Significance
The growth of many cell types combines polarized elongation with directional responses to external cues. We have previously linked Ca2+ influx with directional growth in fungi and show here that Ca2+ influx can rescue phenotypes caused by genetic disruption of two Cdc42 GTPase plasma-membrane trafficking pathways that are required for polarity establishment, hence restoring directional polarization. Constitutive activation of Cdc42 reversed the direction of polarization, which was also enhanced by the provision of exogenous Ca2+. Our model proposes that Ca2+ transport amplifies weak directional growth signals specified by activated Cdc42 by promoting Cdc42 trafficking to the plasma membrane, thereby enhancing its directional regulation of polarized growth.
Abstract
Polarized cells reorient their direction of growth in response to environmental cues. In the fungus Candida albicans, the Rho-family small GTPase, Cdc42, is essential for polarized hyphal growth and Ca2+ influx is required for the tropic responses of hyphae to environmental cues, but the regulatory link between these systems is unclear. In this study, the interaction between Ca2+ influx and Cdc42 polarity-complex dynamics was investigated using hyphal galvanotropic and thigmotropic responses as reporter systems. During polarity establishment in an applied electric field, cathodal emergence of hyphae was lost when either of the two Cdc42 apical recycling pathways was disrupted by deletion of Rdi1, a guanine nucleotide dissociation inhibitor, or Bnr1, a formin, but was completely restored by extracellular Ca2+. Loss of the Cdc42 GTPase activating proteins, Rga2 and Bem3, also abolished cathodal polarization, but this was not rescued by Ca2+. Expression of GTP-locked Cdc42 reversed the polarity of hypha emergence from cathodal to anodal, an effect augmented by Ca2+. The cathodal directional cue therefore requires Cdc42 GTP hydrolysis. Ca2+ influx amplifies Cdc42-mediated directional growth signals, in part by augmenting Cdc42 apical trafficking. The Ca2+-binding EF-hand motif in Cdc24, the Cdc42 activator, was essential for growth in yeast cells but not in established hyphae. The Cdc24 EF-hand motif is therefore essential for polarity establishment but not for polarity maintenance.
Fungal filaments, plant root hairs, pollen tubes, and neurites are specialized cells that grow by continuous extension at a polarized tip. Growth trajectory is determined initially by the site at which cell polarity is established and is subsequently controlled by steering mechanisms within the cell tip. Directional growth is fundamental to the ecology of all fungi, but the sensing and response mechanisms have not been dissected at the molecular level. Ca2+ influx is required for the tropic growth of Candida albicans hyphae, but it is not known how such influxes influence the polarized growth machinery at the hyphal tip (1).
In the budding yeast Saccharomyces cerevisiae, cells lacking intrinsic cortical site markers polarize at a random site through the process of symmetry breaking (2) whereby autocatalytically activated Cdc42 GTPase recruits cytosolic Cdc24 [the guanine exchange factor (GEF) for Cdc42] and the adaptor protein, Bem1, to form the polarity complex and activate formins. Cell polarity becomes fully established when formins nucleate the assembly of actin cables for delivery of exocytic secretory vesicles. Two mechanisms maintain focused polarized growth. First, as Cdc42 diffuses away from the polarity site, it is deactivated by its GTPase activating proteins (GAPs). Second, it is recycled to the polarity site via two trafficking pathways (3). The fast cytosolic route involves extraction of Cdc42 from the membrane by a guanine nucleotide dissociation inhibitor (GDI) for return to the polarity site by an unknown mechanism (4, 5).The slower, membrane-mediated route removes Cdc42 by endocytosis for eventual recycling via the secretory pathway. Thus, Cdc42–GTP–GDP interconversion, extraction of Cdc42–GDP by a GDI, nucleation of actin cables by formins, and reactivation of Cdc42 by Cdc24 form apical gyratory systems that focus Cdc42 activity at the plasma membrane (3, 6–11).We sought to establish whether these trafficking pathways were required for the directional growth responses of C. albicans hyphae and asked whether Ca2+ influx acts on this pathway to provide positional information.
The thigmotropic (contact-sensing) and galvanotropic (alignment in a direct current electric field) responses of the human pathogenic fungus C. albicans serve as tractable systems for the study of normal tip behavior by reverse genetics. Tropisms may also be important for invasive disease (12). Wild-type hyphae exhibit thigmotropism by reorienting their tip growth to follow the contours of small topographical features, a response that requires extracellular Ca2+ (1, 13). The application of an electric field elicits two distinct galvanotropic responses in C. albicans. First, C. albicans polarizes at the cathodal face of the mother yeast cell. This response requires extracellular Ca2+ and the voltage-gated Ca2+ channel, Cch1 (1, 14). Second, mature hyphae reorient toward the cathode when an electric field is applied—a response that seems to be independent of Cch1 (1). All three tropic responses require the Ras-like GTPase Rsr1/Bud1 (12), which localizes Cdc24 to the new growth site in yeast (15, 16). Activation of Cdc42 by Cdc24 at the correct position is therefore also implicated in the regulation of directional growth in hyphae. The presence of a C-terminal EF-hand motif in Cdc24 (17) suggests this protein might be responsive to Ca2+ as a directional signal.
We show here that cathodal polarization depends on the Cdc42 GAPs and the direction of polarization is reversed on expression of constitutively active Cdc42. Cdc42 apical recycling is required for galvanotropic responses, and exogenous Ca2+ can completely rescue defects in these pathways. The Cdc24 EF-hand motif is required for normal directional growth but not for polarity maintenance in C. albicans hyphae. We conclude that Ca2+ influx enhances positional signals by focusing Cdc42 delivery in response to vectorial cues.
