Abstract
B-cell activating factor (BAFF) is involved in not only the physiology of normal B cells, but also the pathophysiology of aggressive B cells related to malignant and autoimmune diseases. However, how excessive BAFF promotes aggressive B-cell proliferation and survival is not well understood. Here we show that excessive human soluble BAFF (hsBAFF) enhanced cell proliferation and survival in normal and B-lymphoid (Raji) cells, which was associated with suppression of PP2A, resulting in activation of Erk1/2. This is supported by the findings that pretreatment with U0126 or PD98059, expression of dominant negative MKK1, or overexpression of PP2A prevented hsBAFF-induced activation of Erk1/2 and cell proliferation/viability in the cells. It appears that hsBAFF-mediated PP2A-Erk1/2 pathway and B-cell proliferation/viability was Ca2+-dependent, as pretreatment with BAPTA/AM, EGTA or 2-APB significantly attenuated these events. Furthermore, we found that inhibiting CaMKII with KN93 or silencing CaMKII also attenuated hsBAFF-mediated PP2A-Erk1/2 signaling and B-cell proliferation/viability. The results indicate that BAFF activates Erk1/2, in part through Ca2+-CaMKII-dependent inhibition of PP2A, increasing cell proliferation/viability in normal and neoplastic B-lymphoid cells. Our data suggest that inhibitors of CaMKII and Erk1/2, activator of PP2A or manipulation of intracellular Ca2+ may be exploited for prevention of excessive BAFF-induced aggressive B-cell malignancies and autoimmune diseases.
Keywords: BAFF, Erk1/2, PP2A, Calcium ion, CaMKII, B cells
1. Introduction
The TNF superfamily plays a crucial role in the regulation of immune response by inducing apoptosis and/or proliferation in lymphocytes [1]. B-cell activating factor of the TNF family (BAFF), also termed BLyS, TALL-1, THANK, and zTNF4, a type II membrane protein that exists in both membrane-bound and soluble forms, is a ligand for three TNF-receptor-family members: BAFF-R (BR3), BCMA, and TACI [2–7]. BAFF has exhibited a strong co-stimulatory function in development, maturation and homeostasis of normal B lymphocytes, as well as in cell proliferation and survival of neoplastic B-lymphoid cells [8–11]. Systemic administration of soluble BAFF results in B-cell expansion and elevated levels of immunoglobulins [7]. More importantly, high levels of BAFF in the serum of mice that express both endogenous and transgenic BAFF especially extend B lymphocyte survival beyond physiological limits, and drive continued production of plasma cells producing pathogenic autoantibodies, which contribute to systemic lupus erythematosus (SLE) pathogenesis [12–14]. The B cells with prolonged lifespan are considered culprits in developing lupus-like autoimmune diseases [9, 14–16]. In humans, increased serum BAFF levels are found in a number of different autoimmune diseases, such as SLE, rheumatoid arthritis (RA), and Sjögren’s syndrome (SS) [1, 14, 17]. These results indicate that increased expression of BAFF is a possible etiological factor of aggressive or neoplastic B-cell disorders and autoimmune diseases. However, how excessive BAFF promotes aggressive B-cell proliferation and survival is not well understood.
Extensive studies have shown that BAFF regulates expression of several Bcl-2 family members, including Bcl-xL, Mcl-1, A1/Bfl-1, Bcl-2, and Bim, via survival-promoting kinase systems such as Pim 1/2 or extracellular signal-related kinases 1/2 (Erk1/2) [11]. Protein phosphatase 2A (PP2A), a ubiquitous and highly conserved serine/threonine (Ser/Thr) protein phosphatase, plays an essential role in multiple cellular processes, including cell proliferation/growth and death, cell mobility, cytoskeleton dynamics, as well as numerous signaling pathways [18–20]. PP2A negatively regulates Erk1/2 pathway through dephosphorylating and inactivating both mitogen-activated protein kinase kinases 1/2 (MEK1/2) and Erk1/2 proteins [18]. Dysregulation of PP2A activity has been implicated in several diseases. For example, in leukemic cells, inhibition of PP2A activity increases proliferation and impairs cellular differentiation [21]. Increased PP2A activity exists in T cells from patients with SLE [22]. PP2A overexpression promotes DNA hypomethylation through suppressing MEK/Erk/DNA methyltransferase 1(DNMT1) pathway in normal and SLE T-cells [20]. However, decreased PP2A activity in the brain of Alzheimer’s patients promotes hyperphosphorylation of tau protein leading to the development of neurofibrillary tangles [23]. Although an important role for PP2A has been established in a number of disease processes, including SLE [20, 24, 25], its effects on BAFF-induced proliferation and survival of aggressive B cells are unclear.
Calcium ion (Ca2+) is a ubiquitous intracellular signal responsible for numerous cellular events, such as proliferation/growth, differentiation, and survival in various immune cells [26, 27]. CaMKII is an ubiquitously expressed multifunctional Ser/Thr kinase that functions through Ca2+ signaling to regulate the development and activity of many different cell types including immune cells [28–30]. CaMKII is activated upon binding of Ca2+/calmodulin (CaM), which undergoes autophosphorylation [31]. Studies have demonstrated that the activation of MEK-Erk1/2 in B lymphocytes is dependent on calcium influx [29, 32]. Activated CaMKII is involved in regulating the activation of Erk1/2 leading to myeloid leukemia cell proliferation [28]. Recently, we have shown that human soluble BAFF (hsBAFF) elevates higher but homeostatic intracellular free Ca2+ ([Ca2+]i), which activates Erk1/2 pathway contributing to the proliferation and survival of cultured mouse splenic B lymphocytes [33]. Excessive hsBAFF-elevated [Ca2+]i phosphorylates CaMKII, which is associated with activation of mTOR signaling and the proliferation and survival in B lymphocytes [34]. These data suggest that Ca2+-dependent CaMKII activity transduces signals to MAPKs and mTOR involved in cell proliferation and survival. Because the disturbances in the expression and/or function of PP2A are linked to autoimmune diseases, this prompted us to study whether excess BAFF inhibits PP2A activity, thereby activating Erk1/2 signaling and promoting B-cell proliferation and survival, through Ca2+ signaling. Here we report that excessive hsBAFF promotes the proliferation and survival in normal and Raji B-lymphoid cells through suppression of PP2A leading to Erk1/2 activation. Furthermore, we demonstrate that hsBAFF activates Erk1/2, at least, partially by Ca2+-CaMKII-dependent inhibition of PP2A. The findings suggest that inhibitors of CaMKII and Erk1/2, activator of PP2A or manipulation of intracellular Ca2+ may be exploited for the prevention of excessive BAFF-induced aggressive B-cell malignancies and autoimmune diseases.
2. Materials and methods
2.1. Reagents
Anti-CD19 magnetic fluorobeads-B was purchased from One Lambda (Canoga Park, CA, USA). RPMI 1640 Medium was from Gibco (Rockville, MD, USA). Fetal bovine serum (FBS) was supplied by Hyclone (Logan, UT, USA). Refolded human soluble BAFF (hsBAFF) was a recombinant form of the extracellular domain of the BAFF synthesized in Escherichia coli from this group [35]. Enhanced chemiluminescence solution was from Millipore (Billerica, MA, USA). CellTiter 96! AQueous One Solution Cell Proliferation Assay kit was from Promega (Madison, WI, USA). Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit was obtained from BD biosciences (San Diego, CA, USA). 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester (BAPTA/AM) and 2-aminoethoxydiphenyl borane (2-APB) were purchased from Calbiochem (San Diego, CA, USA), whereas ethylene glycol tetra-acetic acid (EGTA) was purchased from Sigma (St. Louis, MO, USA). KN93 were from ALEXIS (San Diego, CA, USA), whereas U0126 and PD98059 were from Sigma. The following antibodies were used: PP2ACα(BD Biosciences, San Jose, CA, USA), PP2A-A subunit, PP2A-B subunit (Millipore, Billerica, MA, USA), CaMKII, phospho-CaMKII (Thr286), phospho-Erk1/2 (Thr202/Tyr204) (Cell Signaling Technology, Beverly, MA, USA), β-actin, Erk2, demethylated-PP2A (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho -PP2A (Epitomics, Burlingame, CA, USA), MEK1(Sigma), goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, and rabbit anti-goat IgG-HRP (Pierce, Rockford, IL, USA). Other chemicals were purchased from local commercial sources and were of analytical grade.
