Abstract
We explored the function of endogenous type I interferons (IFN-1) in the colon using the T cell adoptive transfer model of colitis. Colon mononuclear phagocytes (MP) constitutively produced IFN-1 in a TRIF-dependent manner. Transfer of CD4+CD45RBhi T cells from wild type (WT) or interferon α/β receptor subunit 1 knockout (IFNAR1−/−) mice into RAG−/− hosts resulted in similar onset and severity of colitis. In contrast, RAG−/− x IFNAR1−/− double knockout (DKO) mice developed accelerated severe colitis compared to RAG−/− hosts when transferred WT CD4+CD45RBhi T cells. IFNAR signaling on host hematopoietic cells was required to delay colitis development. MPs isolated from the colon lamina propria of IFNAR1−/− mice produced less IL-10, IL-1 receptor antagonist (IL-1RA) and IL-27 compared to WT MPs. Accelerated colitis development in DKO mice was characterized by early T cell proliferation and accumulation of CD11b+CD103− dendritic cells in the mesenteric lymph nodes, both of which could be reversed by systemic administration of IL-1RA (anakinra). Co-transfer of CD4+CD25+ regulatory T cells (Tregs) from WT or IFNAR1−/− mice prevented disease caused by CD4+CD45RBhi T cells. However, WT CD4+CD25+Foxp3GFP+ Tregs co-transferred with CD4+CD45RBhi T cells into DKO hosts failed to expand or maintain Foxp3 expression and gained effector functions in the colon. These data are the first to demonstrate an essential role for IFN-1 in the production of anti-inflammatory cytokines by gut MPs and the indirect maintenance of intestinal T cell homeostasis by both limiting effector T cell expansion and promoting Treg stability.
Introduction
Type I interferons (IFN-1) are a family of cytokines that signal through a common interferon-α/β receptor (IFNAR) and can have both pro- and anti-inflammatory effects. In addition to enhancing NK, B and CD8+ T cell activity, IFN-1 can influence CD4+ T cell differentiation and function via their effects on dendritic cells (DCs). IFN-1 drives DC activation and maturation (1, 2), MHC II expression, and production of IL-12 (3-6) to augment Th1 cell responses. In addition, IFN-1 can act directly on T cells to inhibit their egress from lymph nodes, thus promoting DC-T cell interactions (7). Furthermore, IFNAR signaling on T cells activated in peripheral tissues enhances their survival (8). Consistent with these immune activating effects, type I interferons are essential for driving T cell responses to vaccination with adjuvants, and are themselves being explored as vaccine adjuvants in humans (9).
In contrast, IFN-1 can suppress immune responses by several mechanisms, and are used to treat multiple sclerosis. For example, IFN-1 can drive the production of anti-inflammatory cytokines including IL-10, IL-27 and IL-1 receptor antagonist (IL-1RA) from mononuclear phagocytes (MP), and of regulatory SOCS and PIAS proteins in T cells and MPs (10-14). In addition, IFN-1 can inhibit the secretion of IL-1β, both by suppressing pro-IL-1β production and by inhibiting pro-IL-1β cleavage to mature IL-1β by blocking inflammasome activation (15). Furthermore, IFN-1 can inhibit Th17 cell differentiation by inhibiting IL-1β and osteopontin, and inducing IL-27 production by MPs (13, 16, 17). Finally, IFN-1 can inhibit inflammatory responses that they promote in other contexts. For example, IFN-1 suppresses IFN-γ-induced MHC II expression, perhaps as a negative feedback mechanism (18), and high levels can inhibit IL-12 production during certain viral infections (19). IFN-1 can also either induce or inhibit IFN-γ production by NK and T cells depending on the balance of STAT4 and STAT1 signaling, allowing opposing cell- and context-specific effects on immune cells (20).
The role of IFN-1 in intestinal inflammation is poorly understood. In prior studies of dextran sulfate sodium (DSS)-induced acute colitis in mice, CpG oligodeoxynucleotide administration prevented disease in a IFN-1 and CD11c+ cell dependent manner (21, 22). Furthermore, IFNAR1−/− mice were more susceptible to DSS-induced colitis (22). Similarly, poly (I:C) treatment attenuated T cell-mediated colitis via IFN-1 signaling directly on the T cells (23). Direct treatment of T cells with IFN-1 could also limit their colitogenic potential (24). Although clinical trials using IFN-1 to treat human inflammatory bowel disease (IBD) have been met with limited success (25, 26), a recent genome-wide association study has implicated the locus containing IFNAR in the risk for developing human IBD (27). In the current study, we explored the role of endogenous IFN-1 in regulating chronic colitis, using the T cell adoptive transfer model (28), which more accurately reflects the chronic inflammation of human Crohn’s disease (29). We found a critical role for IFNAR signaling on host innate immune cells in controlling colitis development by regulating T cell accumulation, Treg function, and the production of regulatory cytokines by colon MPs.
Materials and Methods
Mice
WT C57BL/6 (CD45.2) mice were purchased from National Cancer Institute. SJL (CD45.1), IFNAR1−/− and RAG−/− mice, all on a C57BL/6 background were obtained from Taconic Farms bred on contract with NIAID. DKO mice were generated by crossing RAG−/− and IFNAR1−/− mice and screening for offspring homozygous for both genes. Foxp3-GFP mice were originally from V. Kuchroo. TRIF−/−, MyD88−/−, and their WT control mice were purchased from Jackson Labs. TRIF−/−/MyD88−/− mice were a gift from A. Sher. All mouse experimental protocols were approved by the NIAID Animal Care and Use Committee, and mice were housed under SPF conditions in NIAID animal facilities.
