Abstract
Aims
Guanylyl cyclase-cyclic guanosine monophosphate signalling plays an important role in endogenous cardioprotective signalling. The aim was to assess the potential of direct pharmacological activation and stimulation of soluble guanylyl cyclase, targeting different redox states of the enzyme, to limit myocardial necrosis during early reperfusion.
Methods and results
Rat isolated hearts were subjected to reversible left coronary artery occlusion (ischaemia-reperfusion) and infarct size was assessed by the tetrazolium staining technique. Administration during early reperfusion of BAY 41-2272, an NO-independent, haem-dependent stimulator of soluble guanylyl cyclase targeting the reduced state, or BAY 60-2770, an NO-independent, haem-independent activator targeting the oxidized state, significantly limited infarct size. Inhibition of NO synthesis did not abrogate this protection, but exogenous perfusion of NO with BAY 41-2272 produced a synergistic effect. The haem site oxidiser, ODQ abrogated the protection afforded by BAY 41-2272 but potentiated the protection afforded by BAY 60-2770. Targeting both the reduced and oxidized forms of sGC together did not afford additive protection.
Conclusions
Targeting either reduced or oxidized forms of sGC during early reperfusion affords cardioprotection, providing support for the concept that direct sGC manipulation at reperfusion has therapeutic potential for the management of acute myocardial infarction.
Keywords: Ischaemia-reperfusion, cGMP, NO, sGC
1. Introduction
Guanosine-3′,5′-cyclic monophosphate (cyclic guanosine monophosphate, cGMP) is a second messenger generated by soluble and particulate guanylyl cyclases (sGC and pGC). Manipulating cGMP signalling through natriuretic peptide receptors/pGC and NO/sGC can limit the development of myocardial ischaemia-reperfusion injury.1 Most pertinently, activation of these signalling pathways during the early phase of reperfusion is associated with marked limitation of infarct size,2–5 an effect mediated through elevation of intracellular cGMP levels and activation of protein kinase G (PKG).2
sGC is a heterodimeric protein which incorporates a prosthetic haem group, required for stimulation by NO, its major biological activator.6,7 It is known that the balance in the redox state of the haem moiety is shifted from a reduced (Fe2+) state to an NO-insensitive (Fe3+) state under conditions of oxidative stress.8–10 Since the bioavailability of NO may be severely compromised in disease states, especially those associated with oxidative stress,11 a range of directly acting, NO-independent activators and stimulators have been developed for the management of vascular diseases. These compounds include direct NO-independent stimulators of sGC, such as BAY 41-2272, which bind to the haem moiety in its normal Fe2+ state and stimulate GTP catalytic activity independently of NO. Synthetic haem-independent activators of sGC in the oxidized Fe3+ haem state include BAY 60-2770.8,12,13 It is assumed that the redox balance of sGC under conditions of ischaemia-reperfusion is shifted towards the oxidized form which is insensitive to NO stimulation.
Since there is evidence that cGMP signalling is a tractable target for limiting the injurious consequences of reperfusion, we set out to investigate the infarct-limiting properties of this unique class of compounds whose mechanism of action is to stimulate/activate sGC, thereby elevating cGMP levels independently of NO.14–19 The primary objective of the study was to evaluate the effects of agents that target either the reduced or oxidized/haem-free states of sGC. It was hypothesized that perfusion of a sGC stimulator, BAY 41-2272 or activator, BAY 60-2770 during early reperfusion would limit infarct size and this protection would be in part as a result of elevated cGMP concentrations in the myocardium.
2. Methods
2.1. Animals
Male Sprague-Dawley rats (300–350 g; from Charles River, UK; B&K Universal Ltd, UK; or Harlan UK Ltd, UK) were used for these studies. Their care and use were in accordance with the UK Home Office guidelines on the Animals (Scientific Procedures) Act 1986 (The Stationary Office London, UK) and approved by the Cardiff University ethics review board. Rats were housed in the institutional animal house under 12 h on/12 h off light cycles and allowed to acclimatize for at least 7 days with the standard chow containing 4% fat and 18% protein and water available ad libitum.
2.2. Experimental infarction and infarct size measurement
Rats were anaesthetized by pentobarbital sodium (175 mg/kg) with heparin (200 units) given concomitantly by i.p. injection. Hearts were excised and Langendorff perfused with modified Krebs–Henseleit buffer at constant pressure (74 mmHg) as previously described2 (see Supplementary material online for full details). Using a fluid filled balloon, left ventricular end-diastolic pressure (LVEDP) was set between 5 and 10 mmHg and isovolumic developed pressure continuously recorded (Chart, Powerlab data acquisition software, ADInstruments, UK). Coronary flow rate (CFR) was measured by collecting the coronary effluent from the apex of the heart. Before commencing any experimental protocol, hearts were left to equilibrate at 37°C for 10 min using a heated circulator.