Results
Hyphae Expressing GTP-Locked Cdc42 Polarize Anodally.
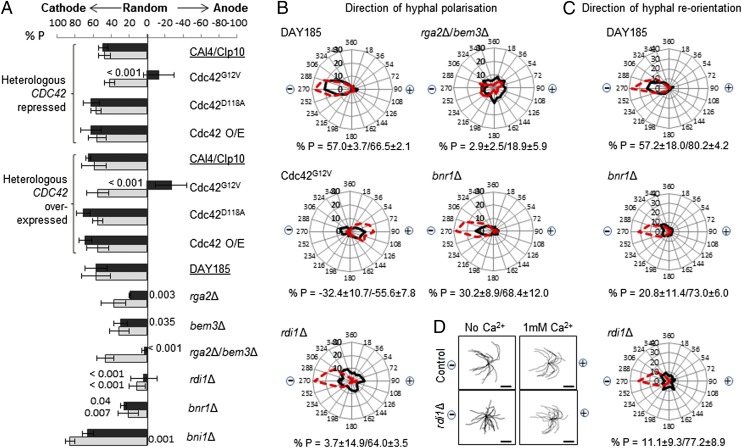
We first asked whether cell polarization or hyphal reorientation in an electric field was affected by altered cellular levels of activated Cdc42. Three Cdc42 mutants expressing one wild-type and one regulatable copy of alleles Cdc42G12V (hyperactive), Cdc42D118A (nonactivatable), or wild-type Cdc42 were used, along with strains lacking either or both Cdc42 GAPs, Rga2 and Bem3. In the GAP mutants, levels of Cdc42–GTP are predicted to be elevated (5, 18, 19). Wild-type cells normally polarize toward the cathode in an electric field. This was not affected by overexpression of wild-type Cdc42 but the direction of polarization was almost random upon deletion of the Cdc42 GAPs, Rga2 and Bem3 (Fig. 1 A and B), as is seen in wild-type cells in the absence of an electric field. When the strain harboring Cdc42G12Vwas grown in glucose to repress its expression, the direction of cell polarization became partially anodal. Repression of the heterologous CDC42 allele did not have this effect, so anodal emergence was not due to CDC42 haploinsufficiency. Cells became more highly polarized toward the anode when Cdc42G12V was overexpressed, indicating that cathodal polarity establishment requires Cdc42 GTP hydrolysis. When an electric field was applied to mature hyphae expressing Cdc42G12V or the GAP mutants, they reoriented cathodally as wild type (Fig. S1), indicating that dependence on Cdc42 GTP hydrolysis for cathodal orientation was limited to the period of polarity establishment.
Fig. 1.
Cdc42 GTPase regulation and trafficking is required for cathodal alignment. (A) The direction of cell polarization (dark bars) was determined after 2 h of growth in an applied electric field. Reorientation of mature hyphae (light bars) was determined after 2 h of pregrowth followed by 3 h of growth in an electric field. % P = 100 denotes cathodal growth, 0 denotes random orientation, and –100 denotes anodal growth. Growth medium for the Cdc42 mutants was supplemented with 2% glucose or 2% sorbitol to repress or induce expression of heterologous CDC42, respectively. CAI4/CIp10 was the control strain for Cdc42 mutants and DAY185 for the other mutants. P values compared with the relevant control strains are shown. Error bars indicate SD, n = 3 (>100 hyphae/strain/independent experiment). (B) Radar plots showing the frequency distribution of polarization angles without (black lines) or with [red broken lines (rga2Δ/bem3Δ, solid red)] 1 mM Ca2+. Relative orientation of the cathode (−) and anode (+) is indicated. % P (growth polarity) values without and with 1 mM Ca2+ are shown. (C) Radar plots showing final reorientation angles of mutant hyphae with defective response to an electric field, without or with 1 mM Ca2+, as for B. (D) Spider diagrams depict reorientation trajectories of wild-type and rdi1Δ hyphae in an electric field. (Scale bars, 10 µm.)
Cdc42 Apical Recycling Is Required for Cathodal Polarization.
Cdc42–GDP is recycled to the polarity site by two pathways; one is mediated by a GDI and the other involves formin-directed actin nucleation. Mutants lacking the C. albicans GDI, Rdi1, or either of the two formins, Bnr1 and Bni1 (5, 20), were analyzed. Cathodal polarization and the reorientation of mature hyphae were abolished in the rdi1Δ strain, indicating that both galvanotropic responses are Rdi1-dependent (Fig. 1 A and B). Cathodal polarization was significantly reduced in the bnr1Δ but not the bni1Δ mutant. Bnr1was therefore required for efficient cathodal polarization. Cathodal reorientation of mature hyphae was again attenuated in the bnr1Δ mutant but enhanced in the bni1Δ strain (Fig. 1 A and B). Together, these results indicate that GDI-mediated extraction of Cdc42 from the membrane and an intact actin network, two of the key elements involved in Cdc42 trafficking, are required for both cathodal polarization and hyphal reorientation in an electric field.
Ca2+ Enhances Electric Field-Induced Directional Growth.