Antibodies and reagents
Antibodies used for staining were against CD4 (RM4-5), CD11b (M1/70), CD11c (N418), CD19 (eBio1D3), CD25 (PC61.5), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD45RB (C363.16A), CD62L (MEL-14), CD103 (2E7), MHC Class II IA/IE (M5/114.15.2), TCRβ (H57-597), TCRγδ (eBioGL3), F4/80 (BM8), Foxp3 (FJK-16s), IL-17A (eBio17B7), IFN-γ (XMG1.2), or isotype controls. All were purchased from eBioscience. Anti-murine IFNAR1 (MAR1-5A3) was purchased from Biolegend. Murine IFN-αA was purchased from PBL. Anakinra (Kineret®, Amgen) was kindly provided by Dr. Raphaela Goldbach-Mansky, NIAMS, NIH.
Induction of colitis
CD4+ T cells were enriched from the spleens of WT, IFNAR1−/− or Foxp3-GFP mice by mechanical disruption followed by ACK red blood cell lysis, and negative selection for CD4+ cells with magnetic beads (CD4+ T cell isolation kit, Miltenyi). CD4+ T cell subpopulations were sorted by flow cytometry based on expression of CD4, CD25, CD45RB and Foxp3-GFP. 4×105 CD4+CD25−CD45RBhi T cells, sorted on the 30% of CD4+CD25− cells with the highest expression of CD45RB, were injected intraperitoneally with or without 1×105 CD4+CD25+CD45RBlo cells. Under these sorting conditions, CD4+CD25−CD45RBhi T cells routinely contained less than 0.02% Foxp3+ cells and CD4+CD25+CD45RBlo cells contained 95% FoxP3+ cells from all mouse strains. For some experiments, CD4+CD25−CD45RBhi T cells and CD4+CD25+CD45RBlo Tregs were sorted based on negative or positive expression of Foxp3-GFP, respectively. For the CFSE dilution studies, 1×106 sorted CD4+CD25−CD45RBhi T cells were labeled with CFSE (Invitrogen, 10 μM) according to the manufacturer’s protocol prior to intraperitoneal injection. For the anakinra treatment experiments, 1 mg of anakinra in PBS was injected i.p. on the same day as the T cell transfer followed by 1 mg daily for nine additional days. Clinical signs of colitis including weight loss and diarrhea were monitored bi-weekly. H&E stained sections from the proximal, mid and distal colon were scored for histopathology as previously described (30).
Generation of bone marrow chimeras
Following 900 rads of γ-radiation, RAG−/− mice were intravenously injected with 3×106 bone marrow cells from RAG−/− or DKO mice. Mice were used for experiments 8 weeks later. >95% of hematopoietic cells were routinely of donor origin.
Isolation of mononuclear cell populations
Mesenteric lymph nodes (MLNs) were minced and placed in digestion buffer [Iscove’s Modified Dulbecco’s Medium (IMDM; Gibco) with 5% fetal calf serum, 0.17 mg/ml liberase TL (Roche) and 30 μg/ml DNAse I (Roche)] for 30 minutes with continuous shaking at 37°C. Digested MLNs were centrifuged and resuspended in warm HBSS with 10% FCS, 5 mM EDTA, and 25 mM HEPES for 5 min with shaking at 37°C. MLNs were then mashed through a 40 μm filter and washed with PBS.
For colon lamina propria (cLP) cell isolation, colons were rinsed of mucus and feces and cut longitudinally and into 2 cm pieces. Colon pieces were incubated in warm HBSS with 10% FCS, 5 mM EDTA, 15 mM HEPES and 0.015% dithiothreitol for 15 minutes with continuous shaking at 37°C. Colon pieces were shaken vigorously and washed at least five times or until the supernatant cleared to remove the epithelial cell layer. Colon pieces were then minced and placed into digestion buffer for one hour with continuous shaking at 37°C. Colon pieces were mashed through a 40 μm filter. In some cases, the cLP cell suspension was enriched for mononuclear phagocytes using a Nycodenz gradient (Accurate Chemical) according to the manufacturer’s instructions as previously described (31). Specific MP populations were sorted or analyzed by flow cytometry by gating on live, Lin (TCRβ, TCRγδ, CD19)−, MHC IIhi, and F4/80+ or CD11c+ cells as indicated, and as previously described (31).
In vitro cell stimulations
Colon or MLN MPs (1×106/ml in complete RPMI media) from steady state mice were cultured for 24 hours with or without FSL-1 (Invivogen; 500 ng/ml), flagellin (Invivogen; 1 μg/ml), anti-IFNAR1 (5 μg/ml) or IFN-αA (500 U/ml) in complete RPMI. Cytokine production was detected by multiplex immunoassay (Aushon Biosystems), ELISAs for IL-10 and IL-1RA (R&D Systems) or ELISA for IFN-β (PBL).
cLP cells (1×106/ml) from colitic mice were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) in complete RPMI. After two hours, Golgi Stop (BD) was added and left in culture for an additional three hours before analysis by flow cytometry.
Quantitative RT-PCR
RNA was extracted using Qiagen RNEasy Mini Kit, converted to cDNA (Quanta qScript cDNA supermix) and used in a PCR reaction with PerfeCTa qPCR FastMix (Quanta), FAM-labeled probes and primers for genes of interest. Quantitative expression was normalized to transcript levels of GAPDH using the formula 1/2ΔCT.
Flow cytometry
Cells were stained with LiveDead conjugated dye (Invitrogen) and antibodies against extracellular markers for 20 minutes at 4°C. Intracellular Foxp3 was stained using a Foxp3 intracellular staining kit (eBioscience) per the manufacturer’s instructions. Cells were analyzed on a LSR II flow cytometer (BD).