Regional ischaemia was induced by placing a reversible suture and snare occluder around the left main coronary artery close to its origin. Ischaemia was confirmed by a CFR reduction of >30%. Hearts were subjected to 35 min coronary artery occlusion followed by 120 min reperfusion at 37°C. Following reperfusion the coronary artery was ligated and hearts were perfused with Evans' Blue dye to stain the non-ischaemic tissue and thereby delineate the ischaemic risk zone by dye exclusion. Hearts were then frozen at −20°C, sectioned transversely from the apex into 2 mm thick sections and incubated in 1% triphenyltetrazolium chloride in phosphate buffer (pH 7.4) at 37°C to determine the unstained necrotic region within the ischaemic risk zone. After fixing for 48 h in 4% formaldehyde, sections were imaged from both sides (ImageJ version 1.45q, NIH, USA) and a graphics tablet (Trust International B.V., Netherlands) was used to measure the total tissue area, risk zone, and necrotic zone of each section. These values were converted into volumes and combined to give total risk zone volume (R) and infarct size as a percentage of the risk zone (I/R %).
Hearts were included for analysis only if they met strict inclusion criteria during the experimental protocols. Hearts were excluded if they failed to develop sinus rhythm during stabilization, if heart rate was <200 b.p.m., baseline CFR >20 mL/min or if left ventricular developed pressure was <50 mmHg during stabilization. Regional ischaemia was confirmed by a CFR reduction >30% during coronary artery occlusion. Following release of the coronary artery snare, successful reperfusion was confirmed by a return towards baseline of CFR.
2.3. Myocardial cGMP assay
cGMP measurements were made in LV and RV myocardial tissue samples, harvested from hearts perfused in separate experiments as described below. Tissue samples were snap frozen, crushed, and immediately added to 200 μL lysis buffer containing 1 mM 3-isobutyl-1-methylxanthine. Samples were centrifuged for 60 min at 10 000 g. Supernatants were re-suspended in 400 μL 10% trichloroacetic acid and left for 30 min followed by a further centrifugation at 2500 g for 10 min. The supernatant was used for the cGMP measurements using the commercially available cyclic nucleotide radioimmunoassay kit (IBL International GMBH, Germany).
2.4. Treatment protocols
The following pharmacological agents were used in this study. BAY 41-2272 (Sigma-Aldrich, UK) is a sGC stimulator; BAY 60-2770 (gift from Bayer Pharma AG, Germany) is a sGC activator; NG-nitro-l-arginine methyl ester (l-NAME, Tocris Bioscience, UK) was used to inhibit NO synthase activity; 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, Tocris Bioscience) was used to selectively inhibit sGC; 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (C-PTIO, Tocris Bioscience) was used as a scavenger of free NO; 1-hydroxy-2-oxo-3-(N-methyl-6-aminohexyl)-3-methyl-1-triazene (NOC-9, Sigma-Aldrich) was used as NO donor. ODQ and BAY 41-2272 were dissolved in DMSO; the final maximum concentration of DMSO; in modified Krebs–Henseleit buffer was 0.05% v/v.
Following stabilization, all hearts were randomized to one of the experimental protocols, illustrated in Figure 1 (see Supplementary material online for details). All drug perfusions were started 5 min before release of the coronary artery snare until 10 min after reperfusion apart from l-NAME, C-PTIO, and ODQ which were perfused from 7 min before release of the snare. In Series 1, the infarct-limiting effects of BAY 41-2272 given at early reperfusion were examined and the relationship to NO and sGC redox state was interrogated by co-administration of l-NAME, C-PTIO, and ODQ. In Series 2, the contribution of exogenous NO was explored using NOC-9. In Series 3, the effects of BAY 60-2770 were examined to explore the targeting of the oxidized Fe3+ or haem-free state of sGC. Allied to each series, separate groups of hearts were prepared, without infarct size determination, for biochemical analysis of tissue cGMP concentration as described in Section 2.3.
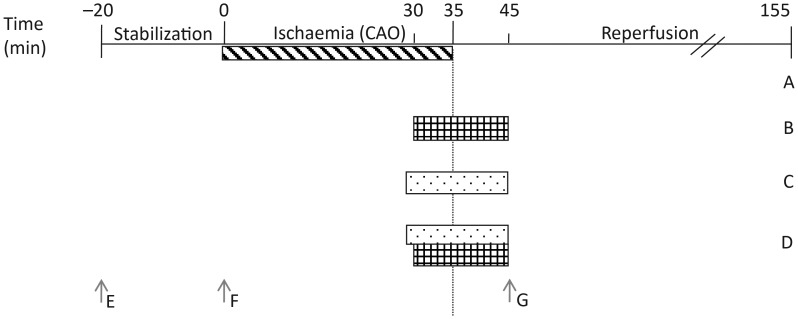
Figure 1.
