Abstract
The consecutive radical/ionic reaction consisting of radical formylation of alkyl bromides and nucleophilic addition of a cyanide ion was investigated, which gave moderate to good yields of cyanohydrin derivatives in one-pot.
Keywords: alkyl bromide, carbon monoxide, cyanohydrin, ethyl cyanoformate, multicomponent, radical reaction
Introduction
Radical carbonylation reactions have been recognized as a versatile tool for the synthesis of a wide variety of carbonyl compounds [1–4]. In 1990, we demonstrated that aldehydes can be prepared from alkyl or aromatic halides and CO under typical radical chain reaction conditions using tributyltin hydride and AIBN [5–6]. Under the reaction conditions where a catalytic amount of fluorous tin hydride and an excess amount of sodium cyanoborohydride were used, initially formed aldehydes can be converted into hydroxymethylated compounds in one-pot [7–9], since borohydride acts not only as the reagent for the regeneration of tin hydride [10–13] but also as the reagent for aldehyde reduction. Later on we found that borohydride reagents can also serve as radical mediator delivering hydrogen to the radical centre [14], thus we developed a hydroxymethylation method using Bu4NBH4 and a radical initiator [15–17]. Recent work in collaboration with Dennis Curran has revealed that, with the use of NHC-borane [18], hydroxymethylation of aromatic iodides can be attained [19]. All these reactions consist of the combination of radical formylation with CO and ionic hydride reduction by hydride reagents (Scheme 1, reaction 1). During the course of our study on borohydride-mediated radical hydroxymethylation of alkyl halides with CO, we found that cyanohydrin was obtained as a byproduct when Bu4NBH3CN was used as a radical mediator [15], which led us to investigate the one-pot synthesis of cyanohydrins based on radical formylation. Thus, we thought that the two step radical/ionic reactions can be extended to the consecutive C–C bond forming reactions.
Scheme 1.
Sequential radical formylation and derivatization.
Cyanohydrins are important subunits frequently found in biologically active compounds and are also versatile building blocks for further synthetic transformations [20–21]. The common method to obtain cyanohydrins is the reaction of aldehydes with a cyanide sources such as TMSCN [22–23], ethyl cyanoformate [24–26] or acyl cyanide [27–28]. We provide here an efficient one-pot method for the synthesis of cyanohydrin derivatives via consecutive radical/ionic C–C bond forming reaction of alkyl bromides, CO and ethyl cyanoformate (Scheme 1, reaction 2).
Results and Discussion
We examined AIBN-induced radical formylation of 1-bromooctane (1a) with Bu3SnH under 80 atm of CO pressure in the presence of a cyanide source (Scheme 2). Under the employed conditions, the reaction using TMSCN (2a') was slow, which gave 16% of 3a' and 51% of nonanal. The use of AcCN (2a'') also gave 3a'' but only in 12% yield. However, when ethyl cyanoformate (2a) was used together with Et3N [29], the cyanohydrin 3a was obtained in 62% yield. When we used higher CO pressure such as 120 atm, the yield of 3a increased to 79%.
Scheme 2.
Examination of cyanide source.
We examined various alkyl bromides 1 in the present radical/ionic three-component coupling reaction (Table 1). Primary alkyl bromides 1b–e containing a chlorine atom, an ester group, a cyano group, or a phenyl group worked well to give the corresponding cyanohydrin derivatives 3b–e in good yields (Table 1, entries 2–5). The reaction of secondary and tertiary alkyl bromides 1f–i also proceeded well to give the corresponding cyanohydrins 3f–i in good yields (Table 1, entries 6–9). The reaction using cyclopropylmethyl bromide (1j) afforded the lowest yield of cyanohydrin 3j, which possessed an olefin structure arising from the ring-opening of a cyclopropylcarbinyl radical (Table 1, entry 10) [30–31].
Table 1.
Three-component coupling reaction leading to cyanohydrin derivatives.
 | ||||
| entry | alkyl bromide | CO (atm) | product | yielda (%) |
| 1b |
 1a |
120 |
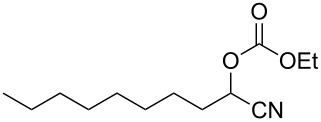 3a |
79 |
| 2 |
 1b |
80 |
 3b |
60 |
| 3 |
 1c |
80 |
 3c |
83 |
| 4 |
 1d |
120 |
 3d |
76 |
| 5 |
 1e |
120 |
 3e |
61 |
| 6 |
 1f |
120 |
 3f |
61 |
| 7 |
 1g |
120 |
 3g |
74 |
| 8 |
 1h |
120 |
 3h |
73 |
| 9 |
 1i |
110 |
 3i |
82 |
| 10 |
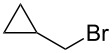 1j |
110 |
 3j |
45 |
aIsolated yield after flash chromatography on SiO2. b0.03 M.