Statistics
Body weight curves were analyzed using one-way ANOVA and Bonferroni post-test corrections. In vivo studies using individual mice were analyzed using the non-parametric Mann-Whitney test. In vitro data using cells pooled from several mice were analyzed using a student t-test. Analysis was performed using Prism 5 software (GraphPad). *p<0.05, **p<0.01, ***p<0.001.
Results
Constitutive type I interferon production is TRIF-dependent
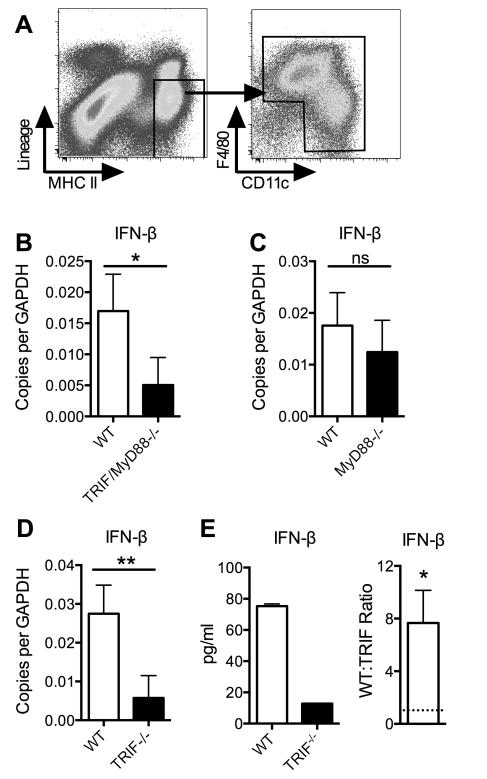
Prior studies showed that lamina propria (LP) DCs from the small intestine constitutively produce IFN-β (32). FACS sorted MP subsets from the colon (as gated in Fig. 1A) also constitutively produced IFN-β (Fig. 1B-E). Transcription of IFN-β was independent of the TLR adapter molecule, MyD88, but dependent on TRIF, an adapter molecule that mediates signaling by TLR3, TLR4, and intracellular helicases that sense dsRNA (33). Isolated, cultured colon MPs also constitutively secreted IFN-β in a TRIF-dependent manner (Fig. 1E).
Figure 1. Constitutive production of IFN-1 by myeloid cells is TRIF-dependent.
(A) Lamina propria cells isolated from the colon were stained for the indicated surface markers. Lineage markers used were TCR-β, TCR-γδ and CD19. (B-D) Cells were sorted as in (A) from WT B6 and specified gene-knockout mice. mRNA from ex vivo cells levels determined by quantitative RT-PCR analysis are shown. Data shown are the mean values ± SD of three or four independent experiments, each with a minimum of three mice per group. (E) Cells were sorted as in (A) from WT B6 and TRIF−/− mice and placed in culture without stimulation. Protein levels in the culture supernatant were measured after 24 hours. Data shown are mean values ± SD for one representative experiment (left panel) and ratios of WT to TRIF−/− MP production of IFN-β (right panel). Ratios from three independent experiments with similar results were compared to a ratio of “1” (dashed line) using the one column t-test. *p<0.05, **p<0.01, ***p<0.001.
IFNAR signaling on RAG−/− host cells suppresses the induction of colitis
We next explored the role of endogenous IFN-1 in immune homeostasis using the T cell transfer model of colitis (28). Adoptive transfer of CD4+CD45RBhi T cells into RAG−/− mice results in intestinal inflammation in 8-10 weeks, driven by naïve T cells responding to IL-23 and commensal bacterial antigens without sufficient control by Tregs (28, 30, 34). While prior studies demonstrated that induced or exogenous sources of IFN-1 can impart regulatory properties directly on CD4+ T cells (23, 24, 35), we found that RAG−/− hosts transferred CD4+CD25−CD45RBhi T cells from either WT or IFNAR1−/− mice resulted in similar weight loss and colon histopathology (Fig. 2A), as well as a similar number and phenotype of pathogenic T helper cells in the colon (data not shown). In contrast, WT CD4+CD45RBhi T cells transferred into RAG−/− x IFNAR1−/− double knockout (DKO) mice resulted in accelerated colitis compared to RAG−/− mice as evidenced by weight loss, histopathology, and accumulation of CD4+ T cells in the colon (Fig. 2B-C). While the proportion of pathogenic colon T cells producing IFN-γ in RAG−/− and DKO mice was similar, the proportion producing IL-17 alone, or producing IL-17 and IFN-γ, was higher among T cells from DKO mice (2D-E). The proportion of CD4+ T cells expressing Foxp3, however, was equivalent in RAG−/− and DKO mice (Fig. 2F).
Figure 2. Host cell type I interferon signaling suppresses colitis.
(A) WT or IFNAR1−/− CD45RBhiCD4+ T cells were transferred into RAG−/− mice. (B) WT CD45RBhiCD4+ T cells were transferred into RAG−/− or DKO mice. For (A) and (B), mice were monitored for weight loss and scored for histopathology. Representative photomicrographs of H&E stained sections are shown in (B). Bar, 100 μm. (C) CD4+ T cells from the cLP of mice in (B) were counted. (D, E) Cells from (C) were restimulated and analyzed for cytokine production by intracellular staining. (F) Cells from (C) were analyzed for Foxp3 expression by intracellular staining. Data shown is pooled from four (A) or three (B,C, E, and F) independent experiments or representative of three independent experiments with similar results (D). Each experiment contained a minimum of three mice per group. Mean values from individual mice ± SD are shown for weight curves and were analyzed by two-way ANOVA with Bonferroni post-test corrections. Median values are shown for all other plots and were analyzed using a Mann-Whitney non-parametric t-test. *p<0.05, **p<0.01, ***p<0.001.