Previous studies showed that raising extracellular [Ca2+] from trace levels (<5 µM) to 1 mM boosts cathodal hypha emergence in wild-type cells (1). We asked whether cathodal polarization could be restored by the addition of Ca2+ in strains rga2Δ/bem3Δ, rdi1Δ, bnr1Δ, and Cdc42G12V. Exogenous Ca2+ restored cathodal polarization in the rdi1Δ and bnr1Δ mutants (Fig. 1B). This was reversed by the further addition of 4 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (Fig. S2A), supporting the notion that Ca2+ influx was compensating for the loss of these proteins. The cathodal reorientation of rdi1Δ and bnr1Δ mature hyphae was also rescued by exogenous Ca2+ (Fig. 1 C and D), but this could not be reversed by 4 mM BAPTA (Fig. S2B), suggesting an alternative mechanism for the rescue of cathodal orientation by the addition of Ca2+. Addition of exogenous Ca2+ did not restore cathodal growth in the strain overexpressing Cdc42G12V, but instead anodal polarization was significantly enhanced. In the rga2Δ/bem3Δ mutant, exogenous Ca2+ did not overcome the loss of Cdc42 GAP activity but generated an anodal and a cathodal population of cells (Fig. 1B). Together, these results suggest that Ca2+ influx amplifies the positional signal mediated by Cdc42 during polarity establishment and, in an electric field, cells polarize increasingly toward the anode as the intracellular proportion of Cdc42–GTP rises.
Cdc42 GTP–GDP Cycling and the Formin Bni1 Are Required for Thigmotropism.
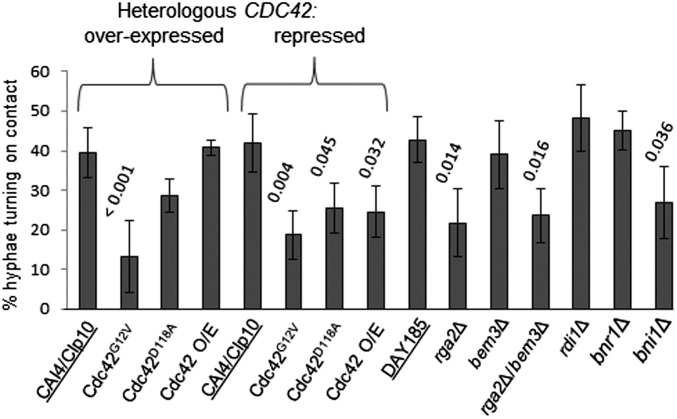
Extending hyphae reorient their growth to become aligned with respect to contours in the substrate. Thigmotropism in the rga2Δ GAP mutant or the strain expressing Cdc42G12V was attenuated but the bem3Δ strain was unaffected (Fig. 2), suggesting that Rga2-mediated Cdc42–GTP hydrolysis is required for this response. Strains harboring heterologous Cdc42G12V, Cdc42D118A, or Cdc42 alleles were all attenuated in contact sensing when the Cdc42 allele was repressed, suggesting that hyphae require wild-type levels of Cdc42 expression to exhibit a full thigmotropic response. In contrast to galvanotropism, the Bni1 formin was required for thigmotropism but Rdi1 and Bnr1 were not (Fig. 2). Together, these results suggest that Cdc42 regulation is required for immediate directional responses to external cues, Bnr1 and Rdi1 are required for cathodal polarization, and Bni1 is required for normal directional responses in mature hyphae.
Fig. 2.
Disruption of Cdc42 dynamics or deletion of the Bni1 formin attenuate hyphal thigmotropism. Hyphal growth of adhered cells was induced on quartz slides with 0.79-µm ridges. The Cdc42 mutants’ growth medium was supplemented with 2% glucose or 2% sorbitol to repress or induce expression of the heterologous copy of CDC42, respectively. The number of hypha–ridge interactions resulting in tip reorientation was expressed as a percentage of total interactions. P values are shown above the relevant bars. Error bars indicate SD, n = 3 (>100 hypha–ridge interactions per strain per experiment).
The Cdc24 Ca2+-Binding Motif Is Required for Polarity Establishment but Not Hyphal Maintenance.
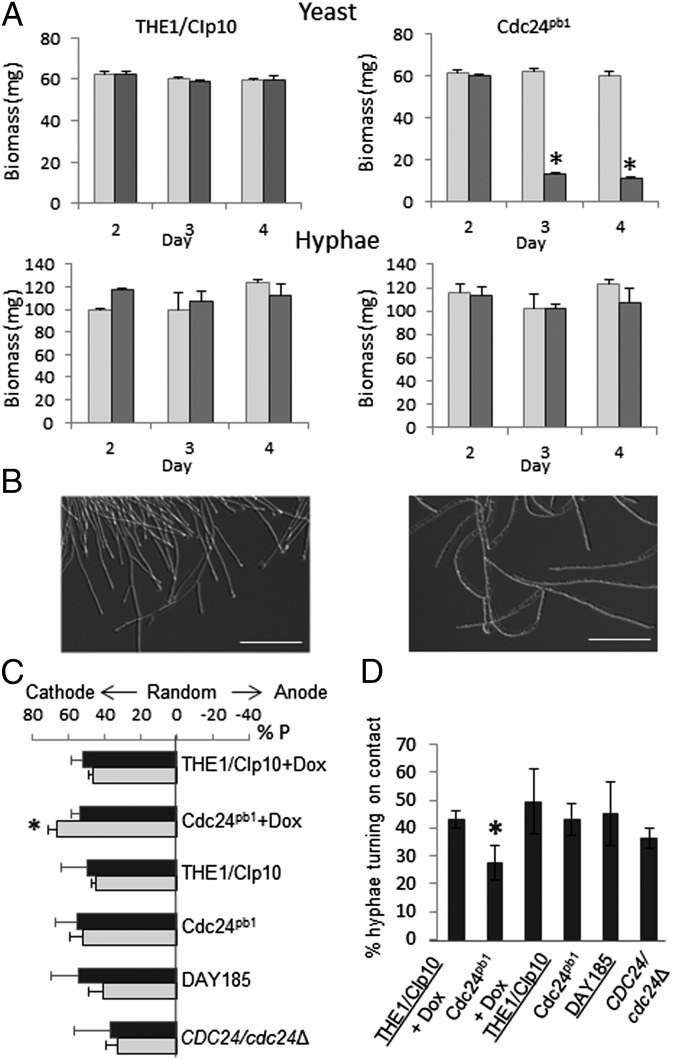
The GEF, Cdc24, is a potential effector of Ca2+-directed growth guidance because it is an essential component of the Cdc42 recycling pathway and its C terminal contains a Ca2+-binding EF-hand motif (Fig. S3A). Strain Cdc24pb1was generated in which this motif was disrupted by site-directed mutagenesis of acidic aspartate residues D802, D806, and D813, which coordinate Ca2+ in the EF-hand domain (Fig. S3B) (21–23). The Tet-repressible promoter was inserted in front of the retained wild-type copy of CDC24, which reduced expression 90-fold within 4 h in the presence of 20 μg/mL doxycycline (Fig. S4). Cdc24pb1 yeast cells lost viability when exposed to 0.25 μg/mL doxycycline or higher (Fig. S5). To distinguish between roles