Treatment protocol for isolated heart perfusion experiments and cGMP measurement sampling. Hearts used for infarct experiments were stabilized for 20 min, followed by 35 min of regional ischaemia and 120 min reperfusion. They were treated with one of four protocols. Control experiments were subjected to no pharmacological intervention (A). Hearts perfused with cGMP elevating compounds from 30 min ischaemia until 10 min reperfusion (B). Hearts perfused with inhibitors from 28 min ischaemia until 10 min reperfusion (C). Hearts perfused with both cGMP elevating compounds and inhibitors (D). Arrows indicate time at which tissue was sampled for cGMP analysis. Naive samples were excised, washed in Krebs–Henseleit (E). Stabilization samples were perfused for 20 min (F). Drug-treated and untreated reperfusion samples were subjected to 35 min left descending coronary artery occlusion and 10 min reperfusion (G).
2.5. Data analysis
Data are presented as means ± SEM and analysed using GraphPad Prism 5.0 (USA). Normal distribution of data was confirmed with the Kolmogorov–Smirnoff test. Differences in mean values for infarct size, cGMP concentrations, and baseline haemodynamic parameters were compared by one-way ANOVA and Newman–Keul's post hoc test. Correlation of cGMP concentration with infarct size was determined using Spearman's rank correlation coefficient. A P-value of <0.05 was considered significant.
3. Results
Two hundred and eighty-five rats were used for this study. In the infarct experiments, 19 hearts failed to meet one or more of the inclusion criteria and were excluded from further experimentation. There were no technical exclusions in the groups prepared for cGMP analysis. Thus, we report data from 205 infarct experiments and from 61 hearts prepared for cGMP analysis.
3.1. Cardioprotective effects of sGC stimulator BAY 41-2272
In Series 1, we investigated the infarct-limiting properties of the sGC stimulator BAY 41-2272 and explored its dependency on endogenous NO and its effects on myocardial cGMP concentration.
3.1.1. Infarct size
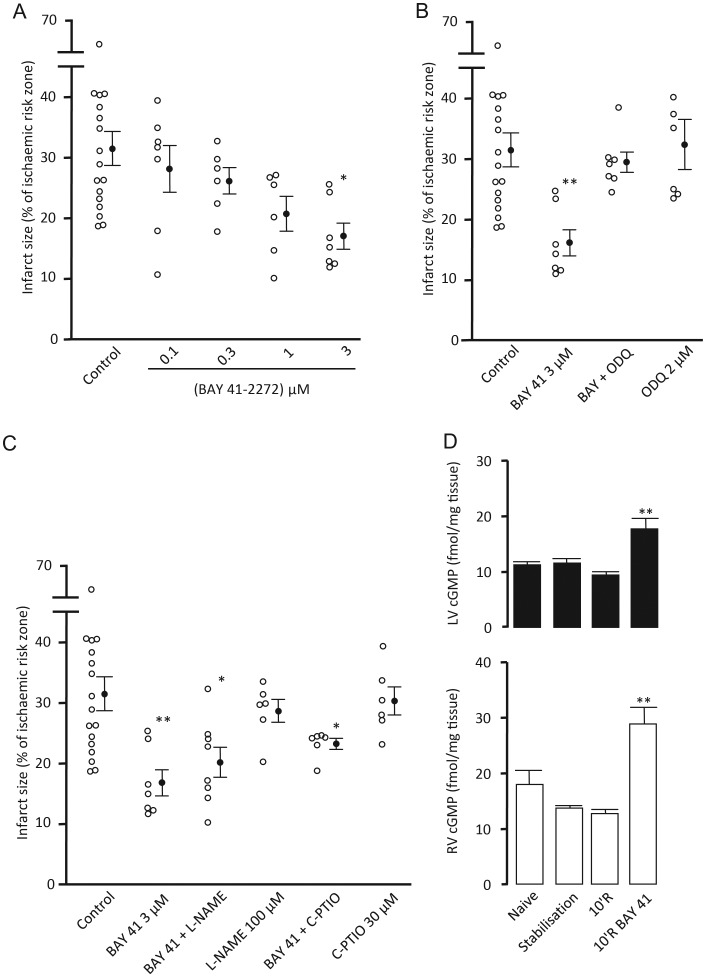
Baseline haemodynamic data are presented in Table 1. All experimental groups displayed comparable flow (CFR) and functional (HR, LVDP, RPP) parameters at the end of the pre-ischaemic stabilization period. The ischaemic area at risk of infarction for these groups was 44–55% of total myocardial volume with no statistically significant differences between groups. Under control conditions, infarct size was 31.5 ± 2.8% of the ischaemic risk zone. Treatment at reperfusion with BAY 41-2272 effected a concentration-dependent reduction in infarct size, maximal at 3 μM the highest concentration employed (17.0 ± 2.1%, P < 0.05) (Figure 2A). The sGC inhibitor, ODQ 2 μM, which oxidizes the haem group of the NO binding site in sGC, abrogated the infarct-limiting effect of BAY 41-2272 (29.6 ± 1.7%) confirming the need for the haem site to be in its reduced state, although ODQ had no effect on infarct size per se (Figure 2B). However, in the presence of the NO synthase inhibitor l-NAME 100 μM, infarct limitation was still afforded by BAY 41-2272 (20.5 ± 2.5%, P < 0.05 vs. control). In the presence of C-PTIO 30 μM, BAY 41-2272 also produced a significant limitation of infarct size (23.6 ± 0.9%, P < 0.05 vs. control). Neither l-NAME nor C-PTIO perfused alone had any effect on infarct size (Figure 2C).