2.2. Cells
Raji cells line (American Type Culture Collection, Manassas, VA, USA) was maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, 100 U/mL streptomycin at 37°C in a humidified incubator containing 5% CO2. Normal mouse B lymphocytes were purified from fresh splenic cells of healthy mice using anti-CD19 magnetic fluorobeads and cultured as described previously [34].
2.3. Recombinant adenoviral constructs and infection of cells
The recombinant adenoviruses encoding N-terminal FLAG-tagged wild-type rat PP2ACα (Ad-PP2A), FLAG-tagged constitutively active MKK1 (Ad-MKK1-R4F), FLAG-tagged dominant negative MKK1 (Ad-MKK1-K97M), and the control virus encoding the green fluorescent protein (GFP) (Ad-GFP) were described previously [36, 37]. For experiments, cells were grown in the growth medium and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI=5). Subsequently, cells were used for experiments. Ad-GFP served as a control. Expression of FLAG-tagged PP2A or MKK1 was determined by western blotting with antibodies to FLAG.
2.4. Lentiviral shRNA cloning, production, and infection
Lentiviral shRNAs to CaMKII and GFP (for control) were generated and used as described [38].
2.5. Cell proliferation and viability assay
Purified mouse B lymphocytes, Raji cells, Raji cells infected with lentiviral shRNA to CaMKII or GFP, or Raji cells infected with Ad-MKK1-R4F, Ad-MKK1-K97M, Ad-PP2A and Ad-GFP, respectively, were seeded in 24-well plates (3×105 cells/well, for cell proliferation assay) or 96-well plates (3×104 cells/well, for cell viability assay) under standard culture conditions and kept overnight at 37°C humidified incubator with 5% CO2. Next day, cells were treated with 0–5 μg/mL hsBAFF for 48 h, with 0, 1 and 2.5 μg/mL hsBAFF for 48 h, or with/without 1 and 2.5 μg/mL hsBAFF for 48 h following pre-incubation with/without U0126 (5 μM), PD98059 (10 μM), BAPTA/AM (20 μM), EGTA (100 μM), 2-APB (100 μM), or KN93 (10 μM) for 1 h with 3–6 replicates of each treatment. Subsequently, cell proliferation was assessed by counting the trypsinized cells with a Beckman Coulter Counter (Beckman Coulter, Fullerton, CA, USA). The viability of the cells, after incubation with MTS reagent (one solution reagent) (20 μL/well) for 4 h, was determined by measuring the optical density (OD) at 490 nm using a SynergyTM 2 Multi-function Microplate Reader (Bio-Tek Instruments, Inc. Winooski, Vermont, USA).
2.6. Live cell assay by trypan blue exclusive and flow cytometry
Raji cells and purified mouse B lymphocytes were seeded in 24-well plates (3×105 cells/well, for trypan blue exclusive) or 6-well plates (2×106 cells/well, for flow cytometry), respectively. Next day, cells were treated with 0–5 μg/mL hsBAFF for 48 h, Then, live cells were monitored by counting viable cells using trypan blue exclusive, and the ratios of death cells, live cells, necrotic and apoptotic cells were calculated by a fluorescence-activated cell sorter (FACS) Vantage SE flow cytometer (Beton Dickinson, California, USA) using annexin-V-FITC and propidium iodide staining.
2.7. Western blot analysis
Purified mouse B lymphocytes, Raji cells, Raji cells infected with lentiviral shRNA to CaMKII or GFP, or Raji cells infected with Ad-MKK1-R4F, Ad-MKK1-K97M, Ad-PP2A and Ad-GFP, respectively, were seeded in 6-well plates at a density of 2 × 106 cells/well under standard culture conditions and kept overnight at 37°C humidified incubator with 5% CO2. Next day, cells were treated with 0–5 μg/mL hsBAFF for 12 h, with/without 2.5 μg/mL hsBAFF for 0–24 h, with 0, 1 and 2.5 μg/mL hsBAFF for 12 h, or with/without 1 and 2.5 μg/mL hsBAFF for 12 h following pre-incubation with/without U0126 (5 μM), PD98059 (10 μM), BAPTA/AM (20 μM), EGTA (100 μM), 2-APB (100 μM), or KN93 (10 μM) for 1 h. After that, The Western blotting was performed as described [39].
2.8. Statistical analysis
Results were expressed as mean values ± standard error (mean ± SE). The Student’s t-test for non-paired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using either one-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicate means. Significance was accepted at P < 0.05.
3. Results
3.1. hsBAFF-enhanced cell proliferation and viability are Erk1/2-dependent in B cells
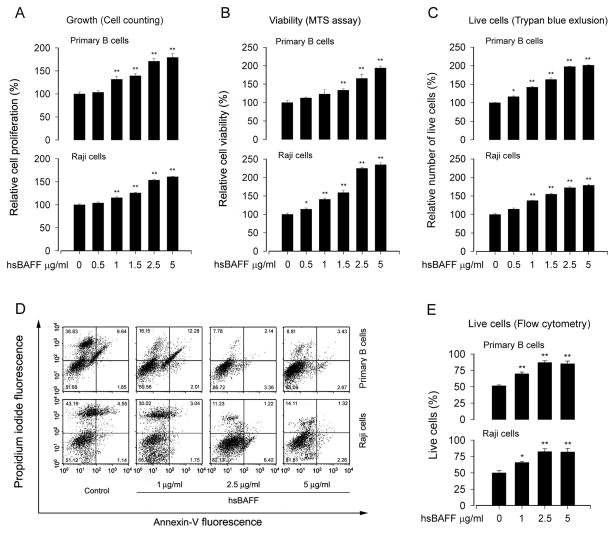
Raji cells and purified mouse splenic B lymphocytes were chosen as a model to study the mechanisms by which excess hsBAFF promotes cell proliferation and survival in B cells. For this, cell proliferation and viability were evaluated by cell counting and MTS assay, respectively. As shown in Fig. 1A and B, treatment with 0.5–5 μg/mL of hsBAFF for 48 h increased cell proliferation and viability, respectively, in a concentration-dependent manner in Raji cells and primary B lymphocytes.
Fig. 1.
hsBAFF promotes normal and Raji B-cell proliferation and viability. Raji cells and purified mouse splenic B lymphocytes were treated with 0–5 μg/mL hsBAFF for 48 h. (A) Cell proliferation was evaluated by cell counting. (B) Cell viability was monitored by measuring OD at 490 nm using MTS reagents. (C) Live cells were detected by counting viable cells using trypan blue exclusion. (D) The ratios of death cells, live cells, necrotic and apoptotic cells were calculated by FACS using annexin-V-FITC and propidium iodide staining. Results from one representative experiment are shown. (E) Quantitative analysis of live cells by FACS assay. Results are presented as mean ± SE (n = 3–6). *P <0.05, ** P <0.01, difference vs control group.
To corroborate the event that hsBAFF does increase cell survival, which is related to the prolonged lifespan of the B cells, we counted viable cells using trypan blue exclusive (Fig. 1C) and calculated the ratios of death cells, live cells, necrotic and apoptotic cells by fluorescence-activated cell sorting (FACS) using annexin-V-FITC and propidium iodide staining. (Fig. 1D and E). As expected, treatment with hsBAFF for 48 h increased the relative number of live cells significantly in Raji cells and primary B lymphocytes in a concentration-dependent manner (Fig. 1C–E). Collectively, the results suggest that hsBAFF may increase the cell number probably by enhancing both cell proliferation and cell survival. As 2.5 μg/mL of hsBAFF was able to increase the cell proliferation and viability almost to a maximal level, this concentration was selected for more studies, as described below.
