Abstract
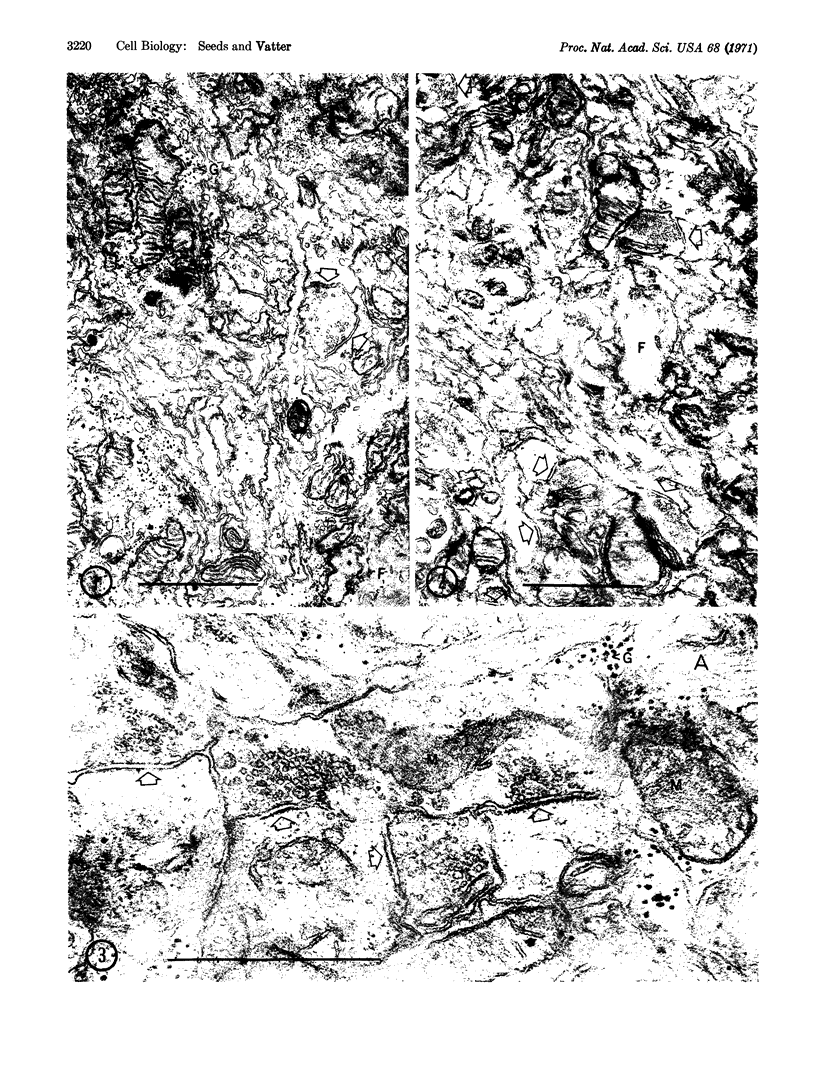
Dissociated cells from embryonic mouse brain reassociate in rotation culture to form highly organized aggregates. Electron microscopic observations of aggregates show the presence of synapses that mature and increase in number during culture. Apparent myelination of axons is observed in the aggregates after 5 weeks of culture. Thus, brain cell aggregates perform two highly specialized morphological events that are characteristic of mouse brain differentiation.
Keywords: embryonic mouse, electron microscopy, axons, synapses, myelin
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967 Dec;6(4):716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R. The onset of synapse formation in spinal cord cultures as studied by electron microscopy. Brain Res. 1967 Dec;6(4):728–749. doi: 10.1016/0006-8993(67)90129-1. [DOI] [PubMed] [Google Scholar]
- DeLong G. R. Histogenesis of fetal mouse isocortex and hippocampus in reaggregating cell cultures. Dev Biol. 1970 Aug;22(4):563–583. doi: 10.1016/0012-1606(70)90169-7. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synaptic potentials recorded in cell cultures of nerve and muscle. Science. 1970 Sep 25;169(3952):1331–1333. doi: 10.1126/science.169.3952.1331. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959 Oct;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. Development of specific neuronal connections. Science. 1969 Feb 7;163(3867):543–547. doi: 10.1126/science.163.3867.543. [DOI] [PubMed] [Google Scholar]
- Karlsson U. Observations on the postnatal development of neuronal structures in the lateral geniculate nucleus of the rat by electron microscopy. J Ultrastruct Res. 1967 Jan;17(1):158–175. doi: 10.1016/s0022-5320(67)80027-3. [DOI] [PubMed] [Google Scholar]
- Seeds N. W. Biochemical differentiation in reaggregating brain cell culture. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1858–1861. doi: 10.1073/pnas.68.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G. Norepinephrine stinulated increase of cyclic AMP levels in developing mouse brain cell cultures. Science. 1971 Oct 15;174(4006):292–292. doi: 10.1126/science.174.4006.292. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Fischman D. A., Moscona A. A. Formation of neuromuscular junctions in embryonic cell cultures. Proc Natl Acad Sci U S A. 1969 Mar;62(3):715–721. doi: 10.1073/pnas.62.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli A., Zacchei A. M., Caravita S., Cataldi A., Ieradi L. A. New-forming retinal synapses in vitro. Experientia. 1967 Mar 15;23(3):199–200. doi: 10.1007/BF02136284. [DOI] [PubMed] [Google Scholar]