Table 1.
Baseline cardiodynamic data for infarct experiments in series 1, 2, and 3
| Series | Treatment group (number) | n | CFR (mL/min) | HR (BPM) | LVDP (mmHg) | RPP (mmHg/min × 103) | Vol. LV and RV (cm3) | Risk zone vol. (cm3) | Risk zone (% vol. LV and RV) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Control (1) | 17 | 14.6 ± 1.0 | 272 ± 8 | 69.3 ± 4.3 | 18.9 ± 1.4 | 0.95 ± 0.03 | 0.39 ± 0.03 | 44.7 ± 2.4 |
| BAY 41 100 nM (2) | 7 | 17.3 ± 1.0 | 296 ± 12 | 62.7 ± 4.6 | 18.7 ± 1.7 | 0.87 ± 0.03 | 0.42 ± 0.04 | 47.9 ± 3.6 | |
| BAY 41 300 nM (3) | 6 | 15.3 ± 0.7 | 295 ± 10 | 59.7 ± 3.9 | 17.6 ± 1.1 | 0.87 ± 0.05 | 0.40 ± 0.06 | 45.7 ± 4.7 | |
| BAY 41 1 μM (4) | 6 | 18.1 ± 1.3 | 293 ± 11 | 69.2 ± 4.5 | 20.3 ± 1.5 | 0.91 ± 0.04 | 0.46 ± 0.08 | 49.2 ± 6.3 | |
| BAY 41 3 μM (5) | 7 | 17.1 ± 1.2 | 296 ± 18 | 69.7 ± 4.7 | 21.0 ± 2.6 | 0.95 ± 0.03 | 0.48 ± 0.04 | 50.1 ± 3.7 | |
| ODQ 2 μM + BAY 41 3 μM (6) | 7 | 15.7 ± 0.8 | 311 ± 10 | 80.0 ± 8.7 | 24.4 ± 2.0 | 1.01 ± 0.04 | 0.46 ± 0.03 | 46.4 ± 2.1 | |
| ODQ 2 μM (7) | 6 | 14.7 ± 1.2 | 315 ± 21 | 70.7 ± 10.5 | 21.7 ± 2.3 | 0.98 ± 0.02 | 0.44 ± 0.04 | 45.4 ± 3.3 | |
| l-NAME 100 μM + BAY 41 3 μM (8) | 8 | 15.3 ± 0.6 | 290 ± 9 | 68.4 ± 6.3 | 19.5 ± 1.3 | 0.96 ± 0.05 | 0.47 ± 0.03 | 49.9 ± 2.0 | |
| l-NAME 100 μM (9) | 6 | 13.8 ± 0.7 | 309 ± 17 | 61.2 ± 1.8 | 18.3 ± 1.0 | 0.89 ± 0.27 | 0.44 ± 0.03 | 50.6 ± 3.8 | |
| C-PTIO 30 μM + BAY 41 3 μM (10) | 6 | 16.8 ± 0.7 | 298 ± 6 | 63.1 ± 3.6 | 18.8 ± 1.0 | 1.07 ± 0.03 | 0.52 ± 0.03 | 49.7 ± 4.0 | |
| C-PTIO 30 μM (11) | 6 | 15.7 ± 0.7 | 313 ± 6 | 64.5 ± 4.8 | 20.2 ± 1.5 | 1.03 ± 0.05 | 0.54 ± 0.04 | 53.4 ± 4.3 | |
| 2 | Control (12) | 12 | 14.6 ± 0.6 | 313 ± 10 | 66.0 ± 6.6 | 20.4 ± 1.7 | 0.81 ± 0.04 | 0.35 ± 0.03 | 42.6 ± 2.9 |
| NOC-9 1 nM (13) | 6 | 14.9 ± 1.1 | 327 ± 11 | 69.9 ± 4.8 | 22.4 ± 1.4 | 0.81 ± 0.06 | 0.39 ± 0.03 | 48.2 ± 2.1 | |
| NOC-9 10 nM (14) | 6 | 15.2 ± 0.8 | 322 ± 8 | 70.1 ± 6.1 | 21.9 ± 1.2 | 0.83 ± 0.04 | 0.41 ± 0.02 | 49.4 ± 3.6 | |
| NOC-9 100 nM (15) | 6 | 14.4 ± 0.2 | 329 ± 14 | 63.9 ± 8.7 | 20.5 ± 1.9 | 0.82 ± 0.03 | 0.45 ± 0.02 | 54.6 ± 3.7 | |
