Summary
Background and Rationale Bortezomib
(PS-341, VELCADE®) is a selective inhibitor of the 26S proteasome, an integral component of the ubiquitinproteasome pathway. This phase II study evaluated the activity and tolerability of bortezomib in unresectable hepatocellular carcinoma (HCC) patients.
Methods
The primary endpoint was confirmed tumor response rate (RR) with secondary endpoints including duration of response, time to disease progression, survival and toxicity. Treatment consisted of bortezomib, 1.3 mg/m2 IV bolus on days 1, 4, 8, and 11 of each 21-day treatment cycle. Eligibility included: no prior systemic chemotherapy, ECOG PS 0-2, Child-Pugh A or B, preserved hematologic, hepatic and neurologic function; prior liver-directed therapy was permitted.
Results
Thirty-five patients enrolled and received a median of 2 cycles of treatment (range 1–12). Overall, 24 and 4 patients had a maximum severity of grade 3 and 4 adverse events (AEs), respectively. No treatment related deaths occurred. Only thrombocytopenia (11%) was seen in greater than 10% of patients. One patient achieved a partial response, lasting 13 weeks during treatment and progressed 11.6 months later; two patients received treatment for greater than 6 months. Median time-to-progression was 1.6 months and median survival was 6.0 months.
Conclusions
This international, multicenter trial evaluated bortezomib as monotherapy in unresectable HCC patients. And, despite the lack of significant activity, this report serves as a baseline clinical experience for the development of future dual biologic approaches including bortezomib.
Keywords: Boronic acids, Antineoplastic agents, Biologic agents, Treatment outcome
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide with approximately 626,000 new cancer cases per year [1]. Unfortunately, its incidence approximates its mortality, 598,000 per year, resulting in HCC being the third most common cause of cancer death. In the United States, the incidence of HCC is increasing due to the rise in hepatitis C virus infection and the subsequent risk of cirrhosis in these individuals [2, 3]. Advances in treatment of HCC have recently been made with sorafenib, an oral multikinase inhibitor which targets the Raf kinase and vascular endothelial growth factor (VEGF) pathways [4–6]. Improvements in overall survival by about 31% have been reported in separate phase III trials conducted in Western countries and in Asia. The survival improvements in predominately Child-Pugh A patients in these trials led to the approval of sorafenib and its acceptance as a new standard therapy in advanced HCC patients.
Similar to the development of sorafenib, an increased understanding of the molecular alterations characteristic of HCC has led to the identification of potential therapeutic targets. One candidate is the 26S proteasome, an ATP-dependent multicatalytic protease of the ubiquitinproteasome pathway that governs the ordered and temporal degradation of key regulatory proteins [7–9]. These mediators (for example, p53, p21, p27, NF-kB) are critical to sustaining HCC cells and regulate transcription and the cell cycle; they are integral to apoptosis, angiogenesis and metastasis. For example, the tumor suppressor gene, p53, has a mutation frequency in HCC ranging from 15% to 42% in HCC [10] and hepatitis B and C viral proteins are known to interfere with p53 function [11, 12]. The HBVx protein hinders p53 entry into the cell nucleus resulting in inhibition of p53-mediated transcription, DNA repair, and apoptosis. These effects can be reversed if p53 concentrations and nuclear localization are increased through inhibition of proteasome-mediated p53 degradation. These types of interactions provide the rationale, from a mechanistic perspective, for targeting the ubiquitin-proteasome pathway in HCC.
Bortezomib, a small molecule, dipeptidyl boronic acid, is a selective and reversible inhibitor of the 26S proteasome. In pre-clinical studies, bortezomib has antitumor activity in a variety of in vitro and in vivo tumor models, either alone or in combination with common chemotherapeutic agents [13–16]. Bortezomib has demonstrated activity in the clinic and is approved for the treatment of multiple myeloma patients, as well as for the treatment of refractory mantle cell lymphoma [17, 18]. The front-line multiple myeloma approval was largely based on an international, multicenter trial in which symptomatic multiple myeloma patients treated with a bortezomib-containing combination experienced a statistically significant improvement in the primary endpoint, time-to-progression. Bortezomib has been evaluated in other cancers and similarly demonstrated some clinical activity. A multicenter, single-arm, phase II trial was conducted to evaluate the activity of bortezomib in HCC.