IFNAR signaling on RAG−/− host hematopoietic cells is required to suppress colitis
Most cells in the body express IFNAR, including both hematopoietic and non-hematopoietic cells. To determine the cell types responsible for the indirect effects of IFNAR signaling on T cell expansion and colitis development, we reconstituted irradiated RAG−/− mice with BM from RAG−/− or DKO mice. Two weeks after the transfer of WT CD4+CD45RBhi T cells, DKO BM chimeric mice exhibited rapid weight loss (Fig. 3A), worse histopathological features (Fig. 3A) and a greater accumulation of CD4+ T cells in the colon than RAG−/− BM chimeric mice (Fig. 3B). No difference was observed in the proportion of cLP CD4+ T cells producing IFN-γ between DKO and RAG−/− BM chimeric mice, but the percentage of CD4+ T cells from DKO BM chimeric mice producing IL-17 or IL-17 and IFN-γ was significantly higher than that from RAG−/− BM chimeric mice (Fig. 3C), consistent with data from DKO recipients (Fig. 2). Also resembling DKO mice, DKO BM chimeras showed no difference in the proportion of Foxp3+ cells among CD4+ T cells (Fig. 3D).
Figure 3. IFNAR signaling is required on hematopoietic cells to suppress colitis.
WT CD45RBhiCD4+ T cells were transferred into irradiation RAG−/− mice reconstituted with BM from RAG−/− or DKO mice. (A) Weight loss and histopathology are shown. cLP cells were isolated, counted (C), and restimulated for intracellular detection of cytokine production (D) or left unstimulated for intracellular Foxp3 expression (E). Data shown is pooled from three independent experiments, each containing a minimum of three mice per group. Mean values from individual mice ± SD are shown for weight curves and were analyzed by two-way ANOVA with Bonferroni post-test corrections. Median values are shown for all other plots and were analyzed using a Mann-Whitney non-parametric t-test. **p<0.01, ***p<0.001.
Accelerated effector T cell expansion was found in the MLNs in the absence of host IFNAR signaling
The increased accumulation of effector T cells in the cLP of DKO or DKO BM chimeric mice may be due to enhanced priming and proliferation of T cells in mucosal lymphoid organs or to local expansion and/or survival in the cLP. To address this issue, we transferred CFSE-labeled CD4+CD45RBhi T cells into RAG−/− or DKO recipients. Seven to ten days post-transfer, before colitis was evident, T cell expansion in the MLNs was greatly enhanced in DKO compared to RAG−/− hosts (Fig. 4A, 4E). The majority of CD4+ T cells recovered from the MLNs of DKO hosts had undergone rapid proliferation, indicated by complete dilution of CFSE. In contrast, RAG−/− recipients had a larger proportion of CD4+ T cells with fewer (0-3) divisions (Fig. 4B). Proliferating CD4+ T cells recovered from RAG−/− recipients expressed CD62L, a marker of naïve T cells and of T cells undergoing homeostatic proliferation, while the vast majority of CD4+ T cells from DKO recipients expressed low levels of CD62L and high levels of CD44 (Fig. 4C-D), indicative of effector and memory T cells.
Figure 4. Accelerated effector T cell proliferation in the MLNs of DKO mice.
MLNs were dissected from RAG−/− and DKO mice 7 days after transfer with CD45RBhiCD4+ T cells. Cells were gated on CD4+ T cells (A) and analyzed for CFSE dilution (B), CD62L expression (C), and CD44 expression (D). Data shown in (A-D) is from one experiment representative of two independent experiments with similar results, each with at least three mice per group. (E) Mean absolute number of CD4+ T cells ± SD, isolated from the MLNs 7-10 days after transfer with CD45RBhiCD4+ T cells, is shown. Data shown is compiled from three independent experiments with similar results. MLNs were pooled from 3-5 mice per group in each experiment. *p<0.05.
Colon mononuclear phagocytes require IFNAR signaling for anti-inflammatory cytokine production
Because the accelerated colitis observed in DKO mice was dependent on a lack of IFNAR signaling on host hematopoietic cells, we next evaluated the role of IFNAR signaling on MPs. We isolated MPs from the cLP of WT and IFNAR1−/− mice (as gated in Fig. 1A). MPs can be further subdivided into distinct populations of macrophages and DCs with specialized functions. F4/80hi colon macrophages produce high levels of IL-10 and IL-1RA and lower levels of IL-27 while CD11c+F4/80int/lo DC subsets stimulate T cell proliferation and produce pro-inflammatory cytokines such as IL-12, Il-23, IL-6, TNF-α, and IL-1 Since the local balance of these regulatory macrophage and stimulatory DC populations could influence immunological homeostasis in the colon (31, 36), we next determined whether the distribution of these distinct MP populations was altered in the absence of IFNAR signaling. However, we did not find a change in either the distribution or level of activation of these populations in the steady state colon (Supplemental Figure 1A-B).
We next investigated cytokine production by cLP MPs in WT and IFNAR1−/− mice (Fig. 5A). IFNAR1−/− MPs produced significantly lower amounts of the anti-inflammatory cytokine, IL-10, either with or without in vitro stimulation with FSL-1 and flagellin, TLR ligands chosen due to the high expression of their receptors, TLR2/6 and TLR5, respectively, on intestinal MPs (31). MPs from IFNAR1−/− mice also showed defects in the production of IL-27 and IL-1RA, two other anti-inflammatory cytokines. Constitutive production of IFN-1 was necessary for optimal anti-inflammatory cytokine production as treatment with an anti-IFNAR1 antibody also decreased IL-10 and IL-1RA production from WT cells (Fig. 5B). Addition of exogenous IFN-1 did not, however, enhance the production of these cytokines.