| NOC-9 1 μM (16) | 9 | 15.9 ± 0.8 | 341 ± 4 | 69.0 ± 4.9 | 22.9 ± 1.8 | 0.79 ± 0.02 | 0.40 ± 0.02 | 50.6 ± 3.3 | |
| 3 | Control (17) | 18 | 16.8 ± 0.6 | 315 ± 6 | 65.9 ± 3.1 | 20.7 ± 0.9 | 0.82 ± 0.02 | 0.39 ± 0.02 | 47.9 ± 2.2 |
| BAY 60 5 nM (18) | 6 | 17.3 ± 1.0 | 319 ± 15 | 69.0 ± 5.0 | 22.1 ± 2.0 | 0.79 ± 0.03 | 0.36 ± 0.02 | 46.0 ± 2.1 | |
| BAY 60 50 nM (19) | 8 | 16.1 ± 1.0 | 331 ± 5 | 68.1 ± 4.0 | 22.5 ± 1.4 | 0.80 ± 0.02 | 0.40 ± 0.03 | 52.3 ± 3.3 | |
| BAY 60 500 nM (20) | 8 | 14.6 ± 0.6 | 322 ± 7 | 68.7 ± 3.7 | 22.1 ± 1.3 | 0.80 ± 0.05 | 0.34 ± 0.02 | 42.7 ± 1.8 | |
| BAY 60 1 μM (21) | 7 | 16.5 ± 1.1 | 336 ± 4 | 69.6 ± 4.8 | 23.4 ± 1.4 | 0.85 ± 0.02 | 0.46 ± 0.04 | 54.2 ± 4.1 | |
| ODQ 2 μM (22) | 6 | 16.9 ± 0.9 | 316 ± 7 | 61.9 ± 1.7 | 19.6 ± 0.7 | 0.85 ± 0.04 | 0.37 ± 0.03 | 55.8 ± 3.5 | |
| BAY 60 5 nM + ODQ 2 μM (23) | 7 | 15.5 ± 1.0 | 310 ± 7 | 69.5 ± 4.8 | 21.5 ± 1.7 | 0.86 ± 0.05 | 0.35 ± 0.05 | 43.1 ± 2.8 | |
| BAY 60 5 nM + C-PTIO 30 μM (24) | 6 | 14.1 ± 0.7 | 333 ± 4 | 73.9 ± 6.6 | 24.7 ± 2.3 | 0.84 ± 0.01 | 0.38 ± 0.03 | 45.6 ± 3.4 | |
| C-PTIO 30 μM (25) | 6 | 14.7 ± 0.8 | 320 ± 6 | 67.4 ± 7.5 | 21.5 ± 2.4 | 0.85 ± 0.05 | 0.35 ± 0.01 | 41.7 ± 2.9 | |
| BAY 41 1 μM (26) | 6 | 14.1 ± 0.6 | 313 ± 9 | 69.5 ± 4.4 | 21.6 ± 0.9 | 0.84 ± 0.02 | 0.32 ± 0.02 | 38.6 ± 1.6 | |
| BAY 60 5 nM + BAY 41 1 μM (27) | 6 | 15.2 ± 1.6 | 323 ± 9 | 68.6 ± 5.3 | 22.1 ± 1.8 | 0.84 ± 0.02 | 0.38 ± 0.02 | 45.5 ± 2.9 |
No statistical difference between groups within each series (P > 0.05).
CFR, coronary flow rate; HR, heart rate; LVDP, left ventricular developed pressure; RPP, rate pressure product (HR × LVDP); LV, left ventricle; RV, right ventricle.
Figure 2.
Infarct size data for BAY 41-2272 concentration response (A), concomitant perfusion of the haem site oxidiser ODQ (B) and NO inhibitors (C) expressed as infarct to-risk ratio %. Data are means ± SEM. *P < 0.05, **P < 0.01 vs. control (one-way ANOVA). Total cGMP concentrations in LV (solid) and RV (open) myocardial tissue samples (D). **P < 0.01 vs. respective reperfusion (10'R) control. (one-way ANOVA) n = 5–17.