Methods
Eligibility criteria
Patients eligible for the study had histologically or cytologically confirmed HCC that was surgically unresectable or metastatic. Measurable disease was required. Other eligibility criteria included Child-Pugh classification of A or B; an Eastern Cooperative Oncology Group performance status (PS) of 0, 1, or 2; an estimated life expectancy of at least 3 months; adequate bone marrow, hepatic, and renal function, indicated by an absolute neutrophil count ≥1,500/μL, platelets ≥75,000/μL, and total bilirubin ≤3× upper limit of normal (UNL), respectively; serum AST or ALT levels ≤5× UNL; serum creatinine ≤2 mg/dL; serum albumin ≥2.5 g/dL; an international normalized ratio ≤1.5 (unless on anticoagulation). Patients had to provide written informed consent. Patients may not have received prior systemic chemotherapy and for individuals having prior liver-directed therapy (chemoembolization, cryotherapy, radiofrequency ablation, ethanol injection, or photodynamic therapy) the following criteria were required: >6 weeks had elapsed since therapy; indicator lesion(s) was/were outside the prior treatment area; or, if the only indicator lesion was inside the prior treatment area, there must have been clear evidence of disease progression associated with that lesion. In addition, edges of the indicator lesion must have been clearly distinct on CT scanning. Exclusion criteria included the presence of >grade 1 sensory peripheral neuropathy of any etiology or grade 1 with neuropathic pain of any etiology, pregnancy, and a history of other malignancy within the previous 3 years (except for adequately treated basal cell or squamous cell skin cancer). Patients were also excluded on the basis of uncontrolled intercurrent illness, CNS metastases, and HIV infection. Institutional Review Board approval from each participating institution was required and the trial was monitored by the Mayo Clinic Data Safety Monitoring Board.
Bortezomib administration
Bortezomib was supplied by Millenium Pharmaceuticals through the National Cancer Institute (NCI; Bethesda, MD), and a dose of 1.3 mg/m2 was administered intravenously as a bolus over 3–5 s. A cycle consisted of this dose administered on days 1, 4, 8, and 11 of a 21-day cycle. Treatment continued until disease progression, unacceptable toxicity, intercurrent illness that did not allow the patient to receive further treatment, or patient refusal. All toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE version 3.0) during treatment. Complete patient histories, physical examinations, complete blood cell counts (CBCs), and serum electrolytes/chemistries were performed at baseline and before each cycle of treatment. CBCs and electrolytes were monitored more frequently during the initial two cycles. Alpha fetoprotein was measured every two cycles.
Disease assessment
The primary endpoint of the trial was the confirmed tumor responses assessed using RECIST criteria [19]. All patients meeting the eligibility criteria who signed a consent form, began treatment, and had at least one post-baseline disease assessment were considered evaluable for assessing response. Radiologic studies were performed at baseline and after every two cycles (i.e., every 6 weeks) of therapy to assess tumor response. Total disappearance of target lesions constituted a complete response (CR); whereas a minimum of a 30% decrease in the sum of the LDs of the lesions was classified as a partial response (PR). New lesions or a 20% increase in the sum of the LDs of the target lesions or any increase in the size of number of non-target lesions, was considered progressive disease (PD). Otherwise, patients were classified as having stable disease (SD). Patients were re-evaluated for disease status after a minimum of 6 weeks of achieving a CR or PR to confirm the assessment. Similarly, an SD was re-assessed at a minimum interval of 6 weeks. Patients with global deterioration of health status requiring discontinuation of treatment without objective evidence of disease progression at that time, and not related to study treatment or other medical conditions, were considered to have PD due to symptomatic deterioration. For the primary endpoint of the study, a confirmed tumor response was defined to be either a CR or PR on 2 consecutive evaluations at least 6 weeks apart during the first 12 cycles of treatment.
Other endpoints included duration of response which was calculated from the first date of a patient’s objective status of either a CR or PR to the date of PD (or last tumor assessment). Duration of SD was calculated from the date of registration to the date of PD (or last tumor assessment if no PD) for patients having achieved a best response of SD. Patients were censored for progression (survival) at their date of last assessment (last contact) if no progression (death) occurred. Time to PD was calculated from the date of registration to the date of PD (or last tumor assessment). Survival or time to death was calculated from the date of registration to the date of death (or last contact). All patients were followed for a maximum of death or 3 years following registration, whichever was earlier.