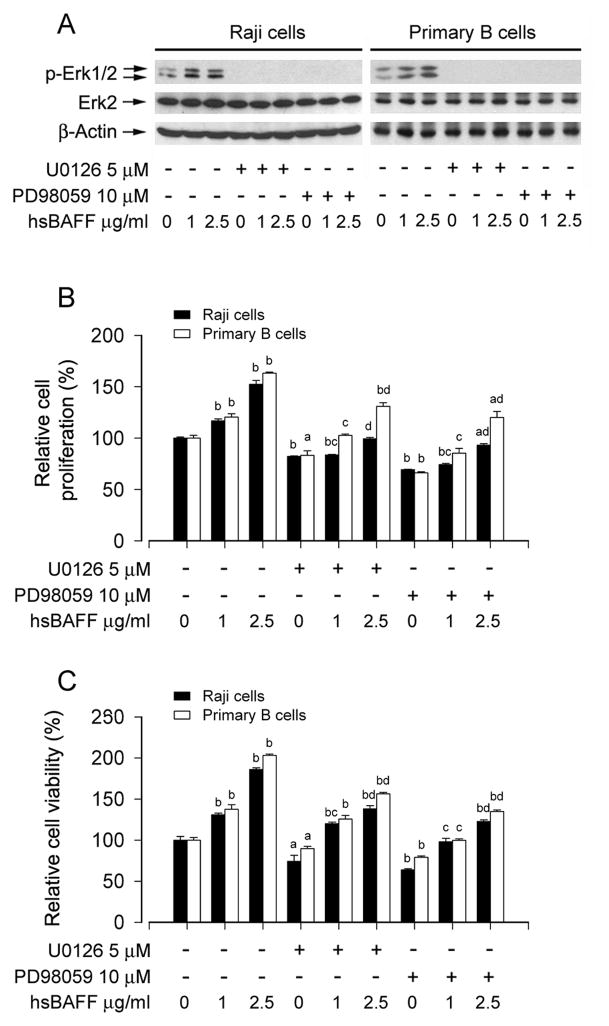
Erk1/2 plays an important role for proliferation and survival of different types of cells [40]. To assess whether hsBAFF-enhanced cell proliferation and survival is dependent on Erk1/2 activation, Raji cells and purified mouse splenic B lymphocytes were preincubated with/without U0126 (5 μM) or PD98059 (10 μM), a selective MKK1/2 (upstream of Erk1/2) inhibitor, for 1 h, followed by treatment with hsBAFF (1 and 2.5 μg/mL) for 12 h or 48 h. Western blot analysis showed that treatment with hsBAFF for 12 h induced remarkable phosphorylation of Erk1/2, which was completely blocked by U0126 or PD98059 in all cells (Fig. 2A). Cell counting and MTS assay revealed that U0126 or PD98059 significantly inhibited the basal or hsBAFF-stimulated cell proliferation (Fig. 2B) and viability (Fig. 2C) in these cells.
Fig. 2.
Inhibition of Erk1/2 blocks hsBAFF-enhanced cell proliferation and viability in B cells. Raji cells and purified mouse splenic B lymphocytes were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay) following pretreatment with/without U0126 (5 μM) or PD98059 (10 μM) for 1 h. Total cell lysates were subjected to Western blotting using indicated antibodies (A). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (B) and the cell viability was determined by the MTS assay (C). (A) hsBAFF-induced phosphorylation of Erk1/2 was severely blocked by U0126 or PD98059. (B and C) U0126 or PD98059 markedly inhibited hsBAFF-stimulated cell proliferation and cell viability, respectively. Results are presented as mean ± SE (n = 6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cp<0.01, difference vs 1 μg/mL hsBAFF group; dp<0.01, difference vs 2.5 μg/mL hsBAFF group.
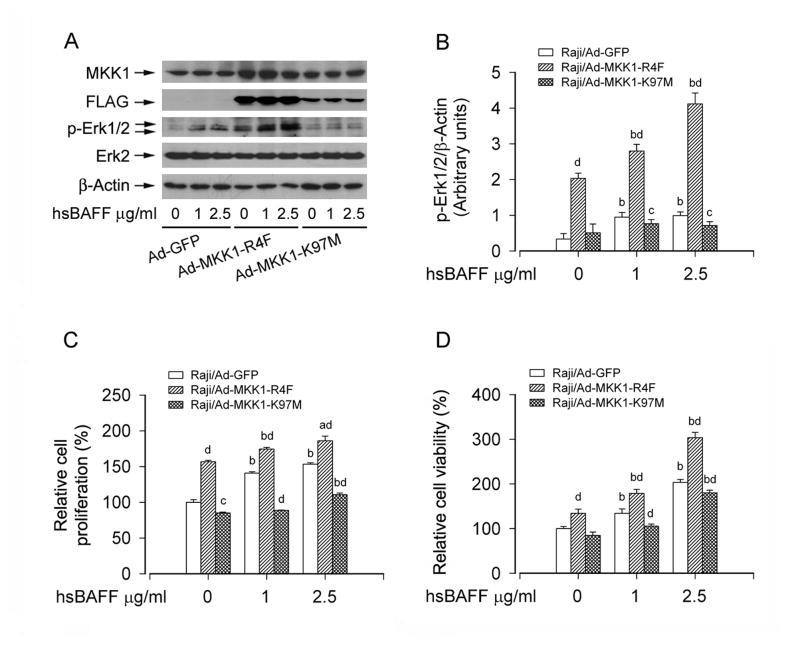
To further underscore a role of Erk1/2 in hsBAFF-stimulated B-cell proliferation and survival, recombinant adenoviruses Ad-MKK1-R4F and Ad-MKK1-K97M, encoding FLAG-tagged constitutively active and dominant negative MKK1, respectively, were employed. Infection of Raji cells with Ad-MKK1-R4F and Ad-MKK1-K97M, but not Ad-GFP (control virus), resulted in expression of high levels of FLAG-tagged MKK1 mutants (Fig. 3A and B). Expression of MKK1-R4F led to robust phosphorylation of Erk1/2 even without stimulation with hsBAFF, whereas expression of MKK1-K97M blocked hsBAFF-stimulated phosphorylation of Erk1/2 (Fig. 3A and B), indicating that the MKK1 mutants function in the cells as expected. Consistently, expression of MKK1-R4F significantly elevated the basal or hsBAFF-stimulated cell proliferation/viability (Fig. 3C and D). In contrast, expression of MKK1-K97M in the cells significantly inhibited the basal or hsBAFF-stimulated cell proliferation/viability (Fig. 3C and D). The results clearly indicate that hsBAFF-stimulated cell proliferation and viability are at least partially dependent on Erk1/2 activation in B cells.
Fig. 3.
Expression of constitutively active or dominant negative MKK1 influences Erk1/2 activity and proliferation/viability in B cells. Raji cells, infected with Ad-MKK1-R4F, Ad-MKK1-K97M, and Ad-GFP (as control), respectively, were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay). Total cell lysates were subjected to Western blotting using indicated antibodies (A). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (C) and the cell viability was determined by the MTS assay (D). (A) Infection of Raji cells with Ad-MKK1-R4F and Ad-MKK1-K97M, but not Ad-GFP, resulted in expression of high levels of FLAG-tagged MKK1 mutants. Expression of MKK1-R4F resulted in robust phosphorylation of Erk1/2 even without stimulation with hsBAFF, whereas expression of MKK1-K97M suppressed hsBAFF-stimulated phosphorylation of Erk1/2. (B) Blots for p-Erk1/2 were semi-quantified using NIH image J. (C and D) Expression of MKK1-R4F significantly elevated the basal or hsBAFF-stimulated cell proliferation and viability, but expression of MKK1-K97M markedly inhibited these events. Results are presented as mean ± SE (n = 3–6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cP <0.05, dP <0.01, Ad-MKK1-R4F group or Ad-MKK1-K97M group vs Ad-GFP group.