Figure 5. Colon mononuclear phagocytes require IFNAR signaling for anti-inflammatory cytokine production.
(A) FACS sorted MPs (as gated in Fig. 1A) from WT or IFNAR1−/− colons were cultured for 24 hours ± FSL-1 or flagellin. Culture supernatants were analyzed for cytokine levels. (B) FACS sorted MPs (as gated in Fig. 1A) from WT colons were cultured for 24 hours in the indicated conditions. Data shown are means ± SD from three independent experiments, each with ten (A) or five (B) mice per group. Statistics were calculated using a student’s t-test. *p<0.05, **p<0.01, ***p<0.001.
In contrast, production of the pro-inflammatory cytokines, IL-1α, IL-23, and TNF-α was not affected by IFNAR signaling, though IFNAR1−/− MPs produced marginally lower quantities of IL-6 upon FSL-1 stimulation (Fig. 5A).
Treatment with IL-1RA can reverse accelerated T cell proliferation seen in DKO mice
We next asked whether the defect in anti-inflammatory cytokine production in IFNAR1−/− MPs could explain the accelerated colitis. We initially treated DKO mice transferred WT CD4+CD45RBhi cells with recombinant IL-10, but were unable to see any effect on colitis development (data not shown). We next treated mice with anakinra, a recombinant form of human IL-1RA used in the treatment of rheumatoid arthritides (37), which also blocks IL-1 activity in mice (38). Treatment with anakinra reduced the expansion of CD4+ T cells in the MLNs of DKO mice ten days after transfer of CD4+CD45RBhi cells, but had little effect on T cell expansion in RAG−/− mice (Fig. 6A and Supplemental Figure 2A, 2C).
Figure 6. Rapid early T cell proliferation is accompanied by accumulation of CD11b+CD103-cells and can be inhibited by IL-1RA.
MLNs were dissected from RAG−/− and DKO mice 10 days after transfer with CD45RBhiCD4+ T cells. Mice were treated with either PBS or anakinra daily for the course of the experiment. (A) FACS plots pre-gated on live cells are shown. (B) FACS plots pre-gated on live CD11c+ cells are shown. Data shown is from one representative experiment with five mice per group. A repeat of this data is shown in Supplemental Figure 3.
The enhanced T cell proliferation in DKO mice may be due to enhanced priming of T cells by DCs in the MLNs. Early after T cell transfer, we found an influx of CD11c+CD11b+CD103− cells into the MLNs of DKO mice (Fig. 6B and Supplemental Figure 2B-C). A similar population of cells also expands in the cLP during inflammation, migrates from the colon tissue to the MLNs and is capable of priming T cells (31, 39). Anakinra treatment reduced the accumulation of CD11c+CD11b+CD103− cells in the MLNs of DKO mice without affecting the accumulation of CD11c+CD11b+CD103+ or CD11c+CD11b-CD103+ cells (Fig. 6B and Supplemental Figure 2B-C). Thus, IL-1RA may control T cell proliferation indirectly by inhibiting migration of this inflammatory antigen presenting cell population from the cLP to the MLNs, consistent with previous reports that IL-1 signaling directly on CD4+ T cells is dispensable for T cell proliferation in the MLNs in this model of colitis (40).
DKO mice cannot maintain the Foxp3+ Treg population
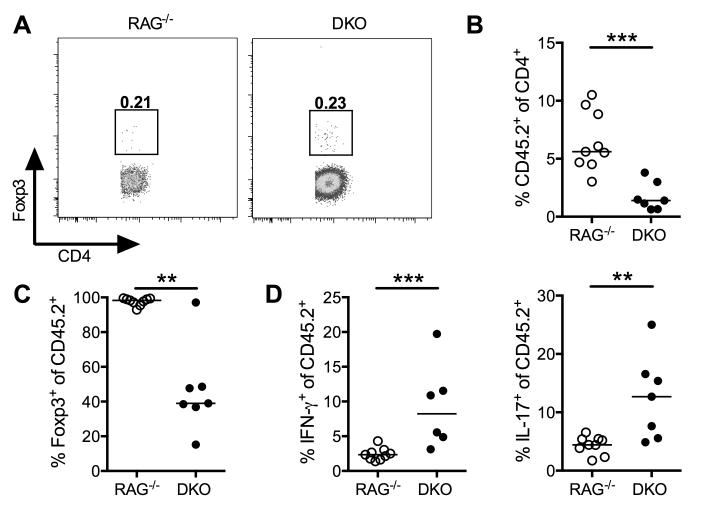
The adoptive T cell transfer model of colitis can also be used to study the function of regulatory T cells since co-transfer of CD4+CD25+CD45RBloFoxp3+ Tregs can inhibit the development of colitis driven by CD4+CD45RBhi T cells (28, 41). Furthermore, de novo induced Tregs can also limit the severity of CD4+ T cell-driven colitis (42, 43). We first checked whether CD4+CD45RBhi T cells transferred into DKO hosts were less likely to differentiate into Tregs. However, ten days after CD4+CD45RBhi T cell transfer, Tregs were equivalently induced in the MLNs of both mouse strains (Fig. 7A).
Figure 7. DKO mice cannot maintain the Foxp3+ Treg population.
