assay, siRNA knockdown of MMP1, proliferation assay, immunofluorescent staining of tissue sections, flow cytometry, and fluorescent, confocal, and electron microscopy methodologies are listed in SI Materials and Methods.
Exosome Purification.
Exosomes were isolated from cell-culture–conditioned medium and plasma samples, as indicated in SI Materials and Methods.
Western Blot Analysis.
Total exosomal and cellular proteins were analyzed as described in SI Materials and Methods.
qRT-PCR.
cDNA synthesis was performed using 1 µg of total RNA with SuperScript III Reverse Transcriptase (Life Technologies). Real-time PCR reactions were conducted using SSoAdvanced SYBR Green Supermix, Biorad in a CFX96 Touch (Biorad), as described in SI Materials and Methods.
Adhesion Assay.
Adhesion of untreated or TEX-treated ULTR cells to collagen-1 and FN after 24 h was determined as described in SI Materials and Methods.
Invasion Assay.
The assay was performed as described in SI Materials and Methods.
Statistics.
Statistical analysis and graphs were generated using the GraphPad Prism-5 software (version 5.01). Statistical analyses were done using unpaired/paired, two-sided t test and Kruskal–Wallis test and Mann–Whitney U posttest for further analysis.
Supplementary Material
Acknowledgments
We are grateful to Dr. Vargheese Chennathukuzhi for providing the primary myometrial cells, Dr. Harsh Pathak for his help with Luminex experiments, and Dr. Jamie Kistler for critical review of the manuscript. We acknowledge support from the University of Kansas (KU) Cancer Center’s Biospecimen Repository Core Facility staff for helping obtain human specimens. The authors also acknowledge support from the KU Cancer Center’s Cancer Center Support Grant (P30 CA168524), the Kansas Bioscience Authority Eminent Scholar Program. This project was supported in part by a grant from the National Cancer Institute R01 CA106588 and from the National Institute of Health UL1 TR000001-02S1 (to A.K.G.) and KU Biomedical Research Training Program (to S.A.). The funders did not have any involvement in the experimental design, data collection, analysis, or interpretation of the data; the writing of the article; or the decision to submit the article for publication. A.K.G. is the Chancellors Distinguished Chair in Biomedical Sciences endowed Professor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310501111/-/DCSupplemental.
References
- 1.Löffek S, Zigrino P, Mauch C. [Tumor-stroma interactions: Their role in the control of tumor cell invasion and metastasis] J Dtsch Dermatol Ges. 2006;4(6):496–502, quiz 503. doi: 10.1111/j.1610-0387.2006.05991.x. [DOI] [PubMed] [Google Scholar]
- 2.Horimoto Y, Polanska UM, Takahashi Y, Orimo A. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adhes Migr. 2012;6(3):193–202. doi: 10.4161/cam.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 4.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adhes Migr. 2007;1(3):156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 7.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Chaput N, Schartz NE, Andre F, Zitvogel L. Exosomes for immunotherapy of cancer. Adv Exp Med Biol. 2003;532:215–221. doi: 10.1007/978-1-4615-0081-0_17. [DOI] [PubMed] [Google Scholar]
- 9.Blay JY, Le Cesne A, Cassier PA, Ray-Coquard IL. Gastrointestinal stromal tumors (GIST): A rare entity, a tumor model for personalized therapy, and yet ten different molecular subtypes. Discov Med. 2012;13(72):357–367. [PubMed] [Google Scholar]
- 10.Godwin AK. Bench to bedside and back again: Personalizing treatment for patients with GIST. Mol Cancer Ther. 2011;10(11):2026–2027. doi: 10.1158/1535-7163.MCT-11-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 12.Raut CP, DeMatteo RP. Prognostic factors for primary GIST: Prime time for personalized therapy? Ann Surg Oncol. 2008;15(1):4–6. doi: 10.1245/s10434-007-9634-y. [DOI] [PubMed] [Google Scholar]
- 13.Sircar K, et al. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23(4):377–389. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 15.Rink L, Godwin AK. Clinical and molecular characteristics of gastrointestinal stromal tumors in the pediatric and young adult population. Curr Oncol Rep. 2009;11(4):314–321. doi: 10.1007/s11912-009-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMatteo RP. The GIST of targeted cancer therapy: A tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571) Ann Surg Oncol. 2002;9(9):831–839. doi: 10.1007/BF02557518. [DOI] [PubMed] [Google Scholar]
- 17.Debiec-Rychter M, et al. EORTC Soft Tissue and Bone Sarcoma Group Italian Sarcoma Group Australasian GastroIntestinal Trials Group KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Joensuu H. [Tyrosine kinase inhibitor as a targeted therapy for GIST tumors] Duodecim. 2002;118(22):2305–2312. [PubMed] [Google Scholar]
- 19.Taguchi T, et al. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab Invest. 2002;82(5):663–665. doi: 10.1038/labinvest.3780461. [DOI] [PubMed] [Google Scholar]
- 20.Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1β production by macrophages. Am J Reprod Immunol. 2011;66(4):259–269. doi: 10.1111/j.1600-0897.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 21.Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol. 2011;65(1):65–77. doi: 10.1111/j.1600-0897.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 22.Thery C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Mehren M, et al. Soft tissue sarcoma, version 2.2012: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(8):951–960. doi: 10.6004/jnccn.2012.0099. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal A, et al. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008;7(9):2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andre F, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 26.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita K, Mori M, Kataoka A, Inoue H, Sugimachi K. The clinical significance of MMP-1 expression in oesophageal carcinoma. Br J Cancer. 2001;84(2):276–282. doi: 10.1054/bjoc.2000.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawatsubashi M, Mizokami H, Tokunaga O, Shin T. Expression of MMP-1, TIMP-1, and type I collagen in laryngeal carcinoma. Mod Pathol. 1998;11(9):878–885. [PubMed] [Google Scholar]
- 29.Pei Y, Harvey A, Yu XP, Chandrasekhar S, Thirunavukkarasu K. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells: Potential role of Runx2 in mediating p38 effects. Osteoarthritis Cartilage. 2006;14(8):749–758. doi: 10.1016/j.joca.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samant RS, Shevde LA. Recent advances in anti-angiogenic therapy of cancer. Oncotarget. 2011;2(3):122–134. doi: 10.18632/oncotarget.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basset P, et al. Matrix metalloproteinases as stromal effectors of human carcinoma progression: Therapeutic implications. Matrix Biol. 1997;15(8-9):535–541. doi: 10.1016/s0945-053x(97)90028-7. [DOI] [PubMed] [Google Scholar]
- 33.Drummond AH, et al. Preclinical and clinical studies of MMP inhibitors in cancer. Ann N Y Acad Sci. 1999;878:228–235. doi: 10.1111/j.1749-6632.1999.tb07688.x. [DOI] [PubMed] [Google Scholar]
- 34.Fingleton B. MMPs as therapeutic targets—Still a viable option? Semin Cell Dev Biol. 2008;19(1):61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.