3.2. hsBAFF-enhanced B-cell proliferation and viability are associated with its suppression of PP2A, resulting in activation of Erk1/2
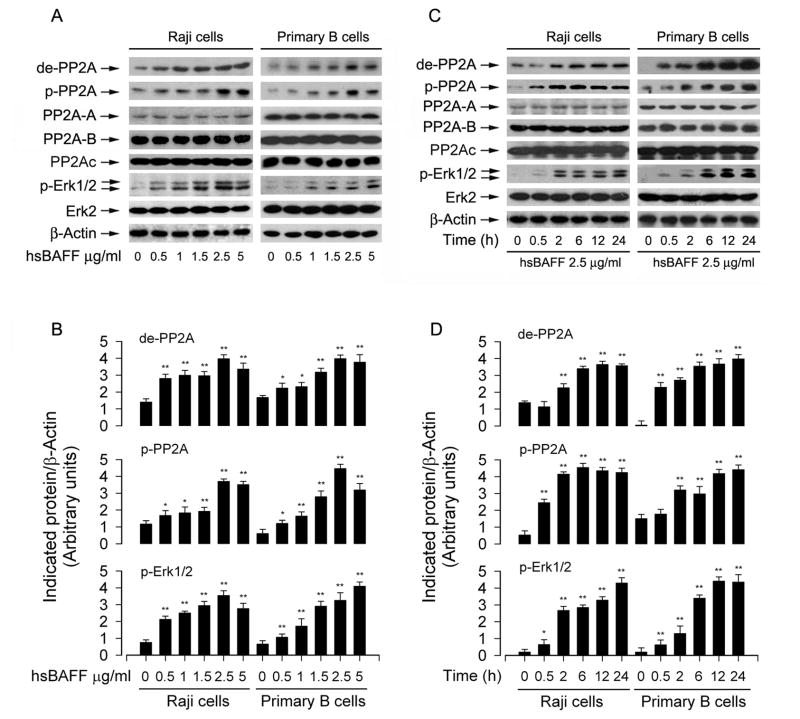
PP2A plays an essential role in cell proliferation/growth, cytoskeletal dynamics, and various signaling pathways [18–20]. It has been reported that PP2A negatively regulates Erk1/2 pathway through dephosphorylation of the Erk1/2 protein [18]. To test whether hsBAFF stimulates B-cell proliferation and viability by regulating PP2A-Erk1/2 pathway, Raji cells and purified mouse splenic B lymphocytes were stimulated with 0–5 μg/mL of hsBAFF for 12 h or with 2.5 μg/mL of hsBAFF for different time (0–24 h). We found that treatment with 0.5–5 μg/mL of hsBAFF for 12 h (Fig. 4A and B) or with 2.5 μg/mL of hsBAFF for 0.5–24 h (Fig. 4C and D) obviously increased expression of demethylated-PP2Ac, phospho-PP2Ac, and phospho-Erk1/2 in a concentration and time-dependent manner. PP2A is a heterotrimeric holoenzyme, which consists of a catalytic subunit (PP2Ac), an A subunit (also termed PR65), and members of the B subunit families, such as B (PR55), B! (PR61), B!! (PR72), and B!!! (PR93/PR110) [41]. As the localization and substrate specificity of PP2Ac is modulated by its association with PP2A-A and -B regulatory subunits [42], we also detected whether hsBAFF affects expression of PP2A-A or PP2A-B. It turned out that hsBAFF did not alter cellular protein levels of PP2A-A or PP2A-B (Fig. 4A and C). The results imply that hsBAFF-induced activation of Erk1/2 might be attributed to suppression of PP2A, by inducing demethylation and phosphorylation of PP2Ac.
Fig 4.
hsBAFF induces inhibition of PP2A and activation of Erk1/2 in B cells. Raji cells and purified mouse splenic B lymphocytes were treated with 0–5 μg/mL hsBAFF for 12 h (A) or with 2.5 μg/mL hsBAFF for different time (0–24 h) (C). Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. (A and C) hsBAFF did not alter cellular protein level of PP2Ac and Erk2, but obviously increased expression of demethylated-PP2A (de-PP2A), phospho-PP2A (p-PP2A), and phospho-Erk1/2 (p-Erk1/2) in a concentration- and time-dependent manner, and (B and D) blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. Results are presented as mean ± SE (n = 3). *p<0.05, **p<0.01, difference vs control group.
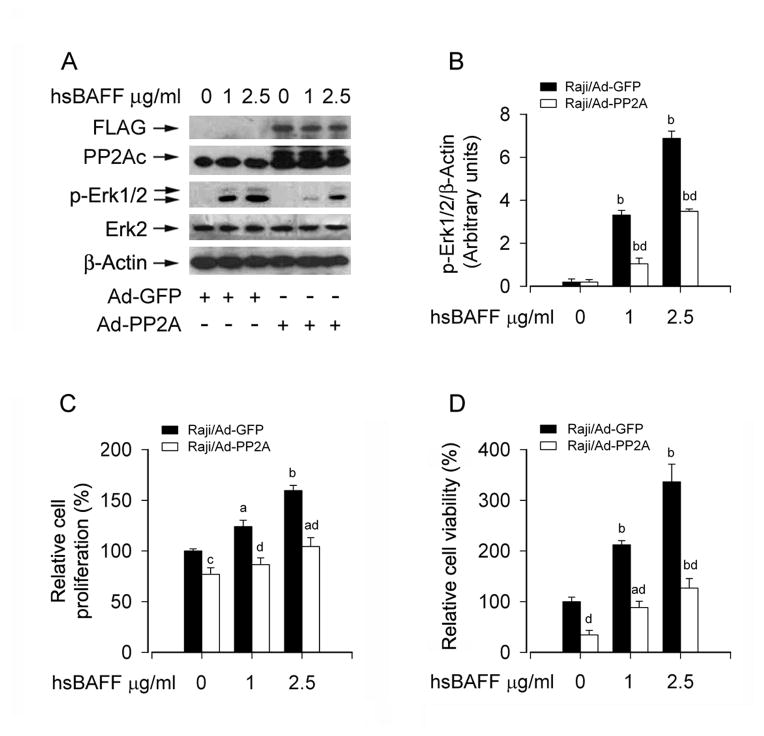
To validate the role and significance of PP2A in hsBAFF-induced activation of Erk1/2 and B-cell proliferation/viability, we next studied whether overexpression of PP2Ac has any impact on hsBAFF activation of Erk1/2 signaling, as well as cell proliferation/viability. To this end, Raji cells, infected with Ad-PP2A and Ad-GFP (as control), were treated with hsBAFF (1 and 2.5 μg/mL) for 12 h or 48 h, then Western blotting, cell counting and MTS assay were carried out. As expected, infection with Ad-PP2A increased the expression of PP2Ac compared with infection with Ad-GFP (Fig. 5A). Of interest, overexpression of PP2Ac markedly prevented hsBAFF-induced phosphorylation of Erk1/2 (Fig. 5A and B). Furthermore, overexpression of PP2Ac also significantly suppressed the basal or hsBAFF-stimulated cell proliferation/viability (Fig. 5C and D). Taken together, these data strongly supported the notion that hsBAFF enhances cell proliferation and viability in B cells, by targeting PP2A-Erk1/2 signaling pathway.
Fig. 5.