3.1.2. Myocardial cGMP concentration
cGMP measurements for Series 1 are presented in Figure 2D. In parallel groups of hearts subjected to 35 min left coronary artery occlusion and 10 min reperfusion, treatment with BAY 41-2272 (from 5 min before until 10 min after reperfusion) elicited an approximately two-fold elevation of cGMP content in both left ventricle subjected to ischaemia-reperfusion (17.76 ± 1.87 vs. 9.44 ± 0.61 fmol/mg tissue, P < 0.01) and normoxically perfused right ventricle (28.79 ± 3.01 vs. 13.69 ± 0.50 fmol/mg tissue, P < 0.01). It is of interest to note that the cGMP content of naive (non-perfused) hearts was significantly higher in right ventricle than in left ventricle samples (17.87 ± 2.56 vs. 11.28 ± 0.54 fmol/mg tissue, P < 0.01).
3.2. Cardioprotective effects of exogenous NO
In Series 2, we explored the effects of exogenously administered NO, from the donor compound NOC-9, on limiting infarct size when administered during early reperfusion.
3.2.1. Infarct size
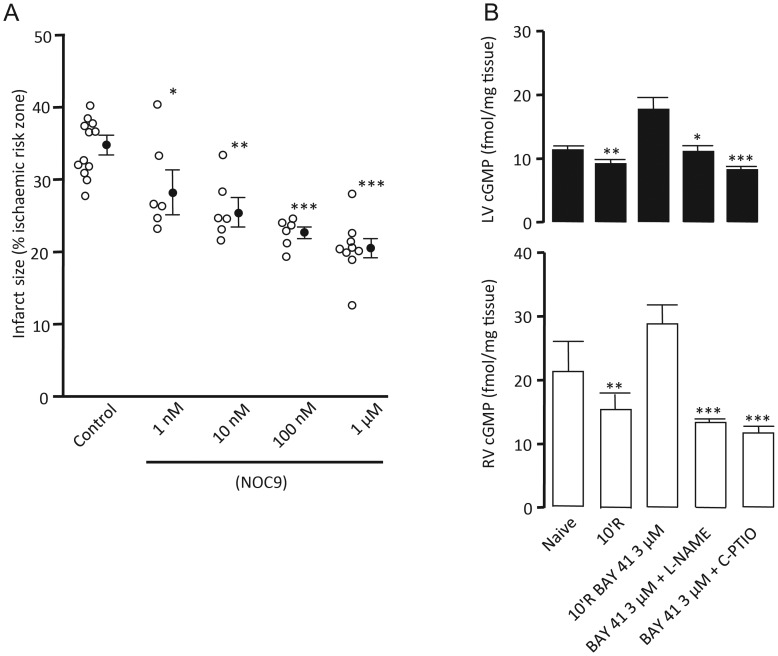
The baseline haemodynamic parameters and area at risk for all groups in this series were comparable between groups (Table 1). NOC-9 perfused across the concentration range 1 nM–1 μM at early reperfusion limited infarct size in a concentration-dependent manner from 34.7 ± 1.6% in control hearts to 20.5 ± 1.3% (P < 0.001) in hearts treated with NOC-9 at the highest concentration (Figure 3A).
Figure 3.
Infarct size data for NOC-9 concentration response (A), expressed as infarct to-risk ratio %. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control (one-way ANOVA). Total cGMP concentrations in LV (solid) and RV (open) myocardial tissue samples (B). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. 10'R BAY 41 (one-way ANOVA) n = 5–12.
3.2.2. Myocardial cGMP concentration
To further explore the relationship between BAY 41-2272-induced cardioprotection and NO, cGMP measurements were made in hearts perfused with BAY 41-2272 and concomitantly with either the NO synthase inhibitor l-NAME or the NO scavenger C-PTIO. Tissue samples from concomitant BAY 41-2272 and l-NAME perfused LV had cGMP levels 48% lower than those perfused with BAY 41-2272 alone (11.15 ± 0.91 vs. 17.76 ± 1.87 fmol/mg tissue, P < 0.05). Similarly, cGMP levels in LV tissue perfused with both C-PTIO and BAY 41-2272 were 54% lower than BAY 41-2272 alone (8.29 ± 0.52 vs. 17.76 ± 1.87 fmol/mg tissue, P < 0.001) (Figure 3B).
3.3. Cardioprotective effects of sGC activator BAY 60-2770
In Series 3, we investigated the infarct-limiting properties of sGC activation of BAY 60-2770 which targets the oxidized Fe3+ and haem-free forms of the protein.