Statistical design
A Simon two-stage phase II study design [20] was used to test whether there was sufficient evidence to determine that the proportion of confirmed tumor responses was at least 30% (i.e., warranted further study) versus 15% (i.e., clinically inactive). Four confirmed responses in the initial 20 evaluable patients warranted the expansion of enrollment to 50 patients. Ten confirmed responses among 50 evaluable patients was considered sufficient evidence of promising activity to recommend further testing of this regimen. This design yielded 88% power at 0.09 level of significance to detect a true response rate of 30%. Confidence intervals (CIs) were calculated using the method of Duffy-Santner [21]. Accrual was not suspended between stages allowing for continued patient entry while the planned early response analysis was performed. Toxicity was closely monitored with a low rate being observed. A toxicity stopping rule was in place, as required by the Mayo Clinic Cancer Center Data Safety Monitoring Board.
Summary statistics and frequency tables were used to summarize baseline patient characteristics and adverse event rates. Adverse events are reported as a maximum severity per patient and type, across all cycles of treatment. All attributions collected for adverse events were reported unless otherwise noted. The Kaplan-Meier method [22] was used to estimate the distributions of time to progression and time to death. All analyses were conducted using SAS version 9.0. Two-sided p-values were reported, and p<.05 is considered statistically significant.
Results
Patient characteristics
Between January, 2004 and November, 2005, a total of 35 patients were accrued onto the study. Twenty-three patients were accrued in North America and twelve patients in Asia. All patients were eligible and began treatment. Baseline patient characteristics are summarized in Table 1. The majority of patients were from the United States (66%) and male (80%). The median age was 61 yrs (21–77). 29 (85%) had Child-Pugh’s classification of A (vs. B). Some patients (20%) had received prior cancer therapy, including: chemoembolization (5 patients), radiofrequency ablation (2 patients), and surgical resection (2 patients). Nearly half (46%) of the patients presented with baseline chronic liver disease such as chronic or viral hepatitis.
Table 1.
Baseline patient characteristics (N=35)
| Characteristic | Frequency |
|---|---|
| Enrollment location | |
| United States | 23 (66%) |
| Pacific | 12 (34%) |
| Median age (Range) | 61 yrs (21–77) |
| ECOG performance status | |
| 0 | 11 (31%) |
| 1 | 23 (66%) |
| 2 | 1 (3%) |
| Male | 28 (80%) |
| Race | |
| Asian | 14 (40%) |
| White | 19 (54%) |
| Black or African American | 1 (3%) |
| Unknown | 1 (3%) |
| Primary disease site—Hepatic | 31 (89%) |
| Locally advanced (vs Metastatic) | 17 (49%) |
| Viral hepatitis | 16 (46%) |
| History of chronic hepatitis | 16 (46%) |
| Pathology | |
| Hepatocellular | 32 (92%) |
| Other | 3 (8%) |
| Child-Pugh’s classification | |
| A | 29 (85%) |
| B | 5 (15%) |
| Prior Therapy | 7 (20%) |
| Types of prior therapy | |
| Chemoembolization | 4 (57%) |
| Surgical resection | 1 (14%) |
| Chemoembolization & radiofrequency | |
| ablation | 1 (14%) |
| Surgical resection & radiofrequency | |
| ablation | 1 (14%) |
Treatment administration
Thirty-five patients completed a total of 88 cycles of treatment (median of 2 cycles, range 1 to 12). At least one bortezomib treatment was omitted for 24 of those cycles and 12 patients required at least one dose reduction on a total of 15 cycles. The reasons for dose reductions were hematologic nadirs (11 cycles) and non-hematologic adverse events (4 cycles- grade 3 diarrhea, skin rash, grade 2 hip pain, grade 1 abdominal pain). Reductions occurred as early as cycle 2 (9 pts) and as late as cycle 10 (1 pt). Reasons for discontinuation of protocol treatment included: progression (60%), refusal (17%), adverse reactions (17%), and miscellaneous reasons (6%, e.g. death during treatment, alternative treatment, or other medical problems).