Overexpression of PP2A partially prevents hsBAFF-induced activation of Erk1/2 and cell proliferation/viability in B cells. Raji cells, infected with Ad-PP2A and Ad-GFP (as control), were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay). Total cell lysates were subjected to Western blotting using indicated antibodies (A). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (C) and the cell viability was determined by the MTS assay (D). (A) Overexpression of PP2Ac partially suppressed hsBAFF-induced phosphorylation of Erk1/2, and (B) blots for p-Erik1/2 were semi-quantified using NIH image J. (C and D) Overexpression of PP2Ac in part inhibited the basal or hsBAFF-stimulated cell proliferation and viability. Results are presented as mean ± SE (n = 3–6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cP<0.05, dP<0.01, Ad-PP2A group vs Ad-GFP group.
3.3. hsBAFF mediates PP2A-Erk1/2 signaling and cell proliferation/viability in B cells in Ca2+-dependent manner
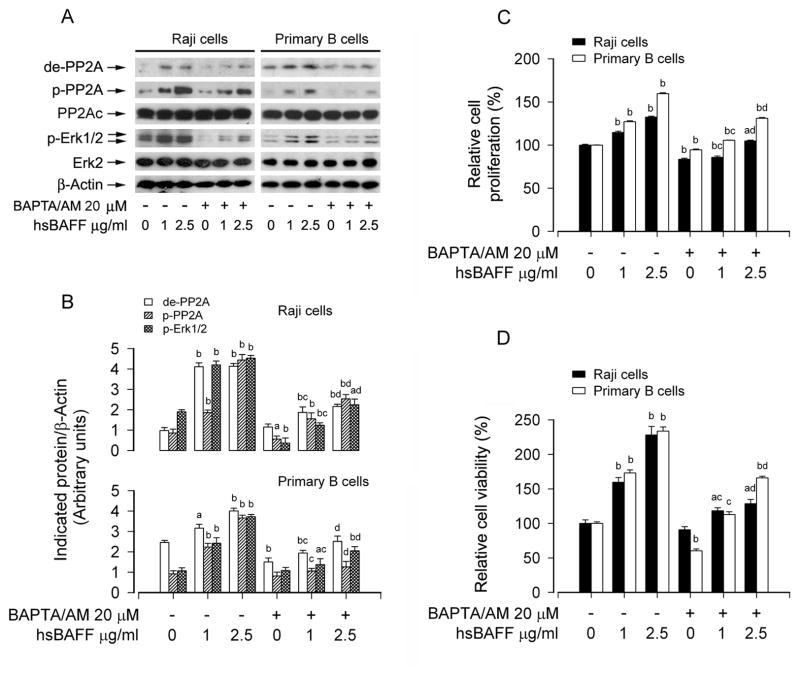
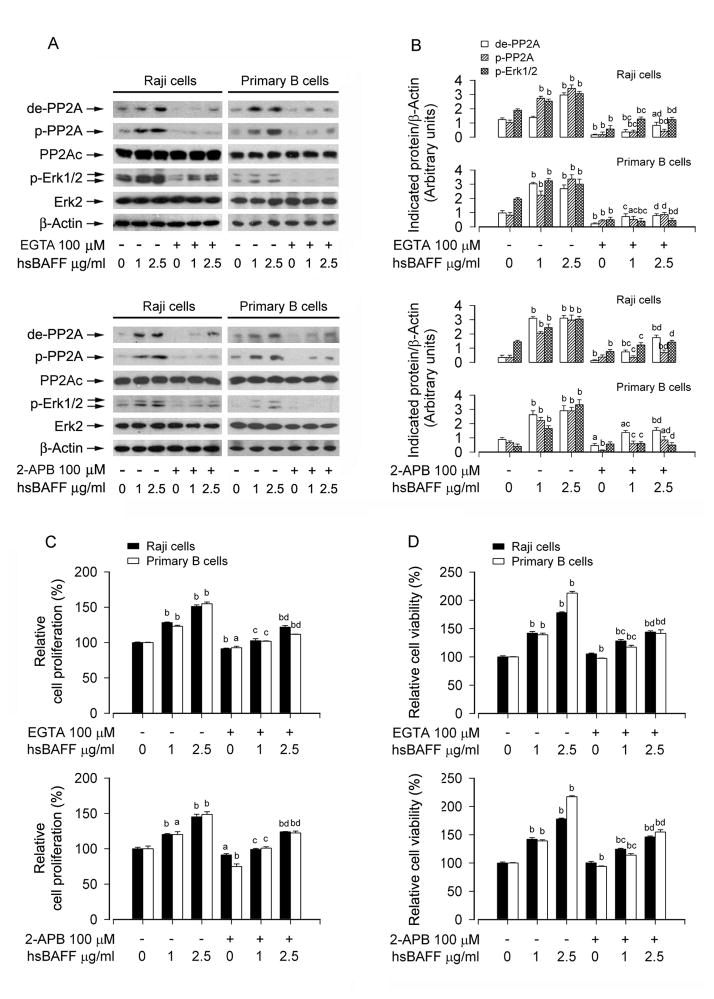
Studies have shown that Erk1/2 activation in B lymphocytes is dependent on calcium flux [29, 32]. hsBAFF elevates intracellular calcium level ([Ca2+]i), which triggers activation of Erk1/2, increasing proliferation of cultured mouse splenic B lymphocytes [33]. We therefore postulated that hsBAFF-mediated PP2A-Erk1/2 pathway and cell proliferation/survival might be dependent on increased [Ca2+]i. Indeed, pretreatment of Raji cells and primary B lymphocytes with BAPTA/AM, an intracellular Ca2+ chelator, significantly prevented both hsBAFF-induced PP2A inhibition involved in Erk1/2 activation (Fig. 6A and B) and B-cell proliferation/viability (Fig. 6C and D), suggesting that hsBAFF mediates PP2A-Erk1/2 signaling and proliferation/survival in the B cells, which depends on an intracellular Ca2+ rise. This is further supported by the findings that preventing hsBAFF-induced [Ca2+]i elevation using EGTA, an extracellular Ca2+ chelator, or 2-APB, an inhibitor for both inositol 1,4,5-trisphosphate (IP3) receptors and the Ca2+ release activated Ca2+ (CRAC) channels, obviously suppressed hsBAFF-induced PP2A inhibition and Erk1/2 activation (Fig. 7A and B), as well as cell proliferation/viability (Fig. 7C and D) in Raji cells and primary B lymphocytes. The results imply that hsBAFF-induced extracellular Ca2+ influx and ER Ca2+ release are involved in hsBAFF-inhibited PP2A, thereby contributing to activation of Erk1/2 and B-cell proliferation/survival.
Fig. 6.
BAPTA/AM attenuates hsBAFF-induced inhibition of PP2A and activation of Erk1/2 as well as cell proliferation/viability in B cells. Raji cells and purified mouse splenic B lymphocytes were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay) following pretreatment with/without 20 μM BAPTA/AM for 1 h. Total cell lysates were subjected to Western blotting using indicated antibodies (A). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (C) and the cell viability was determined by the MTS assay (D). (A) hsBAFF-induced inhibition of PP2A and activation of Erk1/2 were strongly blocked by BAPTA/AM. (B) Blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (C and D) BAPTA/AM markedly inhibited the basal or hsBAFF-stimulated cell proliferation and viability. Results are presented as mean ± SE (n = 3–6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cP<0.01, difference vs 1 μg/mL hsBAFF group; dP<0.01, difference vs 2.5 μg/mL hsBAFF group.
Fig. 7.
EGTA or 2-APB attenuates hsBAFF-induced inhibition of PP2A and activation of Erk1/2 as well as cell proliferation/viability in B cells. Raji cells and purified mouse splenic B lymphocytes were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay) following pretreatment with/without 100 μM EGTA or 100 μM 2-APB for 1 h. Total cell lysates were subjected to Western blotting using indicated antibodies (A). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (C) and the cell viability was determined by the MTS assay (D). (A) hsBAFF-induced inhibition of PP2A and activation of Erk1/2 were strongly blocked by EGTA and 2-APB, respectively. (B) Blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (C and D) EGTA or 2-APB markedly inhibited the basal or hsBAFF-stimulated cell proliferation and viability. Results are presented as mean ± SE (n = 3–6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cP<0.01, difference vs 1 μg/mL hsBAFF group; dP<0.01, difference vs 2.5 μg/mL hsBAFF group.