3.3.1. Infarct size
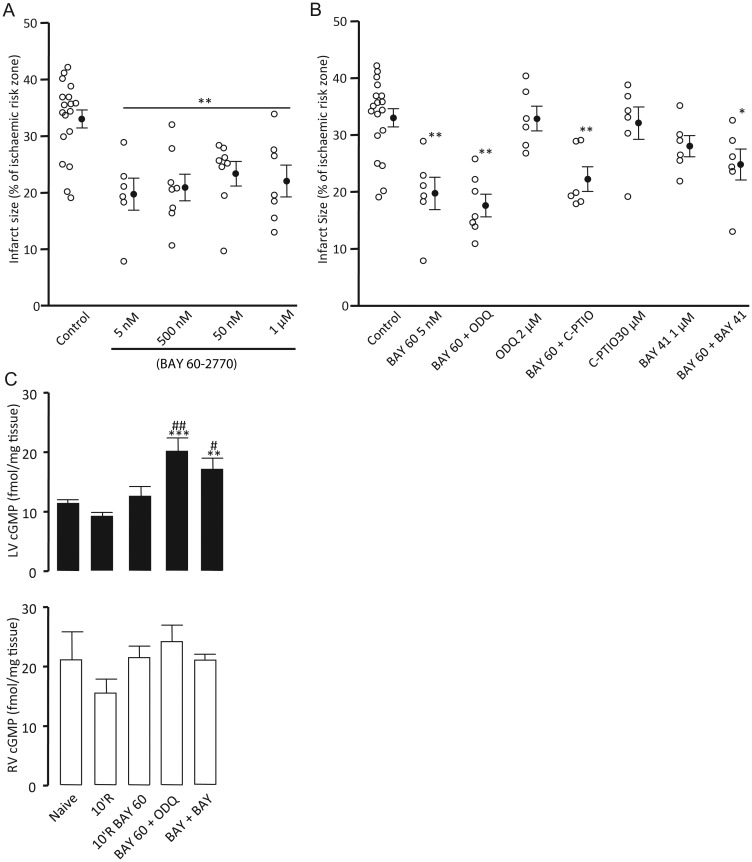
Baseline haemodynamic parameters and area at risk for all groups in this series were comparable between groups (Table 1). The sGC activator BAY 60-2770 afforded protection across the concentration range 5 nM–1 μM during early reperfusion from 33.0 ± 2.6% in control hearts to 22.0 ± 2.8% (P < 0.01) for hearts treated with the highest concentration. However, no concentration effect was observed (Figure 4A).
Figure 4.
Infarct size data for BAY 60-2770 concentration response (A), concomitant perfusion of the haem site oxidiser ODQ, NO scavenger C-PTIO, and BAY 41-2272 (B) expressed as infarct to-risk ratio %. Data are means ± SEM. *P < 0.05, **P < 0.01 vs. control (one-way ANOVA). Total cGMP concentrations in LV (solid) and RV (open) myocardial tissue samples (C). **P < 0.01 and ***P < 0.001 vs. 10'R BAY 60. #P < 0.05 and ##P < 0.01 vs. 10'R (one-way ANOVA) n = 5–18.
To confirm the haem independence of BAY 60-2770's action further hearts were co-perfused with 2 μM ODQ. Concomitant perfusion of ODQ with the lowest concentration of BAY 60-2770 (5 nM) limited infarct size to 17.6 ± 2.0% (P < 0.01 vs. control) (Figure 4B). Perfusion with ODQ 2 μM alone did not afford protection (32.9 ± 2.2%) (Figure 4B), an effect comparable with that seen in Series 1. Reperfusion with the NO scavenger C-PTIO produced infarct sizes similar to controls (32.0 ± 2.8%) (Figure 4B). Furthermore, C-PTIO did not abrogate the protection afforded by BAY 60-2770 (22.2 ± 2.2 vs. 33.0 ± 2.6%, P < 0.01) (Figure 4B). To investigate the protective effect of targeting both the reduced and oxidized/haem-free forms of sGC, we concomitantly perfused BAY 60-2770 5 nM and BAY 41-2272 1 μM. This combination resulted in only a modest 21% reduction in infarct size (24.8 ± 2.7%, P < 0.05 vs. control) (Figure 4B).
3.3.2. Myocardial cGMP concentration
Tissue levels of cGMP were measured in hearts that had been perfused with or without BAY 60-2770 and subjected to 35 min regional ischaemia. Measurements were also made in hearts perfused with BAY 60-2770 concomitantly with ODQ or a sub-maximal concentration of BAY 41-2272. In LV samples, cGMP levels were not elevated in tissue that had been perfused with BAY 60-2770 compared with samples that had not (12.60 ± 1.65 vs. 9.20 ± 0.70 fmol/mg tissue). Tissue samples perfused with concomitant BAY 60-2770 and ODQ had cGMP levels 60% higher than those perfused with BAY 60-2770 alone (20.16 ± 2.25 vs. 12.60 ± 1.65 fmol/mg tissue, P < 0.01). An increase of 36% was also seen in LV samples perfused with both the sGC stimulator and activator compared with the activator alone (17.11 ± 1.90 vs. 12.60 ± 1.65 fmol/mg tissue, P < 0.05), an increase of 86% compared with untreated hearts (17.11 ± 1.90 vs. 9.20 ± 0.70 fmol/mg tissue, P < 0.05) (Figure 4C).