(A) CD45RBhiCD4+ T cells were transferred into RAG−/− or DKO mice. Ten days post-transfer, CD4+ T cells isolated from the MLNs were analyzed for Foxp3 expression. (B-D) CD45.1+RBhiCD4+ T cells were co-transferred with CD45.2+CD4+Foxp3GFP+ Tregs into RAG−/− or DKO mice. Four weeks post-transfer, CD4+ T cells were isolated from the colon. Expansion (B) and Foxp3-GFP expression (C) of CD45.2+ T cells was evaluated. cLP cells were restimulated (D) and analyzed by intracellular flow cytometry for effector cytokine production. Data shown is representative of two independent experiments, each with at least five mice per group (A) or pooled from two independent experiments, each with at least three mice per group (B-D). Median values from individual mice are shown and were analyzed using a Mann-Whitney non-parametric t-test. **p<0.01, ***p<0.001.
Although DKO mice showed no defect in Treg induction, we next determined whether defects in Treg expansion and/or maintenance were observed in the absence of IFNAR signaling. We co-transferred CD45.1+CD4+CD45RBhi T cells with CD45.2+CD25+CD45RBloFoxp3GFP+ Tregs into RAG−/− or DKO recipients. Four weeks after T cell transfer, CD45.2+ T cells constituted a smaller percentage of total CD4+ cells in the cLP of DKO mice compared to RAG−/− mice (Fig. 7B), reflecting a relative impairment in Treg accumulation. Among colon CD45.2+ cells, DKO mice had significantly fewer Foxp3+ cells (Fig. 7C), reflecting a loss of Foxp3 in transferred Foxp3+ Treg cells. Furthermore, a fraction of colon CD45.2+ cells had gained effector function, producing IFN-γ and/or IL-17 (Fig. 7D). However, Tregs by themselves were unable to induce colitis as CD4+CD25+FoxpGFP+ cells were unable to expand in either RAG−/− or DKO hosts without the co-transfer of CD4+CD25−CD45RBhi T cells (data not shown). Together, these studies indicate that DKO mice, while able to generate de novo Foxp3+ Tregs, had a poor capacity to maintain a stable population of Tregs over time.
Finally, we checked whether direct IFNAR signaling on Tregs was necessary for their suppressive function. We co-transferred CD4+CD25+ T cells from WT and IFNAR1−/− mice with WT CD4+CD45RBhi T cells into RAG−/− recipients. CD4+CD25+ T cells from either mouse strain was able to suppress weight loss, development of histopathology, accumulation of CD4+ T cells in the colon, and production of IFN-γ by colon CD4+ T cells (Supplemental Figure 3). Transfer of either genotype Treg enhanced the induction of IL-17-producing CD4+ T cells, as has been shown in other models of in vivo Th17 cell differentiation (44, 45). We also found an equal percentage of Foxp3+ T cells among total CD4+ T cells, showing that WT and IFNAR1−/− Tregs were expanded and maintained equivalently in the lymphopenic environment.
Discussion
Taken together, the data presented here indicate for the first time a clear indirect role for IFNAR signaling in regulating mucosal T cell responses during colitis. Using the adoptive transfer model, we found a predominant effect of IFN-1 on innate immune cells in the RAG−/− host rather than directly on CD4+CD45RBhi naïve T cells or CD4+CD25+ Tregs to affect their colitogenic or protective properties, respectively (Fig. 2A and Supplemental Figure 3). Lack of IFNAR signaling on RAG−/− host hematopoietic cells resulted in accelerated severe colitis following adoptive transfer of WT CD4+CD45RBhi T cells. Colitis in DKO hosts was associated with rapid T cell proliferation in the MLNs and preferential accumulation of Th1 cells in the cLP. In addressing the effects of IFNAR signaling on innate immune cells, we found that colon mononuclear phagocytes from IFNAR1−/− mice were defective in their ability to produce the regulatory cytokines IL-10, IL-27, and IL-1RA in response to TLR activation. Furthermore, the enhanced expansion of T cells in the MLNs of DKO mice, as well as increased accumulation of CD11c+CD11b+CD103− mononuclear cells, was inhibited by the exogenous administration of soluble IL-1RA (anakinra), suggesting that control of the IL-1/IL-1RA balance by endogenous IFN-1 may be an important regulatory mechanism in the colon to maintain immune homeostasis. Finally, we found that the stability of Foxp3+ Tregs when co-transferred with CD4+CD45RBhi T cells was negatively affected by the absence of IFNAR signaling on host innate immune cells, resulting in outgrowth of Th1 and Th17 effector cells from the Foxp3+ Treg pool in the inflamed colon.
Our finding that a direct effect of IFNAR signaling on CD4+ T cells is dispensable in this model is not consistent with prior reports indicating that direct IFNAR signaling modulates the colitogenic and regulatory potential of CD4+ T cells. In particular, in a different T cell adoptive transfer model, in vitro pre-treatment of CD4+CD62Lhi T cells with IFN-1 prior to their transfer into BALB/c RAG−/− mice reduced their colitogenic potential, and the inhibitory effects of CpG administration on the colitogenic potential of CD4+CD62Lhi T cells was dependent on IFNAR signaling on the T cells (24). These discrepant results may be due to the differences between acute effects of high dose exogenous IFN-1 treatment of donor T cells and the effects of constitutive IFN-1 on the ability of innate immune cells to indirectly regulate the colitogenic potential of CD4+ T cells. However, in other studies, CD4+CD45RBlo cells from IFNAR1−/− mice were poorly protective and actually pathogenic upon transfer to RAG-deficient hosts (35). These results are inconsistent with the current findings, and cannot be easily explained, except to suggest that differences in endogenous commensal bacteria or the possibility of occult viral infection in the absence of IFNAR signaling in some mouse colonies may affect the regulatory potential of CD4+CD45RBlo T cell populations. In the current studies, we clearly demonstrate that CD4+CD25+CD45RBlo T cells from IFNAR1−/−mice suppress clinical disease and IFN-γ production by effector T cells (Supplemental Figure 3).