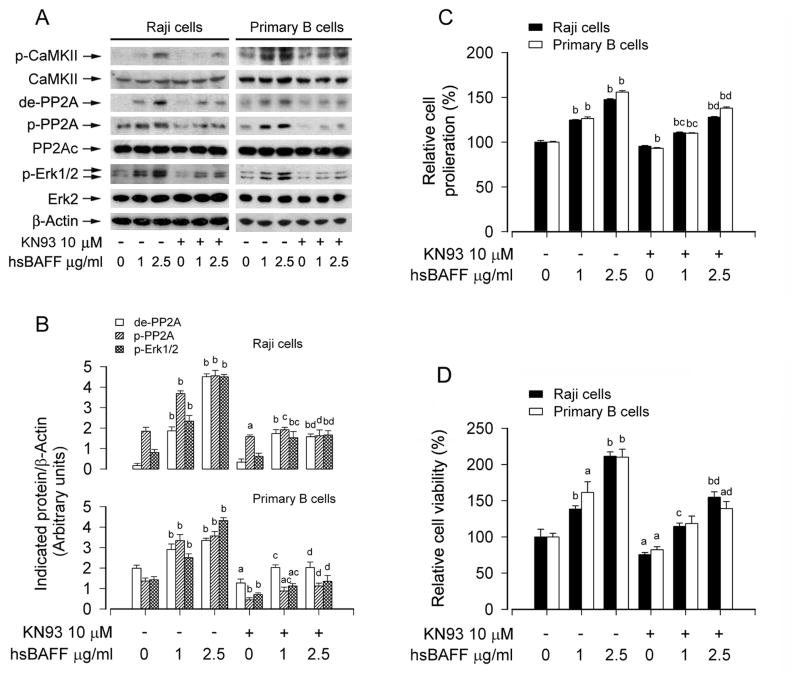
3.4. hsBAFF activates Erk1/2 leading to increased cell proliferation/viability by stimulating CaMKII-dependent inhibition of PP2A in B cells
CaMKII is a general integrator of Ca2+ signaling [43, 44]. The developing Ca2+/calmodulin complex may activate CaMKII, leading to intramolecular autophosphorylation at several sites, including Thr286, Thr305, and Thr306 [28, 45]. This prompted us to further test whether the Ca2+-dependent effects of hsBAFF on PP2A-Erk1/2 signaling as well as proliferation/survival in B cells are through stimulating CaMKII phosphorylation. For this, Raji cells and purified mouse splenic B lymphocytes were treated with/without hsBAFF (1 and 2.5 μg/mL) for 12 h or 48 h after pre-treatment with CaMKII inhibitor KN93 (10 μM) for 1 h. As shown in Fig. 8A and B, hsBAFF-induced phosphorylation at Thr286 of CaMKII was obviously attenuated by KN93, and KN93 markedly inhibited hsBAFF-induced expression of demethylated-PP2Ac, phospho-PP2Ac, and phospho-Erk1/2 in the cells. Furthermore, cell counting and MTS assay revealed that KN93 suppressed the basal and hsBAFF-stimulated B-cell proliferation/viability (Fig. 8C and D).
Fig. 8.
hsBAFF elicits CaMKII phosphorylation, promoting inhibition of PP2A and activation of Erk1/2, as well as proliferation and survival in B cells. Raji cells and purified mouse splenic B lymphocytes were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay) following pretreatment with/without 10 μM KN93 for 1 h. Total cell lysates were subjected to Western blotting using indicated antibodies (A). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (C) and the cell viability was determined by the MTS assay (D). (A) hsBAFF-induced CaMKII phosphorylation was associated with increased expression of de-PP2A, p-PP2A and p-Erk1/2 in B cells, which was strongly blocked by KN93. (B) Blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (C and D) KN93 inhibited the basal or hsBAFF-stimulated cell proliferation and viability. Results are presented as mean ± SE (n = 3–6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cP<0.01, difference vs 1 μg/mL hsBAFF group; dP<0.01, difference vs 2.5 μg/mL hsBAFF group.
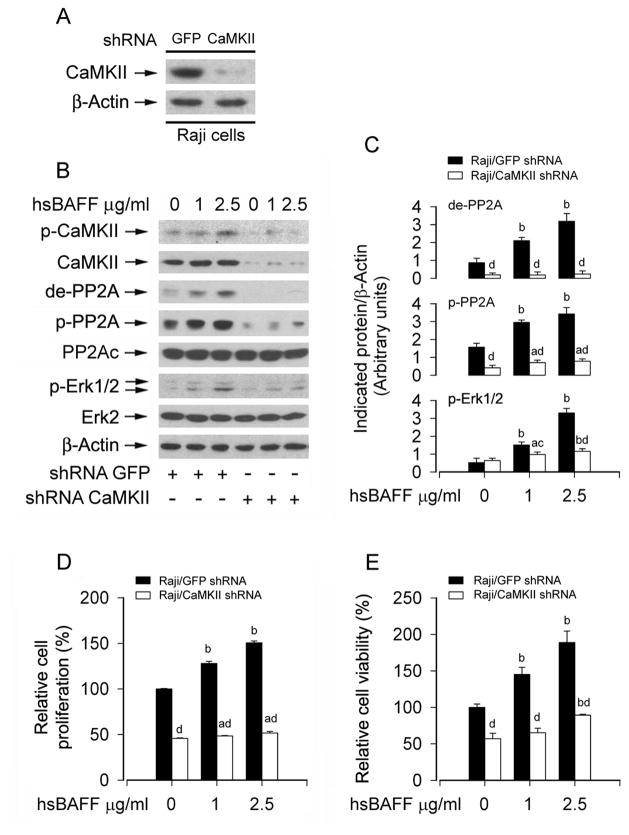
To further corroborate the role of CaMKII in hsBAFF-mediated PP2A-Erk1/2 signaling and B-cell proliferation/survival, expression of CaMKIIα was silenced by RNA interference. As shown in Fig. 9A, lentiviral shRNA to CaMKIIα, but not to GFP, down-regulated CaMKII expression by ~90% in Raji cells. Silencing CaMKII obviously attenuated hsBAFF-induced phosphorylation of CaMKII (Fig. 9B). Consistently, down-regulation of CaMKII conferred partial resistance to hsBAFF-induced inhibition of PP2A and activation of Erk1/2 (Fig. 9B and C). Furthermore, down-regulation of CaMKII significantly prevented hsBAFF-stimulated proliferation/viability in Raji cells (Fig. 9D and E). Taken together, the results strongly support that hsBAFF activates Erk1/2 by Ca2+-CaMKII-dependent inhibition of PP2A, leading to increased B-cell proliferation/viability.
Fig. 9.
Down-regulation of CaMKII prevents hsBAFF-induced inhibition of PP2A and activation of Erk1/2 as well as proliferation/viability in B cells. Raji cells, infected with lentiviral shRNA to CaMKII or GFP (as control), were treated with 0, 1, 2.5 μg/mL hsBAFF for 12 h (for Western blotting) or 48 h (for cell proliferation/viability assay). Total cell lysates were subjected to Western blotting using indicated antibodies (A and B). The blots were probed for β-actin as a loading control. Similar results were observed in at least three independent experiments. The cell proliferation was evaluated by cell counting (D) and the cell viability was determined by the MTS assay (E). (A) Lentiviral shRNA to CaMKIIa, but not to GFP, down-regulated CaMKII expression by ~90% in Raji cells. (B) Silencing CaMKII obviously attenuated hsBAFF-induced phosphorylation of CaMKII, and conferred partial resistance to hsBAFF-induced inhibition of PP2A and activation of Erk1/2 in Raji cells. (C) Blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (D and E) Down-regulation of CaMKII in part suppressed the basal or hsBAFF-stimulated cell proliferation and viability. Results are presented as mean ± SE (n = 3–6). aP <0.05, bP <0.01, difference vs 0 μg/mL hsBAFF group; cP<0.05, dp<0.01, CaMKII shRNA group vs GFP shRNA group.