for the EF-hand motif in polarity establishment and maintenance, yeast or hyphal growth of Cdc24pb1 cells was established for 2 h before wild-type CDC24 expression was repressed. Cells were subcultured for 4 d. By day 2, the biomass of yeast grown with 20 µg/mL doxycycline was 80% lower than the yield from cultures that permitted wild-type Cdc24 expression (Fig. 3A). Hyphal biomass and polarized growth were not affected (Fig. 3 A and B). Thus, cells expressing only mutated Cdc24 could not grow as yeast but wild-type Cdc24 could be repressed without effect once hyphal growth had been established, indicating that the Cdc24 EF-hand is required for polarity establishment but not for sustained polarized growth.
Fig. 3.
Cdc24pb1 cannot grow as yeast and has altered tropic responses. (A) Cells were grown under yeast or hyphal growth conditions for 2 h. Cells were inoculated into fresh medium with (dark bars) or without (light bars) 20 µg/mL doxycycline and subcultured for 4 d. Cells were harvested daily and dried for determination of biomass. *P ≤ 0.001 compared with the control strain, THE1/CIp10. Error bars indicate SD, n = 3. (B) Morphologies of control strain (Left) and Cdc24pb1 (Right) hyphae at day 4 of subculture with 20 µg/mL doxycycline. (Scale bars, 20 µm.) (C) Hyphal polarization angles (dark bars) were determined after 2 h of growth without or with 0.125 µg/mL doxycycline in an electric field. Hyphal reorientation (light bars) was determined after hyphae were pregrown for 2 h, followed by 3 h of growth in an electric field, as above. *P = 0.011. Error bars indicate SD, n = 3 (>100 hyphae per strain per experiment). (D) Thigmotropism is attenuated by mutation of the Cdc24 EF-hand but not deletion of one CDC24 allele. *P = 0.018. Error bars indicate SD, n = 3 (>100 hypha–ridge interactions per strain per experiment).
The Cdc24 EF-Hand Is Required for Cell-Wall Deposition and Growth Guidance.
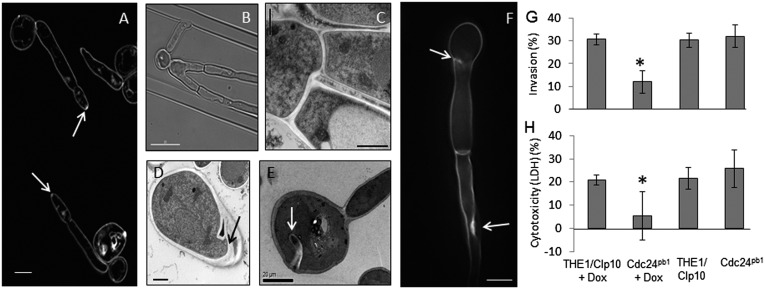
The tropic responses and morphology of strain Cdc24pb1 were examined in the presence of 0.125 μg/mL doxycycline, a level that permitted growth but reduced wild-type Cdc24 expression fivefold (Fig. S4). Because the Cdc24 EF-hand is essential for polarity establishment, it was assumed that, during polarization in an electric field, residual wild-type Cdc24 expression supported growth in Cdc24pb1 and no conclusions as to the requirement for the EF-hand in cathodal polarization could be drawn. However, the Cdc24 EF-hand was not essential in mature Cdc24pb1 hyphae, which reoriented strongly toward the cathode compared with wild-type cells (% P = 66.3 ± 4.8 vs. % P = 46.5 ± 2.2, respectively) (Fig. 3C and Fig. S1). In the CDC24/cdc24Δ heterozygous strain, reorientation toward the cathode was weaker (% P = 32.7 ± 6.7), suggesting that heightened Cdc24pb1 cathodalorientation was facilitated by the Cdc24 EF-hand mutation. The thigmotropic response of Cdc24pb1 hyphae was slightly attenuated (Fig. 3D), and microscopy suggested that this may be due to aberrant positional control of the growth site. Hyphae were wider than wild-type hyphae, typical of reduced focusing of the hyphal expansion site within the tip, but Spitzenkörper formation occurred normally (Fig. 4A). Occasional bifurcation events were observed, demonstrating that more than one polarity site could be formed in Cdc24pb1, unlike in wild-type hyphae (Fig. 4 B and C). Aberrant localized deposits of cell wall material formed invaginations and multiple layers in yeast and hyphal cells (Fig. 4 D–F), but there was no change in the general thickness of the wall or the ratios of its chitin, glucan, or mannan (Fig. S6), indicating that the Cdc24 EF-hand is required for the spatial control of wall synthesis but probably not for its coordination or composition. Attenuated thigmotropism may therefore be due to the inability of Cdc24pb1hyphae to localize the growth site normally.