Toxicity
All 35 patients were evaluable for toxicity. The most common all grade AEs considered possibly related to treatment were thrombocytopenia (71%), diarrhea (46%), anemia (43%), fatigue and leukopenia (both 40%) and nausea (31%). A frequently reported bortezomib related side effect, neuro-sensory changes, was seen at grade 1 (11%) and 2 (6%) intensities. Grade 3 or higher AEs considered at least possibly related to study treatment are summarized in Table 2. Only thrombocytopenia was observed in greater than 10% of patients. Fifteen individuals experienced grade 3 AEs and four patients experienced grade 4 AEs considered at least possibly related to study treatment. Grade 4 events included: thrombosis (2), hepatic failure (1), and hypoglycemia (1).
Table 2.
Maximum severitya (Grade 3+) AEs, Per patient (N=35)
| Adverse event | Grade 3 | Grade 4 |
|---|---|---|
| Thrombocytopenia | 4 (11%) | 0 |
| Anemia | 2 (6%) | 0 |
| Diarrhea | 2 (6%) | 0 |
| Fatigue | 2 (6%) | 0 |
| Rash/Desquamation | 1 (3%) | 0 |
| Thrombosis | 0 | 2 (6%) |
| Hepatic failure | 0 | 1 (3%) |
| SGOT (AST) | 3 (9%) | 0 |
| SGPT (ALT) | 2 (6%) | 0 |
| Hyponatremia | 2 (6%) | 0 |
| Ileus | 1 (3%) | 0 |
| Hyperglycemia | 1 (3%) | 0 |
| Hypoglycemia | 0 | 1 (3%) |
| Hypotension | 1 (3%) | 0 |
| Muscle weakness | 1 (3%) | 0 |
| Dizziness | 1 (3%) | 0 |
| Cognitive disorder | 1 (3%) | 0 |
NCI CTCAE V3.0. Reported are AEs deemed at least possibly related to BORTEZOMIB
Efficacy
Twenty-seven of the 35 patients were evaluable for the primary endpoint, confirmed tumor response. One confirmed partial response was observed in a 71 year old white male who achieved a PR at cycle 6 and continued on bortezomib until cycle 11 (sustained 3.1 months), before moving to an alternate treatment. He ultimately died from this cancer 8.5 months after discontinuing bortezomib while under hospice care at home. The CT scans (Fig. 1) from this patient suggest some degree of tumor necrosis (an effect similarly reported in the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) sorafenib trials)[4]. The confirmed response rate for this trial therefore was 4% (95% CI 0–19%). The pre-study interim analysis criteria dictated that at least 30% of patients (4 of the initial 20 evaluable patients) achieve confirmed tumor responses to warrant continuation of accrual. The trial was thereby permanently closed at the interim analysis although additional patients were accrued while the analysis was being completed. Nine patients experienced a best response of SD, lasting a median of 2.8 months (95% CI 2.8–9.0 months). Overall, the majority (77%) of patients experienced disease progression, and thirty-one patients have died. Of the surviving patients, median follow-up time was 11.4 months (range 1.7–20.2). The estimated time to progression was 1.6 months (95% CI 1.4–2.2 months). The estimated median survival time for all patients was 6.0 months (95% CI 3.9–7.8 months). Table 3 and Fig. 2 provide estimates of the distributions of time to progression and survival.
Fig. 1.
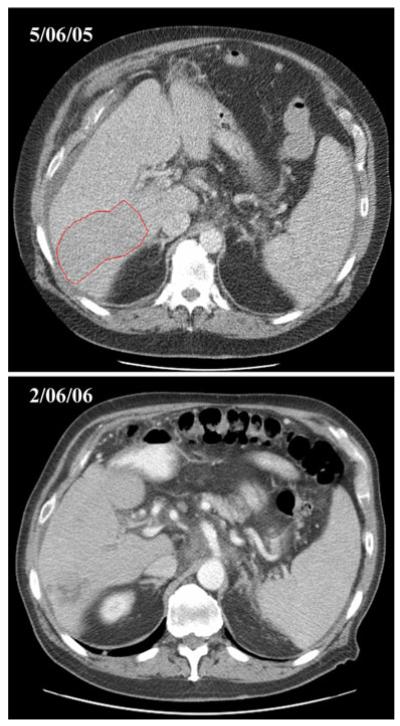
Representative example of baseline and serial follow-up scans demonstrating tumor necrosis in responding hepatocellular carcinoma patient
Table 3.