We determined that rapid early proliferation of CD4+ T cells in MLNs of DKO mice was followed by accumulation of effector CD4+ T cells in the cLP (Fig. 4). Rapid proliferation suggests foreign antigenic stimulation and/or “spontaneous proliferation,” an IL-7 independent mechanism of proliferation seen in chronic lymphopenic conditions (46). Slow proliferation, as was exhibited by the T cells transferred into RAG−/− mice, is more characteristic of homeostatic proliferation driven by self MHC-peptide ligands, IL-7 and IL-15 (47). Accordingly, the rapidly proliferating T cells isolated from DKO mice were CD44hiand CD62Llo, characteristic of effector T cells, while the slowly proliferating T cells isolated from RAG−/− mice were CD44lo and CD62Lhi, characteristic of naïve T cells.
T cell proliferation in the MLNs is most likely driven by migratory DCs. Consistent with this possibility, we found that a population of CD11c+CD11b+CD103− cells infiltrated the MLNs of DKO mice, coincident with rapid T cell proliferation. This cell population in the colon consists of both CX3CR1hi macrophages and CX3CR1int dendritic cells (31). CX3CR1int cells infiltrate the colon during inflammation in this model (31), and become a migratory population that can prime T cell responses during DSS-induced colitis (39). Therefore, these data are consistent with a role for host IFNAR signaling in inhibiting the trafficking of monocyte-derived inflammatory DCs to the MLNs during T cell adoptive transfer colitis.
IL-1α, IL-1β and TNF-α can each drive the migration of tissue-resident DCs to the draining lymph node where they can prime T cells (48, 49). Although production of these cytokines was not increased from colon MPs from IFNAR1−/− mice, IFNAR1−/− MPs produced lower levels of IL-1RA, an inhibitor of IL-1 activity (Fig. 5 and data not shown). Accordingly, treatment of DKO mice with IL-1RA was able to inhibit the accumulation of both CD4+ T cells and the CD11c+CD11b+CD103− cell population in the MLNs of DKO mice. Thus, while others have found that IFN-1 inhibits inflammasome activation and IL-1β secretion (15), our data suggests that IFN-1 in the colon may control the IL-1 axis by inducing its negative regulator, IL-1RA. Also consistent with an effect of IFN-1 on blocking IL-1 function in vivo are the findings that Th17 cell differentiation and/or accumulation was enhanced in the absence of IFNAR signaling (Fig. 2D-E, 3C). IL-1β contributes to Th17 differentiation (50), and recent findings indicate that Th17 cells lacking the IL-1R fail to accumulate following transfer into RAG−/− hosts (40).
Constitutive IFN-1 signaling also conditioned colon MPs for the optimal production of IL-10 and IL-27 (Fig. 5). Local production of IL-10 by LP MPs is critical for both the expansion and maintenance of Foxp3+ Tregs in the intestine (41, 51). We previously identified a subset of colon macrophages that constitutively produce IL-10 via flora dependent and independent mechanisms (31). We demonstrate here an essential role for IFNAR signaling on colon MPs in inducing their production of IL-10. IL-27 potentiates IL-10 production by macrophages in response to LPS-induced IFN-1 signaling (52) and inhibits Th17 differentiation (13, 16). Thus, our finding that T cells transferred into DKO hosts were more likely to differentiate into IL-17-producing subsets may also be due to a lack of IL-27-mediated inhibition of Th17 differentiation. Furthermore, IL-27 was recently shown to be a key cytokine that specifically promotes the development and function of mucosal Tregs (53). Thus, poor production of IL-27, in addition to IL-10, may be responsible for the impaired maintenance of Tregs in the colon.
Our data also show a more important role for constitutive IFN-1 production in inducing anti-inflammatory cytokines than treatment with exogenous IFN-1. When IFNAR signaling was blocked using a monoclonal neutralizing antibody, FSL-1-induced IL-10 and IL-1RA production was reduced. FSL-1 does not induce IFN-1 (54), suggesting that the IFN-1 found in the in vitro cell stimulations was constitutively produced. Furthermore, exogenous IFN-1 did not augment the production of IL-10 or IL-1RA.
Finally, the fact that DKO BM chimeras (Fig. 3) and RAG−/− mice treated with anti-IFNAR1 antibodies (data not shown) also developed severe and accelerated colitis indicate a microbiome-independent role for IFN-1 in regulating intestinal inflammatory T cell responses. Because both RAG−/− and DKO BM chimeras were created using RAG−/− recipients, they were likely to have the same microbiome. Whether alterations in the microbiome are responsible for the more severe disease observed in the DKO mice when compared to the DKO BM chimeric mice is not yet clear and currently under investigation.
Taken together, our studies indicate for the first time that endogenous IFN-1 act on host innate immune cells to induce the production of anti-inflammatory cytokines by intestinal MPs and indirectly regulate the accumulation of both effector and regulatory T cells in the colon during the course of inflammation. The impaired ability of IFNAR1−/− colonic MP to produce IL-10, IL-1RA, and IL-27 in the context of normal production of pro-inflammatory cytokines provides an explanation for the poor expansion and maintenance of Tregs and enhanced expansion of effector T cells observed in the cLP of DKO mice during colitis. These data also suggest that further studies addressing the factors that drive physiological IFN-1 production and regulate their signaling in the colon will help to elucidate their pleiotropic effects on immune cells and lead to more targeted therapies for colitis and other inflammatory diseases.