Fig. 4.
Growth and tissue penetration defects of Cdc24pb1 cells. (A) Hyphae stained with FM4-64 to show the Spitzenkörper (arrows). (Scale bars, 5 µm.) (B and C) Bifurcation of hyphae. (Scale bars, 10 µm and 1 µm.) (D and E) Mislocalized cell wall material in yeast and pseudohyphal cells (arrows). (Scale bars, 1 µm and 20 µm.) (F) Hypha stained with Calcofluor White with deposits of cell-wall material (arrows). (Scale bar, 5 µm.) (G) Yeast cells (1 × 105) were inoculated onto confluent monolayers of H4 intestinal epithelial cells and incubated with or without 0.125 µg/mL doxycycline. Using a fluorescent anti-Candida antibody, hyphal cells were categorized as penetrating (no fluorescence) or nonpenetrating (fluorescent staining). (H) Cdc24pb1 and control strain yeast cells (2 × 105 cells) were coincubated for 8 h with H4 cells with or without 0.125 µg/mL doxycycline and supernatants analyzed for lactate dehydrogenase release. *P ≤ 0.01. Error bars indicate SD.
Tissue Damage and Invasion Are Attenuated in the Cdc24pb1 Mutant.
Previous studies suggested that abnormal thigmotropic or galvanotropic responses positively correlate with the reduced ability of C. albicans to invade underlying host cell epithelia (12). The ability of the Cdc24pb1 strain to invade and damage intestinal epithelial cells was significantly reduced compared with the control strain (Fig. 4 G and H). Thus, the regulatory mechanisms required for hyphal tropic responses are also necessary for tissue invasion and damage.
Discussion
Cdc42 GTPase Activity and the Cathode–Anode Polarization Switch.
Unlike in yeasts, the mechanism of site selection for polarity establishment in C. albicans hyphae is not cell cycle-dependent and is only partially determined by the location of previous budding (24–26). In wild-type cells, an electric field overrides intrinsic signals and causes polarized growth to occur at the cathodal face of the cell. Here we show that this phenotype is dependent on Cdc42–GTP hydrolysis. When this activity was partially disabled through deletion of two Cdc42 GAPs, Rga2 and Bem3, cells polarized but at random sites, whereas expression of a population of GTP-locked Cdc42 caused polarization toward the anode. These observations suggest that an electric field provides vectorial signals at both cathodal and anodal poles of cells, which is amplified by exogenous Ca2+ and only becomes apparent as the proportion of cellular Cdc42-GTP increases. Although polarity establishment of the Cdc42G12V strain was anodal, its mature hyphae reoriented toward the cathode. The factors that respond to the electric field directional signal are therefore regulated differently during these two growth phases.
Ca2+ Influx Focuses Directional Signals by Enhancing Cdc42 Trafficking.
Here we show that components of the fast and slow Cdc42 trafficking pathways are required for focusing the Cdc4 polarity signal at the cathodal face of the cell, and defects in these pathways can be overcome by exogenous Ca2+, rendering the GDI and Bnr1 formin functions redundant during polarity establishment. An electric field is predicted to artificially generate Ca2+ influx at the cathodal membrane by depolarizing the plasma membrane and activating Cch1, a voltage-gated Ca2+ channel (1). Spatial Ca2+ gradients provide a vectorial cue by promoting localized fusion of exocytic vesicles with the plasma membrane (27). We therefore speculate that an electric field promotes cathodal symmetry breaking through localized Ca2+ influx, vesicle fusion, and increased delivery of Cdc42 (Fig. 5). It is likely that, under physiological conditions, localized Ca2+ is an integral component of the Cdc42 trafficking loop. The addition of Ca2+ only partially restored cathodal polarization of the rga2Δ/bem3Δ GAP mutant, suggesting that Cdc42 inactivation is Ca2+-independent and a potentially rate-limiting step during Cdc42 recycling. Extracellular Ca2+ did not rescue cathodal growth in the Cdc42G12V mutant, but instead enhanced anodal growth. This suggests that Ca2+ influx was not the primary determinant of the polarity site, but instead acts as a ‟directional enhancer” by amplifying an existing signal. In summary, Ca2+ influx promotes Cdc42 trafficking and suggests a mechanism whereby Ca2+ influx amplifies and reinforces Cdc42-mediated directional growth signals.