Patient outcome (N=35)
| Outcome | Estimate |
|---|---|
| Best objective response | |
| Evaluablea | 27 (77%) |
| CR | 0 |
| PR | 1 (4%) |
| STAB | 9 (33%) |
| PROG | 17 (63%) |
| Confirmed CR/PR | 1 (4%) |
| Time to Progression | |
| Progressions | 27 |
| Medianb (95% CI) | 1.6 mos (1.4–2.2) |
| % Progression-Free | |
| 6 mos | 10% (3–32%) |
| 12 mos | 5% (1–30%) |
| Survival | |
| Deaths | 31 |
| Medianb (95%CI) | 6.0 mos (3.9–7.8) |
| % Alive | |
| 6 mos | 47% (33–67%) |
| 12 mos | 29% (17–50%) |
| 18 mos | 16% (7–36%) |
Patients having completed 6-week restaging or having disease progression prior to their initial 6-week restaging. Confidence intervals are based on all evaluable patients, while the final decision to discontinue the study was based on the first 20 evaluable patients and according to the study design
Kaplan-Meier method
Fig. 2.
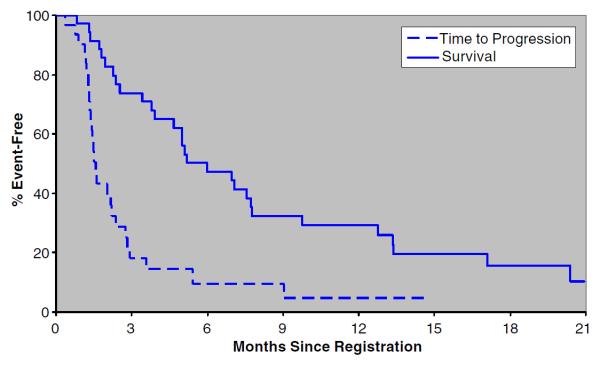
Time to progression and survival (N=35)
Six patients died within 60 days of initiating cycle 1 of study treatment; all were considered unlikely related to therapy. A 61 year old Caucasian male presenting with a history of chronic viral hepatitis, Child-Pugh B, a primary hepatic site of disease and distant nodal metastases, refused further treatment after 18 days and having only grade 1 adverse events reported. This patient subsequently died 23 days later and of reasons secondary to their cancer. A 74 year old Caucasian male, Child-Pugh B, with a primary hepatic site of disease experienced clinical deterioration precluding further treatment by day 21, after experiencing grade 4 liver failure based on encephalopathy considered secondary to study treatment, combined with grade 3 fatigue and thrombocytopenia considered unrelated to study treatment. This patient died 19 days later and of cancer related causes. A 70 year old Caucasian male with a history of kidney stones, symptomatic gout, smoking, hypertension, hemochromatosis managed by phlebotomies, Child-Pugh A function, received only two of the treatments during cycle 1 discontinued treatment due to left upper quadrant pain caused by hepatic and pulmonary metastases. Nearly a month later, this patient was hospitalized due to pain caused from an upper lobe pulmonary embolism. A week later, a CT scan ordered due to extreme shortness of breath and abdominal pain showed catastrophic intraabdominal bleeding, most likely coming from a hyper-vasularized hepatic tumor. The patient died in the emergency room, 25 days after being registered to this study and of a lower GI hemorrhage. A 43 year old Asian male, Child-Pugh A, with advanced localized hepatic disease experienced grade 3 adverse events considered unrelated to treatment (abdominal pain and vomiting) and after receiving 2 cycles of treatment. This patient died 52 days after going on study following the appearance of nodal and hepatic lesions as well as clinical deterioration. A 77 year old Asian male, Child-Pugh B, with localized advanced disease discontinued after receiving one course of treatment due to grade 5 bilirubin considered secondary to the deranged hepatic function. This patient died 55 days after registration and of reasons secondary to their cancer. Finally, a 74 year old Caucasian male with Child-Pugh B function and metastasis on the right adrenal gland discontinued study treatment during their second cycle due to increasing hepatic and metastatic lesions, as well as new lesions. This patient died 59 days after registration.