Supplementary Material
Acknowledgements
The authors thank the NIAID Research Technologies Branch, especially Elina Stregevsky, for help with cell sorting, and Philip Murphy, Joshua Farber, Warren Strober, Fiona Powrie, and Yasmine Belkaid for their helpful discussions of the data.
Abbreviations used
- DKO
double knockout
- IFN-1
type I interferons
- IFNAR
interferon-alpha/beta receptor
- cLP
colon lamina propria
- MLN
mesenteric lymph node
- MP
mononuclear phagocytes
- Treg
regulatory T cell
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Research was supported by the Intramural Research Program of the NIAID, NIH.
References
- 1.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. The Journal of experimental medicine. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 3.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 4.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 5.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. The Journal of experimental medicine. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 7.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 8.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. The Journal of experimental medicine. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizza P, Capone I, Moretti F, Proietti E, Belardelli F. IFN-alpha as a vaccine adjuvant: recent insights into the mechanisms and perspectives for its clinical use. Expert review of vaccines. 2011;10:487–498. doi: 10.1586/erv.11.9. [DOI] [PubMed] [Google Scholar]
- 10.Chang EY, Guo B, Doyle SE, Cheng G. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. Journal of immunology. 2007;178:6705–6709. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
- 11.Molnarfi N, Hyka-Nouspikel N, Gruaz L, Dayer JM, Burger D. The production of IL-1 receptor antagonist in IFN-beta-stimulated human monocytes depends on the activation of phosphatidylinositol 3-kinase but not of STAT1. Journal of immunology. 2005;174:2974–2980. doi: 10.4049/jimmunol.174.5.2974. [DOI] [PubMed] [Google Scholar]
- 12.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Miyakawa Y, Fox N, Kaushansky K. Interferon-alpha directly represses megakaryopoiesis by inhibiting thrombopoietin-induced signaling through induction of SOCS-1. Blood. 2000;96:2093–2099. [PubMed] [Google Scholar]
- 15.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. Journal of immunology. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 18.Ling PD, Warren MK, Vogel SN. Antagonistic effect of interferon-beta on the interferon-gamma-induced expression of Ia antigen in murine macrophages. Journal of immunology. 1985;135:1857–1863. [PubMed] [Google Scholar]
- 19.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. The Journal of experimental medicine. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 21.Abe K, Nguyen KP, Fine SD, Mo JH, Shen C, Shenouda S, Corr M, Jung S, Lee J, Eckmann L, Raz E. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci U S A. 2007;104:17022–17027. doi: 10.1073/pnas.0708469104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radulovic K, Manta C, Rossini V, Holzmann K, Kestler HA, Wegenka UM, Nakayama T, Niess JH. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. Journal of immunology. 2012;188:2001–2013. doi: 10.4049/jimmunol.1100765. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann C, Dunger N, Grunwald N, Hammerling GJ, Hoffmann P, Scholmerich J, Falk W, Obermeier F. T cell-dependent protective effects of CpG motifs of bacterial DNA in experimental colitis are mediated by CD11c+ dendritic cells. Gut. 2010;59:1347–1354. doi: 10.1136/gut.2009.193177. [DOI] [PubMed] [Google Scholar]
- 25.Mannon PJ, Hornung RL, Yang Z, Yi C, Groden C, Friend J, Yao M, Strober W, Fuss IJ. Suppression of inflammation in ulcerative colitis by interferon-beta-1a is accompanied by inhibition of IL-13 production. Gut. 2011;60:449–455. doi: 10.1136/gut.2010.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pena Rossi C, Hanauer SB, Tomasevic R, Hunter JO, Shafran I, Graffner H. Interferon beta-1a for the maintenance of remission in patients with Crohn’s disease: results of a phase II dose-finding study. BMC Gastroenterol. 2009;9:22. doi: 10.1186/1471-230X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBDGC, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 29.te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:325–330. doi: 10.1002/ibd.20079. [DOI] [PubMed] [Google Scholar]
- 30.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. The Journal of experimental medicine. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. European journal of immunology. 2005;35:1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. The Journal of experimental medicine. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SE, Li X, Kim JC, Lee J, Gonzalez-Navajas JM, Hong SH, Park IK, Rhee JH, Raz E. Type I Interferons Maintain Foxp3 Expression and T-Regulatory Cell Functions under Inflammatory Conditions in Mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Current opinion in immunology. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colmegna I, Ohata BR, Menard HA. Current understanding of rheumatoid arthritis therapy. Clinical pharmacology and therapeutics. 2012;91:607–620. doi: 10.1038/clpt.2011.325. [DOI] [PubMed] [Google Scholar]
- 38.Liao Z, Grimshaw RS, Rosenstreich DL. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. The Journal of experimental medicine. 1984;159:126–136. doi: 10.1084/jem.159.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. The Journal of experimental medicine. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. Journal of immunology. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valatas V, He J, Rivollier A, Kolios G, Kitamura K, Kelsall BL. Host-dependent control of early regulatory and effector T-cell differentiation underlies the genetic susceptibility of RAG2-deficient mouse strains to transfer colitis. Mucosal immunology. 2012 doi: 10.1038/mi.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, Li MO, Cua DJ, McGeachy MJ. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. Journal of immunology. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 47.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 48.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. The Journal of experimental medicine. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nature immunology. 2013;14:246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Iyer SS, Ghaffari AA, Cheng G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. Journal of immunology. 2010;185:6599–6607. doi: 10.4049/jimmunol.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nature immunology. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.