Conclusion
In summary, we have demonstrated a three-component coupling reaction comprising alkyl bromides 1, CO and ethyl cyanoformate (2a) in the presence of Bu3SnH, AIBN, and Et3N, which gave moderate to good yields of cyanohydrin derivatives 3. This protocol represents a one-pot method [32–33] based on radical carbonylation and ionic cyanation.
Experimental
Typical procedure for radical/ionic three-component coupling reaction leading to cyanohydrin derivatives 1-cyanononyl ethyl carbonate (3a) [34] (Table 1, entry 1): A mixture of 1-bromooctane (1a, 96.6 mg, 0.5 mmol), ethyl cyanoformate (2a, 79.3 mg, 0.8 mmol), tributyltin hydride (174.6 mg, 0.6 mmol), triethylamine (13.2 mg, 0.13 mmol), and AIBN (24.6 mg, 0.15 mmol) in C6H6 (17 mL) were placed in a 100 mL stainless steel autoclave. The reaction mixture was degassed 3 times with 10 atm of CO and charged with 90 atm of CO at −40 °C (MeCN–dry ice bath). Then the autoclave was allowed to warm to room temperature, which caused the pressure gauge to indicate 120 atm. Then the reaction was conducted at 80 °C for 3 h. After cooling to room temperature, the reaction mixture was concentrated and purified by silica gel flash chromatography (hexane/EtOAc 97:3) to afford 3a (95.3 mg, 79%). 1H NMR (CDCl3, 500 MHz) δ 5.18 (t, J = 6.8 Hz, 1H), 4.4–4.2 (m, 2H), 2.0–1.9 (m, 2H), 1.6–1.5 (m, 2H), 1.4–1.2 (m, 13H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 153.56, 116.51, 65.27, 64.66, 32.31, 31.68, 29.12, 28.99, 28.71, 24.34, 22.53, 14.05, 13.93.
Acknowledgments
We acknowledge a Grant-in-Aid for Scientific Research on Innovative Areas (No. 2105) from the Ministry of Education, Culture, Sports, and Technology (MEXT), Japan.
This article is part of the Thematic Series "Multicomponent reactions II".
References
- 1.Ryu I, Sonoda N. Angew Chem, Int Ed Engl. 1996;35:1050. doi: 10.1002/anie.199610501. [DOI] [Google Scholar]
- 2.Ryu I, Sonoda N, Curran D P. Chem Rev. 1996;96:177. doi: 10.1021/cr9400626. [DOI] [PubMed] [Google Scholar]
- 3.Chatgilialoglu C, Crich D, Komatsu M, Ryu I. Chem Rev. 1999;99:1991. doi: 10.1021/cr9601425. [DOI] [PubMed] [Google Scholar]
- 4.Ryu I. Chem Soc Rev. 2001;30:16. doi: 10.1039/a904591k. [DOI] [Google Scholar]
- 5.Ryu I, Kusano K, Ogawa A, Kambe N, Sonoda N. J Am Chem Soc. 1990;112:1295. doi: 10.1021/ja00159a088. [DOI] [Google Scholar]
- 6.Ryu I, Kusano K, Masumi N, Yamazaki H, Ogawa A, Sonoda N. Tetrahedron Lett. 1990;31:6887. doi: 10.1016/S0040-4039(00)97198-3. [DOI] [Google Scholar]
- 7.Gupta V, Kahne D. Tetrahedron Lett. 1993;34:591. doi: 10.1016/S0040-4039(00)61627-1. [DOI] [Google Scholar]
- 8.Ryu I, Niguma T, Minakata S, Komatsu M, Hadida S, Curran D P. Tetrahedron Lett. 1997;38:7883. doi: 10.1016/S0040-4039(97)10076-4. [DOI] [Google Scholar]
- 9.Matsubara H, Yasuda S, Sugiyama H, Ryu I, Fujii Y, Kita K. Tetrahedron. 2002;58:4071. doi: 10.1016/S0040-4020(02)00256-9. [DOI] [Google Scholar]
- 10.Corey E J, Suggs W. J Org Chem. 1975;40:2554. doi: 10.1021/jo00905a039. [DOI] [Google Scholar]