Discussion
HCC represents one of the most challenging cancers to treat especially in the advanced setting. In general, HCC cells are inherently resistant to most cytotoxic chemotherapy. Patients are afflicted with a tumor burden that affects their performance status but also have compromised hepatic function that limits their tolerance of conventional chemotherapy. In addition, variations in the types of HCC exist and can be attributed to the different etiologies of the disease, for example, HCV versus HBV, and their distinct roles in hepatocarcinogenesis. These distinctions impact which specific molecular derangements are present in a given patient’s cancer and how they will ultimately respond to a specific therapeutic agent. Targeted agents are potentially more useful in HCC treatment since they hypothetically have a more favorable therapeutic index-more direct inhibition of tumor growth with less associated side effects.
The multikinase inhibitor, sorafenib, has increased survival in HCC patients and represents an important new targeted treatment that inhibits signaling of the raf kinase and the VEGF pathway [4–6]. In the pivotal Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol trial [5] that led to sorafenib’s approval, the overall response rate was 2% with only seven patients achieving a partial response and no individuals experiencing a complete response. The positive findings of the trial can be attributed to the ability of sorafenib to delay tumor growth and progression as opposed to producing substantial tumor responses. In the examination of this trial in this context, bortezomib treatment did not result in a significant response rate nor was tumor growth delayed as reflected by the short 1.6-month time to progression. This translated into a median survival equivalent to the control arm of the SHARP trial. It can thus be argued that early pilot investigational trials in HCC may be better served with the endpoint of time to progression or measure of disease stabilization as opposed to a primary endpoint of response as was done in this trial.
The reasons bortezomib exhibited little activity in HCC patients in this trial requires examination. Bortezomib may not significantly inhibit ubiquitin-proteasome pathway in HCC cells, or alternatively, this specific pathway may not be as critical to HCC proliferation and survival as proposed. Additionally, the bortezomib dose and schedule used in this study may be a reason for the lack of activity. Despite sound pre-clinical data that reversal of viral protein-mediated cell proliferation and other malignant processes can be achieved with proteasome inhibition, these alterations may not occur to a significant enough extent to be clinically meaningful. Interestingly, tumor necrosis was observed in the sole responding patient, similar to what was seen the early investigations with sorafenib [4]. Relationships between survival and either hepatitis status or site of enrollment were explored (data not shown). Intriguingly, individuals with underling viral hepatitis had a one-month longer TTP and non-Asia patients experienced a two month longer survival. No difference in outcome was observed based on specific hepatitis viral infection, B vs. C.
In the study, patients received only two cycles on average, one confirmed response was observed, and 77% of individuals experienced disease progression. This either suggests that the enrolled patients had rapidly progressive disease or that bortezomib has essentially no activity in delaying tumor growth. Patients, on average, survived 5 months beyond progression suggesting that subsequent, second-line therapies influenced the observed median survival. It is intriguing to note that in the SHARP trial, up to 48% of patients had prior local or liver-directed therapy, such as radiofrequency ablation or chemoembolization. In our study, only 7 (20%) patients had received prior liver-directed treatment. In both trials, patients had to have exhibited disease progression to be eligible which can be challenging to determine in previously treated hepatic lesions. Progression of only one of several metastases constitutes progression and thus qualifies a patient for study entry. This is important for understanding treatment effects of targeted agents which in general are cytostatic as opposed to cytotoxic.
The observed toxicity was not excessive as the only grade 3 or higher toxicity reported in more than 10% of patients was thrombocytopenia. Six patients died within 60 days of receiving bortezomib, with two individuals dying during active treatment. Furthermore, worsening hepatic function appeared to be a common occurrence in these six individuals. Whether this can directly be attributed to bortezomib is unknown. Interestingly, laboratory studies suggest that proteasome inhibition may attenuate hepatic injury by reducing hepatocyte apoptosis and liver fibrosis [23, 24]. This however occurs with concomitant hepatocyte proliferation. In addition, bortezomib is primarily metabolized by cytochrome P-450 enzymes and decreased clearance may result in patients with hepatic dysfunction.