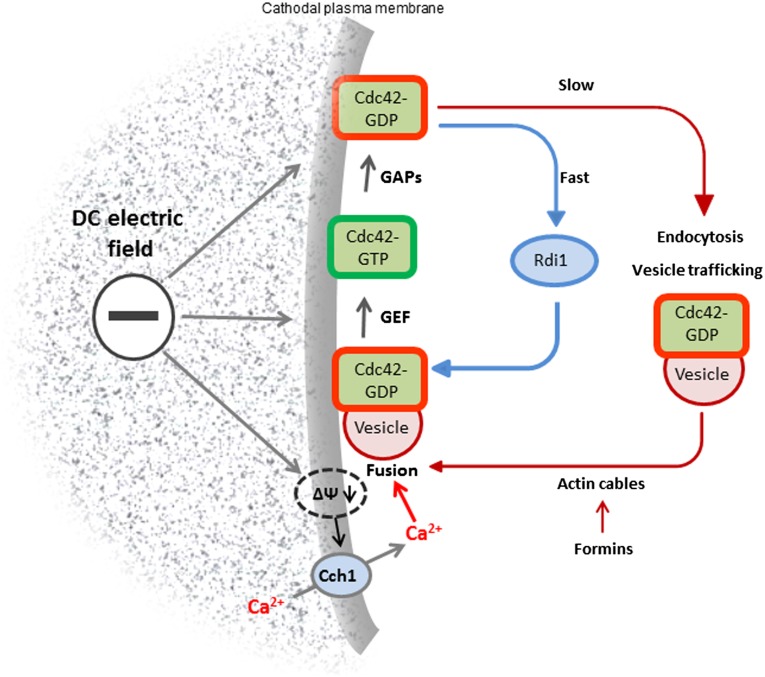
Fig. 5.
Proposed positional influence of electric field-induced Ca2+ influx and enhancement of Cdc42–GDP apical trafficking during cell polarization. Cdc42–GTP diffuses laterally from the polarity site and is deactivated by its GAPs. Cdc42–GDP is recycled cytosolically by Rdi1-mediated extraction from the plasma membrane (blue arrows) or via endocytosis and vesicle delivery on formin-nucleated actin cables (red arrows), where it is reactivated by its GEF, Cdc24. Application of an electric field depolarizes the cathodal plasma membrane and activates Cch1, the voltage-gated [Ca2+] channel (gray arrows), asymmetrically raising intracellular [Ca2+] and promoting vesicle fusion at the cathodal face of the cell, thereby reinforcing weak Cdc42-mediated polarity signals. Deletion of Rdi1 or Bnr1disables the cytosolic and actin-mediated Cdc42 trafficking pathways, respectively, and in low extracellular [Ca2+] cathodal polarization in an electric field is lost. Increased extracellular [Ca2+] in an electric field enhances Cdc42 delivery by promoting localized vesicle fusion, compensating for loss of the Rdi1-mediated pathway or defective actin-cable organization to restore cathodal polarization.
Contrasting Formin Phenotypes.
The tropism data for the Cdc42 trafficking mutants highlighted contrasting phenotypes for the two formins, Bnr1 and Bni1. The growth rate of bni1Δ was 25% slower than that of wild type (Fig. S7) but its hyphae were hyperresponsive to an electric field. Anodal growth of the for3Δ formin mutant in Schizosaccharomyces pombe was proposed to be due to the ability of the negatively charged glucan synthase subunits to electrophorese within the membrane through loss of anchorage to cytoskeletal actin (28). Deletion of Bni1 in C. albicans may allow a similar movement of proteins within the plasma membrane. Loss of Bnr1 attenuated galvanotropism, so this formin seems to be a major influence on directional growth, even though Bnr1–GFP has been localized primarily at the septa (29). Thigmotropism was normal in bnr1Δ but attenuated in bni1Δ. The contrasting phenotypes of the two formin mutants indicate they have distinct functions during hyphal development and tropic responses.
The Cdc24-PB1 Domain in Yeast and Hyphal Growth.
Cdc24 contains a putative Ca2+-binding site within the C-terminal PB1 domain that interacts with Bem1 (7, 30–32). Addition of 2 mM Ca2+ inhibited binding between ScCdc24 and ScBem1 in vitro, suggesting a mechanism whereby Ca2+ might modulate the activity of the Cdc42 complex (Fig. S3A) (8). The C. albicans Cdc24 EF-hand motif was essential for yeast viability but, in hyphal growth conditions, wild-type Cdc24 could be repressed without affecting biomass once hyphal growth had been established. In S. cerevisiae, a Cdc24–Bem1 fusion construct lacking the cognate PB1 domains rescued cell polarization (12). Our findings suggest that the essential function of the PB1 domains lies in mediating Cdc24–Bem1 interactions before their association with Cdc42.
The hyphae of Cdc24pb1 strongly responded to an electric field compared with wild type but showed attenuated thigmotropism. Because the Cdc24 EF-hand lies within the Bem1-interacting domain, it is not known whether these phenotypes resulted from loss of Ca2+ binding or defective Bem1 interactions, but these aberrant tropic responses are consistent with the observed inability to direct other vectorial functions such as cell wall growth in the Cdc24pb1 mutant (Fig. 4). Thus, the Cdc24 EF-hand motif seems to be essential for polarity establishment and influences directional growth by correctly positioning the growth site. Together, these data suggest that Ca2+ fluxes act as transducers of positional guidance cues by focusing the GTPase signaling elements that direct the vectorial axis of secretory-vesicle exocytosis to the fungal hyphal apex.
Attenuated Tropisms As Predictors of Avirulence.
Tropic behavior allows organisms to navigate in their environment in relation to local advantageous or detrimental stimuli. C. albicans mutants with polarity maintenance defects are attenuated in virulence in mice and have reduced capacity to cause endothelial cell damage (12, 20). The relationship between galvanotropism and growth in vivo is less clear, although weak direct current electric fields influence the migration of a variety of mammalian cell types (33). The present study demonstrates that such tropisms are underpinned by regulators of Cdc42 activity, suggesting that pharmacological intervention targeted to this mechanism may present therapeutic opportunities.
Materials and Methods
Strains and Growth Conditions.