- 11.Giese B, González-Gómez J A, Witzel T. Angew Chem, Int Ed Engl. 1984;23:69. doi: 10.1002/anie.198400691. [DOI] [Google Scholar]
- 12.Stork G, Sher P M. J Am Chem Soc. 1986;108:303. doi: 10.1021/ja00262a024. [DOI] [Google Scholar]
- 13.Curran D P, Hadida S, Kim S-Y, Luo Z. J Am Chem Soc. 1999;121:6607. doi: 10.1021/ja990069a. [DOI] [Google Scholar]
- 14.Ryu I, Uehara S, Hirao H, Fukuyama T. Org Lett. 2008;10:1005. doi: 10.1021/ol7031043. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Kawamoto T, Uehara S, Fukuyama T, Ryu I. Org Lett. 2010;12:1548. doi: 10.1021/ol1002847. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Kinoshita T, Kawamoto T, Wada M, Kuroda H, Masuyama A, Ryu I. J Org Chem. 2011;76:7096. doi: 10.1021/jo201064h. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto T, Ryu I. Chimia. 2012;66:372. doi: 10.2533/chimia.2012.372. [DOI] [PubMed] [Google Scholar]
- 18.Curran D P, Solovyev A, Makhlouf Brahmi M, Fensterbank L, Malacria M, Lacôte E. Angew Chem, Int Ed. 2011;50:10294. doi: 10.1002/anie.201102717. See for a review on NHC-borane. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto T, Okada T, Curran D P, Ryu I. Org Lett. 2013;15:2144. doi: 10.1021/ol4006294. [DOI] [PubMed] [Google Scholar]
- 20.Gregory R J H. Chem Rev. 1999;99:3649. doi: 10.1021/cr9902906. [DOI] [PubMed] [Google Scholar]
- 21.Brunel J-M, Holmes I P. Angew Chem, Int Ed. 2004;43:2752. doi: 10.1002/anie.200300604. [DOI] [PubMed] [Google Scholar]
- 22.Lidy W, Sundermeyer W. Chem Ber. 1973;106:587. doi: 10.1002/cber.19731060224. [DOI] [Google Scholar]
- 23.Evans D A, Truesdale L K, Carroll G L. J Chem Soc, Chem Commun. 1973:55. doi: 10.1039/c39730000055. [DOI] [Google Scholar]
- 24.Poirier D, Berthiaume D, Boivin R P. Synlett. 1999:1423. doi: 10.1055/s-1999-2859. [DOI] [Google Scholar]
- 25.Berthiaume D, Poirier D. Tetrahedron. 2000;56:5995. doi: 10.1016/S0040-4020(00)00535-4. [DOI] [Google Scholar]
- 26.Tian S-K, Deng L. J Am Chem Soc. 2001;123:6195. doi: 10.1021/ja010690m. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann H M R, Ismail Z M, Hollweg R, Zein A R. Bull Chem Soc Jpn. 1990;63:1807. doi: 10.1246/bcsj.63.1807. [DOI] [Google Scholar]
- 28.Okimoto M, Chiba T. Synthesis. 1996:1188. doi: 10.1055/s-1996-4361. [DOI] [Google Scholar]
- 29.Baeza A, Nájera C, de Garcia Retamosa M, Sansano J M. Synthesis. 2005:2787. doi: 10.1055/s-2005-872096. [DOI] [Google Scholar]
- 30.Bowry V W, Ingold K U. J Am Chem Soc. 1991;113:5699. doi: 10.1021/ja00015a025. [DOI] [Google Scholar]
- 31.Newcomb M. Tetrahedron. 1993;49:1151. doi: 10.1016/S0040-4020(01)85808-7. [DOI] [Google Scholar]
- 32.Suga S, Yamada D, Yoshida J-i. Chem Lett. 2010;39:404. doi: 10.1246/cl.2010.404. [DOI] [Google Scholar]
- 33.Yoshida J-i, Saito K, Nokami T, Nagaki A. Synlett. 2011:1189. doi: 10.1055/s-0030-1259946. [DOI] [Google Scholar]
- 34.Khan N H, Agrawal S, Kureshy R I, Abdi S H R, Sadhukhan A, Pillai R S, Bajaj H C. Catal Commun. 2010;11:907. doi: 10.1016/j.catcom.2010.04.005. [DOI] [Google Scholar]