4. Discussion
Increasing evidence has demonstrated the importance of BAFF in the development, maturation and homeostasis of normal B lymphocytes, and in the cell growth and survival of neoplastic B-lymphoid cells [8–11]. High levels of BAFF prolong the lifespan of B lymphocytes or autoimmune B cells [12–14, 46, 47]. Increased serum levels of BAFF have been documented in patients with autoimmune diseases, including RA, SS, and SLE [1, 14, 17, 48]. Therefore, it is of great importance to find a novel therapeutic strategy to control BAFF levels in individuals with autoimmune diseases and other aggressive/neoplastic B-cell disorders. Recently, we have demonstrated that excessive hsBAFF elevates [Ca2+]i, which activates CaMKII, Erk1/2, and mTOR signaling network, thereby increasing proliferation and survival of cultured mouse splenic B lymphocytes [33, 34]. Here we provide further evidence that hsBAFF activates Erk1/2 and enhances cell proliferation and survival by suppression of PP2A, which is Ca2+-CaMKII-dependent in normal and Raji B-lymphoid cells.
We observed that hsBAFF inhibits PP2A not through altering cellular protein expression of the catalytic subunit (PP2Ac) and the regulatory proteins (PP2A-A and PP2A-B) (Fig. 4A and C). The phosphatase activity of PP2A can be turned “on” and “off” by post-translational modification such as phosphorylation and carboxyl methylation of PP2Ac [42, 49]. In this study, we found that hsBAFF dramatically increased expression of demethylated-PP2Ac and phospho-PP2Ac (Tyr307) in a concentration- and time-dependent manner in normal and Raji B-lymphoid cells. These data indicate that hsBAFF inhibits the phosphatase activity of PP2A at least by enhancing demethylation and phosphorylation of PP2Ac (Fig. 4A–D), two events responsible for PP2A inactivation [41]. PP2A is a well-known negative regulator of Erk1/2 [18]. As hsBAFF concurrently induced remarkable phosphorylation of Erk1/2, this led us to hypothesize that hsBAFF may induce Erk1/2 activation by suppressing PP2A. To test this hypothesis, genetic manipulation of PP2A activity was utilized. As expected, overexpression of wild-type PP2A prevented hsBAFF from activation of Erk1/2 and cell proliferation/viability in the B cells. Therefore, our findings strongly suggest the involvement of PP2A-Erk1/2 pathway in BAFF-enhanced cell proliferation and survival in normal and B lymphoid cells.
Ca2+ signaling is an important component of signal transduction pathways regulating B and T lymphocyte proliferation and survival [28]. Our previous study has revealed that hsBAFF-upregulated [Ca2+]i promotes the proliferation in B lymphocytes [33] and CD4+ T lymphocytes [27]. This prompted us to test whether hsBAFF inhibits PP2A and activates Erk1/2 in Ca2+ dependent manner. As expected, we found that pretreatment with BAPTA/AM, an intracellular Ca2+ chelator, did obviously attenuate hsBAFF-induced PP2A inhibition involved in Erk1/2 activation and cell proliferation/survival in normal and Raji B-lymphoid cells. Recently, we have observed that preventing [Ca2+]i elevation using EGTA, an extracellular Ca2+ chelator, or 2-APB, an inhibitor for both IP3 receptors and CRAC channels, dramatically inhibited hsBAFF activation of mTOR signaling, as well as cell proliferation and survival, suggesting that hsBAFF-induced extracellular Ca2+ influx and ER Ca2+ release elevates [Ca2+]i contributing to B lymphocyte proliferation and survival via activation of mTOR signaling [34]. In the present study, we noticed that EGTA or 2-APB also suppressed hsBAFF-activated Erk1/2 and B-cell proliferation/survival by preventing hsBAFF from reducing PP2A activity. Therefore, our findings suggest that BAFF-induced inhibition of PP2A, leading to activation of Erk1/2 and B-cell proliferation/survival, is dependent on an intracellular Ca2+ rise, which is involved in extracellular Ca2+ influx and Ca2+ release from ER.
CaMKII, as a general integrator of Ca2+ signaling, is activated upon binding of Ca2+-containing CaM, which regulates the development and activity of many different cell types including immune cells [28–31, 43, 44]. It has been reported that activated CaMKII is involved in regulating the activation of Erk1/2 leading to myeloid leukemia cell proliferation [28]. CaMKII plays a bridging role between hsBAFF induction of [Ca2+]i elevation and activation of mTOR signaling, leading to increased proliferation and survival in B lymphocytes [34]. In this study, to demonstrate that CaMKII is essential for hsBAFF-induced inhibition of PP2A and activation of Erk1/2 contributing to B-cell proliferation/survival, pharmacological inhibition or genetic manipulation of CaMKII activity was utilized. Pre-treatment with KN93, a specific inhibitor of CaMKII [50], blocked hsBAFF-induced CaMKII phosphorylation, and partially prevented hsBAFF from enhancing cell proliferation and survival in normal and Raji B-lymphoid cells (Fig. 8A–D). Consistently, hsBAFF-induced inhibition of PP2A and activation of Erk1/2 signaling were obviously blocked by KN93 in the cells as well (Fig. 8A and B). Furthermore, down-regulation of CaMKII by RNA interference also attenuated hsBAFF-induced inhibition of PP2A, activation of Erk1/2 and cell proliferation/survival in the cells. These findings underscore the notion that BAFF elicits PP2A inhibition contributing to activation of Erk1/2 signaling by CaMKII phosphorylation, enhancing B-cell proliferation and survival.
Multiple studies have documented excess BAFF extends B lymphocyte lifespan beyond physiological limits, which results in aggressive B lymphocyte disorders and autoimmune diseases [9, 14–16, 46]. B lymphocytes carry out central roles in the pathogenesis of the autoimmune diseases through a combination of antibody-mediated and antibody-independent actions [13]. In the present study, we observed a decreased PP2A activity in excessive hsBAFF-stimulated normal and Raji B-lymphoid cells, which was associated with activation (phosphorylation) of Erk1/2 contributing to cell proliferation and survival in the cells. We also demonstrated a link between Ca2+-dependent CaMKII phosphorylation and inhibition of PP2A in normal and Raji B-lymphoid cells induced by hsBAFF. A proposition that arises from this work is that excessive BAFF-induced inhibition of PP2A and activation of Erk1/2 by Ca2+ signaling may be an important mechanism in aggressive or neoplastic B-cells and autoimmune B cells. Our results provide an expanded conceptual view of excess BAFF signaling, which should contribute to a better understanding of molecular mechanisms involved in the physiology of normal B-cell proliferation, as well as in the pathophysiology of aggressive or neoplastic B-cell disorders associated with autoimmune diseases.
In summary, here we identify that excess hsBAFF promotes cell proliferation and survival via suppression of PP2A leading to Erk1/2 activation in normal and neoplastic B-lymphoid cells. Furthermore, hsBAFF activates Erk1/2, at least, in part by Ca2+-CaMKII-dependent inhibition of PP2A in the cells. Our findings suggest that inhibitors of CaMKII and Erk1/2, activator of PP2A or manipulation of intracellular Ca2+ may be exploited for the prevention of excessive BAFF-induced aggressive B-cell malignancies and autoimmune diseases.