Strains, primers, and plasmids are listed in Tables S1–S3. Unless stated otherwise, experiments involving Cdc24pb1 were carried out in the presence of 0.125 μg/mL doxycycline using cells that had undergone >13 doublings. Strains expressing heterologous Cdc42G12V, Cdc42D118A, or Cdc42 mutants were grown on modified Soll’s medium (MSM) supplemented with 2% (wt/vol) glucose or 2% (wt/vol) sorbitol to repress or induce expression of the heterologous copy of Cdc42, respectively. For galvanotropism assays, MSM (resistivity 800–1,000 Ω⋅cm) was used, as described previously (1). For cell invasion assays, strains were cultured in synthetic dextrose complete medium (34) supplemented or not with 0.125 μg/mL doxycycline.
Galvanotropism Assay.
Adhered hyphae were grown in a Biorad midi-sub cell electrophoresis tank, as described previously (1). Polarization angle relative to the cathode was determined after growth for 2 h at 10 V/cm and 33 ± 2 mA using Improvision Openlab 2.0 software. For hyphal reorientation, hyphae grew for 2 h with no electric field, followed by 3 h in an electric field, as above. The percentage cathodal orientation (% P) was calculated using % P = Σ (− sin θ/n) × 100, for n measurements, where n = >100 per sample. Results were reported as the mean % P ± SD for three independent experiments, where % P = 100 denotes cathodal growth, 0 denotes random orientation, and –100 denotes anodal growth. Radar plots were generated in Excel. “Spider” diagrams of hyphal growth trajectories in an electric field were generated in Photoshop CS5 using images captured by light microscopy (1).
Thigmotropism Assay.
Hypha formation was induced in 20% bovine serum (or MSM supplemented with 2%(wt/vol) glucose or sorbitol for Cdc42 mutants, as above) on poly-l-lysine–coated quartz slides featuring ridges of 0.79 μm ± 40 nm and a pitch of 25 μm (Kelvin Nanotechnology), as previously described (1). The number of hyphae reorienting on contact with a ridge was expressed as a percentage of the total observed interactions. A minimum of 100 interactions was observed per sample and results reported as the mean value from a minimum of three independent experiments ± SD.
Generation of Strain Cdc24pb1.
Generation of Cdc24pb1 is described in Supporting Information. Briefly, the Cdc24 ORF was cloned into plasmid pBS-URA3 to generate plasmid ABp71 and underwent site-directed mutagenesis to introduce point mutations D802A, D806A, and D813A into the PB1 C-terminal domain of Cdc24. The plasmid was transformed into one allele of Cdc24 in C. albicans strain THE1 (21). After selection for loss of URA3, the tetracycline-regulatable (Tet-Off) promoter was inserted 5′ to the wild-type Cdc24 allele to generate strain Cdc24pb1.
Microscopy.
Methods for bright field, fluorescence, and transmission electron microscopy are given in Supporting Information.
Cdc24pb1 Yeast and Hyphal Viability.
Cells were grown as yeast or hyphae for 2 h and 100 µL was subcultured into 10 mL fresh medium with or without 20 µg/mL doxycycline for 4 d. The remaining culture was freeze-dried daily and the biomass concentration determined. The maximum doxycycline dose tolerated by strain Cdc24pb1 was derived from the OD600 of cells incubated in the presence of 0, 0.125, 0.25, 5, or 20 µg/mL doxycycline.
Epithelial Cell Invasion Assay.
Confluent H4 intestinal epithelial cells (35) on glass coverslips in 12-well plates were inoculated with yeast cells (1 × 105) and incubated for 3 h at 37 °C with 5% CO2 with or without 0.125 µg/mL doxycycline. Fungal penetration was analyzed by immunocytochemistry using an anti-Candida cell-wall antibody, as described previously (36).
Cytotoxicity Assay.
Lactate dehydrogenase release by H4 epithelial cells was measured after exposure to C. albicans strains cells (2 × 105 cells) with or without 0.125 µg/mL doxycycline, using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) as previously described (36). Results were reported as the mean ± SEM.
Statistical Analyses.
SPSS 20 was used to analyze data generated from a minimum of three independent experiments. ANOVA with a post hoc Dunnett’s t test was used to compare multiple samples with a control strain. For two-sample comparisons, two-tailed independent Student t tests were used. Invasion and cytotoxicity results were analyzed using a blocked ANOVA.
Supplementary Material
Acknowledgments
We thank Doug Johnson, Peter Sudbery, Malcolm Whiteway, Yue Wang, and Martine Bassilana for gifts of strains; Andrew Goryachev for useful discussions; Kathy Lee, Claire Walker, and Kevin Mackenzie for assistance with HPLC analysis, Southern blots, and transmission electron microscopy, respectively; and Heather Podgorski for assistance with epithelial invasion and cytotoxicity assays. This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/E008371/1 (to A.C.B. and N.A.R.G.), National Institutes of Health Grant A1057440 (to C.A.G.), Royal Society Grant UF080611 and Medical Research Council New Investigator Research Grant 90671 (to A.C.B.), and by the Ariadne European Commission Initial Training Networks (N.A.R.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307264111/-/DCSupplemental.