The issue remains that despite intriguing preclinical rationale for the use of proteasome inhibitors for the treatment of HCC, does bortezomib have a future in HCC? Bortezomib has minimal activity in other cancers including breast, renal cell, colorectal and pancreatic cancers [25–29]. In HCC, a phase I/II trial with bortezomib with the same schedule has been performed [30]. In this trial, 18 patients with measurable, unresectable disease were treated; both Child-Pugh A and B (13/5 patients) and hepatitis B and/or C (7 patients) were included. Doses of 1.0 and 1.3 mg/m2 were evaluated with the latter being declared the MTD. A mean of 4.5 cycles was administered and no responses were observed while seven patients experienced stable disease lasting greater than 8 weeks. Similar to approaches in other cancers and because preclinical data reveals additive interactions, bortezomib combined with adriamycin (15–20 mg/m2 every 21 days) was evaluated in HCC in a phase II by Berlin and colleagues [31]. Only one response (2.3%) was seen while 10 (25.6%) patients had stable disease. Similar to historical reports of adriamycin alone in HCC, the median OS was 5.7 months and PFS was 2.4 months leading the authors to conclude that the combination did not show sufficient activity to warrant further investigation.
In summary, this trial demonstrated that bortezomib, administered alone at a dose of 1.3 mg/m2 twice weekly for two of every 3 weeks, has minimal single-agent clinical activity in patients with advanced, unresectable HCC.
Acknowledgement
Supported by the Phase 2 Consortium Contract (NCI N01 CM17104).
Abbreviations
- HCC
Hepatocellular carcinoma
- RR
Response rate
- AEs
Adverse events
- VEGF
Vascular endothelial growth factor
- UNL
Upper normal limit
- CTCAE
Common terminology criteria for adverse events
- CBCs
Complete blood cell counts
- CR
Complete response
- PR
Partial response
- PD
Progressive disease
- SD
Stable disease
- CIs
Confidence intervals
- SHARP
Sorafenib hepatocellular carcinoma assessment randomized protocol
Contributor Information
George P. Kim, Mayo Clinic Florida, Jacksonville, FL, USA
Michelle R. Mahoney, Mayo Clinic Rochester, Rochester, MN, USA
Daniel Szydlo, Mayo Clinic Rochester, Rochester, MN, USA.
Tony S. K. Mok, National University Hospital, Singapore, Singapore
Robert Marshke, Mayo Clinic Arizona, Scottsdale, AZ, USA.
Kyle Holen, University of Wisconsin Carbone Cancer Center, Madison, WI, USA.
Joel Picus, Washington University, St. Louis, MO, USA.
Michael Boyer, Royal Prince Alfred Hospital, Camperdown, Australia.
Henry C. Pitot, Mayo Clinic Rochester, Rochester, MN, USA
Joseph Rubin, Mayo Clinic Rochester, Rochester, MN, USA.
Philip A. Philip, Karmanos Cancer Institute, Detroit, MI, USA
Anna Nowak, Sir Charles Gairdner Hospital, Perth, Australia.
John J. Wright, National Cancer Institute, Rockville, MD, USA
Charles Erlichman, Mayo Clinic Rochester, Rochester, MN, USA.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the united states. N Engl J Med. 1999;340(10):745–750. doi: 10.1056/NEJM199903113401001. doi:10.1056/nejm199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl 2):S88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase ii study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(26):4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A, Kang Y, Chen Z, Tsao C, Qin S, Kim J, Burock K, Zou J, Voliotis D, Guan ZZ. Randomized phase iii trial of sorafenib versus placebo in asian patients with advanced hepatocellular carcinoma. J Clin Oncol (Meeting Abstracts) 2008;26(15_suppl):4509. [Google Scholar]
- 7.Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor ps-341 in cancer therapy. Clin Cancer Res. 1999;5(9):2638–2645. [PubMed] [Google Scholar]
- 8.Rolfe M, Chiu MI, Pagano M. The ubiquitin-mediated proteolytic pathway as a therapeutic area. J Mol Med. 1997;75(1):5–17. doi: 10.1007/s001090050081. [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22(5):442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Ozturk M. Genetic aspects of hepatocellular carcinogenesis. Semin Liver Dis. 1999;19(3):235–242. doi: 10.1055/s-2007-1007113. [DOI] [PubMed] [Google Scholar]
- 11.Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC. Hepatitis b virus x protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ercc3. Proc Natl Acad Sci USA. 1994;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray RB, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis c virus core protein. J Biol Chem. 1997;272(17):10983–10986. doi: 10.1074/jbc.272.17.10983. [DOI] [PubMed] [Google Scholar]