Acknowledgments
This work was supported in part by the grants from National Natural Science Foundation of China (No.31172083; L.C.), NIH (CA115414; S.H.), American Cancer Society (RSG-08-135-01-CNE; S.H.), Project for the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (L.C.), Louisiana Board of Regents (NSF-2009-PFUND-144; S.H.), and Innovative Research Program of Jiangsu College Graduate of China (No.CXLX13_387; L.G.).
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- BAFF
B-cell activating factor of the TNF family
- BAPTA/AM
1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester
- BLyS
B lymphocyte stimulator
- BCMA
B cell maturation antigen
- CaM
calmodulin
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CRAC
Ca2+-release activated Ca2+
- EGTA
ethylene glycol tetra-acetic acid
- ER
endoplasmic reticula
- Erk1/2
extracellular signal-related kinase 1/2
- FBS
fetal bovine serum
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- PBS
phosphate buffered saline
- PP2A
protein phosphatases 2A
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- SS
Sjögren’s syndrome
- TACI
transmembrane activator and cyclophilin ligand interactor
- TALL-1
TNF and apoptosis ligand-related leukocyte-expressed ligand1
- THANK
TNF homologue that activates apoptosis, nuclear factor κB, and c-Jun NH2-terminal kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang J, Roschke V, Baker KP, Wang Z, Alarcon GS, Fessler BJ, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Henley T, Kovesdi D, Turner M. B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur J Immunol. 2008;38:3543–8. doi: 10.1002/eji.200838618. [DOI] [PubMed] [Google Scholar]
- 3.Mueller CG, Boix C, Kwan WH, Daussy C, Fournier E, Fridman WH, et al. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J Leukoc Biol. 2007;82:567–75. doi: 10.1189/jlb.0706481. [DOI] [PubMed] [Google Scholar]
- 4.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–75. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999;65:680–3. [PubMed] [Google Scholar]
- 6.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 8.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–36. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, et al. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–88. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Tschopp J. BAFF and the regulation of B cell survival. Immunol Lett. 2003;88:57–62. doi: 10.1016/s0165-2478(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 11.Fu L, Lin-Lee YC, Pham LV, Tamayo AT, Yoshimura LC, Ford RJ. BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood. 2009;113:4627–36. doi: 10.1182/blood-2008-10-183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 13.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–37. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009;158:155–63. doi: 10.1111/j.1365-2249.2009.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–62. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosello S, Youinou P, Daridon C, Tolusso B, Bendaoud B, Pietrapertosa D, et al. Concentrations of BAFF correlate with autoantibody levels, clinical disease activity, and response to treatment in early rheumatoid arthritis. J Rheumatol. 2008;35:1256–64. [PubMed] [Google Scholar]
- 18.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–84. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Sunahori K, Nagpal K, Hedrich CM, Mizui M, Fitzgerald LM, Tsokos GC. The Catalytic Subunit of Protein Phosphatase 2A (PP2Ac) Promotes DNA Hypomethylation by Suppressing the Phosphorylated Mitogen-activated Protein Kinase/Extracellular Signal-regulated Kinase (ERK) Kinase (MEK)/Phosphorylated ERK/DNMT1 Protein Pathway in T-cells from Controls and Systemic Lupus Erythematosus Patients. J Biol Chem. 2013;288:21936–44. doi: 10.1074/jbc.M113.467266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace AM, Hardigan A, Geraghty P, Salim S, Gaffney A, Thankachen J, et al. Protein phosphatase 2A regulates innate immune and proteolytic responses to cigarette smoke exposure in the lung. Toxicol Sci. 2012;126:589–99. doi: 10.1093/toxsci/kfr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 24.Crispin JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, et al. Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism. J Immunol. 2012;188:3567–71. doi: 10.4049/jimmunol.1200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispin JC, Hedrich CM, Tsokos GC. Gene-function studies in systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:476–84. doi: 10.1038/nrrheum.2013.78. [DOI] [PubMed] [Google Scholar]
- 26.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–35. [PubMed] [Google Scholar]
- 27.Wen L, Chen SJ, Zhang W, Ma HW, Zhang SQ, Chen L. hsBAFF regulates proliferation and response in cultured CD4+ T lymphocytes by upregulation of intracellular free Ca2+ homeostasis. Cytokine. 2011;53:215–22. doi: 10.1016/j.cyto.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Si J, Collins SJ. Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation. Cancer Res. 2008;68:3733–42. doi: 10.1158/0008-5472.CAN-07-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MY, Zal T, Ch’en IL, Gascoigne NR, Hedrick SM. A pivotal role for the multifunctional calcium/calmodulin-dependent protein kinase II in T cells: from activation to unresponsiveness. J Immunol. 2005;174:5583–92. doi: 10.4049/jimmunol.174.9.5583. [DOI] [PubMed] [Google Scholar]
- 30.Bui JD, Calbo S, Hayden-Martinez K, Kane LP, Gardner P, Hedrick SM. A role for CaMKII in T cell memory. Cell. 2000;100:457–67. doi: 10.1016/s0092-8674(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 31.Schworer CM, Colbran RJ, Soderling TR. Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem. 1986;261:8581–4. [PubMed] [Google Scholar]
- 32.Chao TS, Byron KL, Lee KM, Villereal M, Rosner MR. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992;267:19876–83. [PubMed] [Google Scholar]
- 33.Liang JQ, Zhang W, Wen L, Gao W, Zhang SQ, Chen L. hsBAFF-upregulated intracellular free Ca2+ homeostasis regulates ERK1/2 activity and cell proliferation in B cells in vitro. Physiol Res. 2009;58:411–8. doi: 10.33549/physiolres.931522. [DOI] [PubMed] [Google Scholar]
- 34.Ke Z, Liang D, Zeng Q, Ren Q, Ma H, Gui L, et al. hsBAFF promotes proliferation and survival in cultured B lymphocytes via calcium signaling activation of mTOR pathway. Cytokine. 2013;62:310–21. doi: 10.1016/j.cyto.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Cao P, Mei JJ, Diao ZY, Zhang S. Expression, refolding, and characterization of human soluble BAFF synthesized in Escherichia coli. Protein Expr Purif. 2005;41:199–206. doi: 10.1016/j.pep.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, et al. Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS ONE. 2010;5:e10578. doi: 10.1371/journal.pone.0010578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Liu L, Yin J, Luo Y, Huang S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol. 2009;41:1284–95. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Xu Y, Xu B, Guo M, Zhang Z, Liu L, et al. CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J Neurochem. 2011;119:1108–18. doi: 10.1111/j.1471-4159.2011.07493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Liu L, Luo Y, Huang S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J Neurochem. 2008;105:251–61. doi: 10.1111/j.1471-4159.2007.05133.x. [DOI] [PubMed] [Google Scholar]
- 40.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–43. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–39. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X, Xu B, Beevers CS, Odaka Y, Chen L, Liu L, et al. Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Carcinogenesis. 2012;33:868–75. doi: 10.1093/carcin/bgs029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Templeton DM. Cadmium activates CaMK-II and initiates CaMK-II-dependent apoptosis in mesangial cells. FEBS Lett. 2007;581:1481–6. doi: 10.1016/j.febslet.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–27. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Wayman GA, Tokumitsu H, Davare MA, Soderling TR. Analysis of CaM-kinase signaling in cells. Cell calcium. 2011;50:1–8. doi: 10.1016/j.ceca.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 48.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakema JE, Bakker A, de Haij S, Honing H, Bracke M, Koenderman L, et al. Inside-out regulation of Fc alpha RI (CD89) depends on PP2A. J Immunol. 2008;181:4080–8. doi: 10.4049/jimmunol.181.6.4080. [DOI] [PubMed] [Google Scholar]
- 50.Choi SS, Seo YJ, Shim EJ, Kwon MS, Lee JY, Ham YO, et al. Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res. 2006;1108:28–38. doi: 10.1016/j.brainres.2006.06.048. [DOI] [PubMed] [Google Scholar]