References
- 1.Brand A, et al. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol. 2007;17(4):347–352. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol. 2003;5(12):1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev Cell. 2009;17(6):823–835. doi: 10.1016/j.devcel.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiedje C, Sakwa I, Just U, Höfken T. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol Biol Cell. 2008;19(7):2885–2896. doi: 10.1091/mbc.E07-11-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Court H, Sudbery P. Regulation of Cdc42 GTPase activity in the formation of hyphae in Candida albicans. Mol Biol Cell. 2007;18(1):265–281. doi: 10.1091/mbc.E06-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell AS, et al. Singularity in polarization: rewiring yeast cells to make two buds. Cell. 2009;139(4):731–743. doi: 10.1016/j.cell.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DI, Pringle JR. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111(1):143–152. doi: 10.1083/jcb.111.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Bender A, Cerione RA. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J Biol Chem. 1995;270(2):626–630. doi: 10.1074/jbc.270.2.626. [DOI] [PubMed] [Google Scholar]
- 9.Goryachev AB, Pokhilko AV. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 2008;582(10):1437–1443. doi: 10.1016/j.febslet.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Kozubowski L, et al. Symmetry-breaking polarization driven by a Cdc42p GEF-PAK complex. Curr Biol. 2008;18(22):1719–1726. doi: 10.1016/j.cub.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166(6):889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand A, et al. An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans. Eukaryot Cell. 2008;7(4):712–720. doi: 10.1128/EC.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts HJ, Véry AA, Perera TH, Davies JM, Gow NA. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology. 1998;144(Pt 3):689–695. doi: 10.1099/00221287-144-3-689. [DOI] [PubMed] [Google Scholar]
- 14.Crombie T, Gow NAR, Gooday GW. Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J Gen Microbiol. 1990;136(2):311–317. doi: 10.1099/00221287-136-2-311. [DOI] [PubMed] [Google Scholar]
- 15.Park HO, Kang PJ, Rachfal AW. Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J Biol Chem. 2002;277(30):26721–26724. doi: 10.1074/jbc.C200245200. [DOI] [PubMed] [Google Scholar]
- 16.Yaar L, Mevarech M, Koltin Y. A Candida albicans RAS-related gene (CaRSR1) is involved in budding, cell morphogenesis and hypha development. Microbiology. 1997;143(Pt 9):3033–3044. doi: 10.1099/00221287-143-9-3033. [DOI] [PubMed] [Google Scholar]
- 17.Toenjes KA, Simpson D, Johnson DI. Separate membrane targeting and anchoring domains function in the localization of the S. cerevisiae Cdc24p guanine nucleotide exchange factor. Curr Genet. 2004;45(5):257–264. doi: 10.1007/s00294-004-0485-9. [DOI] [PubMed] [Google Scholar]
- 18.Ushinsky SC, et al. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot Cell. 2002;1(1):95–104. doi: 10.1128/EC.1.1.95-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulver R, et al. Rsr1 focuses Cdc42 activity at hyphal tips and promotes maintenance of hyphal development in Candida albicans. Eukaryot Cell. 2013;12(4):482–495. doi: 10.1128/EC.00294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li CR, et al. The formin family protein CaBni1p has a role in cell polarity control during both yeast and hyphal growth in Candida albicans. J Cell Sci. 2005;118(Pt 12):2637–2648. doi: 10.1242/jcs.02393. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama H, et al. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect Immun. 2000;68(12):6712–6719. doi: 10.1128/iai.68.12.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terasawa H, et al. Structure and ligand recognition of the PB1 domain: A novel protein module binding to the PC motif. EMBO J. 2001;20(15):3947–3956. doi: 10.1093/emboj/20.15.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Drogen-Petit A, Zwahlen C, Peter M, Bonvin AMJJ. Insight into molecular interactions between two PB1 domains. J Mol Biol. 2004;336(5):1195–1210. doi: 10.1016/j.jmb.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 24.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 25.Chaffin WL. Site selection for bud and germ tube emergence in Candida albicans. J Gen Microbiol. 1984;13(1):431–440. [Google Scholar]
- 26.Hazan I, Liu H. Hyphal tip-associated localization of Cdc42 is F-actin dependent in Candida albicans. Eukaryot Cell. 2002;1(6):856–864. doi: 10.1128/EC.1.6.856-864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse A, Lee AK, Tse FW. Ca2+ signaling and exocytosis in pituitary corticotropes. Cell Calcium. 2012;51(3-4):253–259. doi: 10.1016/j.ceca.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Minc N, Chang F. Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol. 2010;20(8):710–716. doi: 10.1016/j.cub.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dünkler A, Wendland J. Candida albicans Rho-type GTPase-encoding genes required for polarized cell growth and cell separation. Eukaryot Cell. 2007;6(5):844–854. doi: 10.1128/EC.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretsinger RH. Crystallographic studies of calmodulin and homologs. Ann N Y Acad Sci. 1980;356(1):14–19. doi: 10.1111/j.1749-6632.1980.tb29594.x. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto S, Ohya Y, Ohsumi Y, Anraku Y. Nucleotide sequence of the CLS4 (CDC24) gene of Saccharomyces cerevisiae. Gene. 1987;54(1):125–132. doi: 10.1016/0378-1119(87)90354-4. [DOI] [PubMed] [Google Scholar]
- 32.Bassilana M, Blyth J, Arkowitz RA. Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans. Eukaryot Cell. 2003;2(1):9–18. doi: 10.1128/EC.2.1.9-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122(Pt 23):4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- 34.Sherman F. In: Methods in Enzymology: Guide to Yeast Genetics and Molecular Biology. Guthrie C, Fink GR, editors. San Diego: Academic; 1991. pp. 3–20. [PubMed] [Google Scholar]
- 35.Sanderson IR, et al. Human fetal enterocytes in vitro: Modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93(15):7717–7722. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falgier C, et al. Candida species differ in their interactions with immature human gastrointestinal epithelial cells. Pediatr Res. 2011;69(5 Pt 1):384–389. doi: 10.1203/PDR.0b013e31821269d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.