- 13.Frankel A, Man S, Elliott P, Adams J, Kerbel RS. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor ps-341. Clin Cancer Res. 2000;6(9):3719–3728. [PubMed] [Google Scholar]
- 14.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C. Novel proteasome inhibitor ps-341 inhibits activation of nuclear factor-kappa b, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7(5):1419–1428. [PubMed] [Google Scholar]
- 15.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS., Jr Enhanced chemosensitivity to cpt-11 with proteasome inhibitor ps-341: Implications for systemic nuclear factor-kappab inhibition. Cancer Res. 2001;61(9):3535–3540. [PubMed] [Google Scholar]
- 16.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26s proteasome. J Surg Res. 2001;100(1):11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 17.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos M-V, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG, The VTI. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. doi:10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 18.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase ii clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Duffy D, Santner T. Confidence intervals for a binomial parameter based on multistage tests. Biometrics. 1987;43:81–93. [Google Scholar]
- 22.Kaplan MP. Nonparametirc estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Chang L, Kamata H, Solinas G, Luo J-L, Maeda S, Venuprasad K, Liu Y-C, Karin M. The e3 ubiquitin ligase itch couples jnk activation to tnf[alpha]-induced cell death by inducing c-flipl turnover. Cell. 2006;124(3):601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Anan A, Baskin-Bey ES, Isomoto H, Mott JL, Bronk SF, Albrecht JH, Gores GJ. Proteasome inhibition attenuates hepatic injury in the bile duct-ligated mouse. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G709–716. doi: 10.1152/ajpgi.00126.2006. doi:10.1152/ajpgi.00126.2006. [DOI] [PubMed] [Google Scholar]
- 25.Davis NB, Taber DA, Ansari RH, Ryan CW, George C, Vokes EE, Vogelzang NJ, Stadler WM. Phase ii trial of ps-341 in patients with renal cell cancer: a university of chicago phase ii consortium study. J Clin Oncol. 2004;22(1):115–119. doi: 10.1200/JCO.2004.07.165. doi:10.1200/jco.2004.07.165. [DOI] [PubMed] [Google Scholar]
- 26.Engel RH, Brown JA, Von Roenn JH, O’Regan RM, Bergan R, Badve S, Rademaker A, Gradishar WJ. A phase ii study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Investig. 2007;25(8):733–737. doi: 10.1080/07357900701506573. [DOI] [PubMed] [Google Scholar]
- 27.Mackay H, Hedley D, Major P, Townsley C, Mackenzie M, Vincent M, Degendorfer P, Tsao M-S, Nicklee T, Birle D, Wright J, Siu L, Moore M, Oza A. A phase ii trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11(15):5526–5533. doi: 10.1158/1078-0432.CCR-05-0081. doi:10.1158/1078-0432.ccr-05-0081. [DOI] [PubMed] [Google Scholar]
- 28.Kozuch PS, Rocha-Lima CM, Dragovich T, Hochster H, O’Neil BH, Atiq OT, Pipas JM, Ryan DP, Lenz H-J. Bortezomib with or without irinotecan in relapsed or refractory colorectal cancer: results from a randomized phase ii study. J Clin Oncol. 2008;26(14):2320–2326. doi: 10.1200/JCO.2007.14.0152. doi:10.1200/jco.2007.14.0152. [DOI] [PubMed] [Google Scholar]
- 29.Alberts SR, Foster NR, Morton RF, Kugler J, Schaefer P, Wiesenfeld M, Fitch TR, Steen P, Kim GP, Gill S. Ps-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a north central cancer treatment group (ncctg) randomized phase ii study. Ann Oncol. 2005;16(10):1654–1661. doi: 10.1093/annonc/mdi324. [DOI] [PubMed] [Google Scholar]
- 30.Hegewisch-Becker S, Sterneck M, Schubert U, Rogiers X, Guerciolini R, Pierce JE, Hossfeld DK. Phase i/ii trial of bortezomib in patients with unresectable hepatocellular carcinoma (hcc) J Clin Oncol (Meeting Abstracts) 2004;22(14_suppl):4089. [Google Scholar]
- 31.Berlin JD, Powell ME, Su Y, Horton L, Short S, Richmond A, Kauh JS, Staley CA, Mulcahy M, Benson AB., III Bortezomib (b) and doxorubicin (dox) in patients (pts) with hepatocellular cancer (hcc): A phase ii trial of the eastern cooperative oncology group (ecog 6202) with laboratory correlates. J Clin Oncol (Meeting Abstracts) 2008;26(15_suppl):4592. [Google Scholar]