4. Discussion
The principal findings of these studies can be summarized as follows.
Targeting the reduced form of sGC during early reperfusion with the stimulator BAY 41-2272 afforded concentration-dependent infarct limitation and this protection was independent of endogenous NO, demonstrated with concomitant perfusion of BAY 41-2272 and the NOS inhibitor l-NAME or NO scavenger C-PTIO. Tissue cGMP concentrations during early reperfusion were elevated by the sGC stimulator. The observed elevations were partly independent of NO.
Perfusion of the NO donor NOC-9 during early reperfusion demonstrated concentration-dependent infarct limitation. Targeting the oxidized state of sGC with BAY 60-2770 also afforded infarct limitation. However, the protection observed was not increased beyond that seen with the sGC stimulator. Concomitant perfusion of both the sGC stimulator and activator did not afford greater protection than either treatment alone.
Concomitant perfusion of BAY 60-2770 and ODQ elevated total cGMP concentration beyond BAY 60-2770 only perfused myocardium.
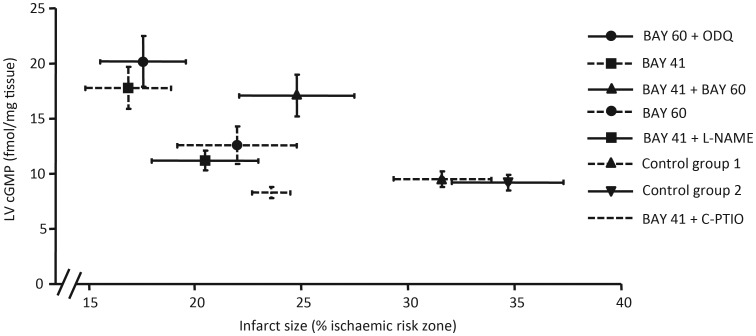
Infarct size and left ventricular cGMP content measured at 10 min reperfusion analysed for correlation did not meet significance, suggesting that elevation of total LV cGMP levels are not imperative to afford protection (Figure 5).
Figure 5.
Analysis of infarct size and total LV cGMP concentration in corresponding experimental groups. Spearman's rank correlation coefficient test did not reach significance with an r-value of −0.7 (P = 0.07). SEM shown for both infarct size and LV cGMP concentrations.
NO/sGC/cGMP signalling during early reperfusion has been shown to be a tractable target to limit infarct size. However, the literature is inconsistent in reporting the protective effects of NO donors.2,20,21 Elevating cGMP levels during the first minutes of reperfusion is recognized as a contributory mechanism in the reperfusion injury salvage kinase signalling paradigm.1,22,23 Previous work by us and others has documented that administration of natriuretic peptides during early reperfusion limits infarct size in both the rabbit and rat.2–4,22 Furthermore, this protection was mediated through elevated cGMP concentration and PKG-dependent mechanisms. The sGC activator BAY 58-2667 (Cinaciguat) has been shown to limit infarct size at reperfusion, again by elevating cGMP concentrations.20,23,24 This demonstrates that the oxidized and/or haem-free forms of sGC are present in the myocardium during reperfusion and can be targeted to elevate cGMP.
To explore the infarct-limiting properties of pharmacological agents that elevate cGMP, we used both a sGC stimulator and a sGC activator. Our initial results demonstrated that perfusion of the sGC stimulator during early reperfusion limited infarct size in a concentration-dependent manner. It was then demonstrated that the protection afforded was at least in part as a result of elevated cGMP levels during early reperfusion. To characterize the mechanistic action of BAY 41-2272 further, ODQ was concomitantly perfused which abrogated the protection afforded by the stimulator alone. This is in agreement with the cell-based studies reported by Stasch et al.14 who reported that sGC stimulation by BAY 41-2272 was haem-dependent requiring the haem moiety to be in the Fe2+ state for enzymatic activity.19,25 The relaxant effects of BAY 41-2272 to rat tracheal rings was dampened in preparations pre-incubated with l-NAME or ODQ.26 To investigate the NO component of BAY 41-2272 action, the sGC stimulator was perfused concomitantly with either l-NAME or C-PTIO. In both instances the infarct-limiting effects of BAY 41-2272 could not be completely abrogated, confirming that in terms of infarct limitation, the stimulator affords protection independently of NO. The results do however suggest that there is an NO component to the protection observed. Stasch et al.14 reported that PTIO at high concentrations could not block sGC stimulation.
Since reports that exogenous NO can afford infarct limitation when perfused during early reperfusion have been inconsistent, we carried out experiments using NOC-9, a rapid release NO donor.27 We were able to demonstrate a concentration-dependent action of the NO donor, limiting infarct size by half at the highest concentration. The current data are supported by previous experiments that demonstrate the need to recruit eNOS in reperfusion salvage.3,20
Using the sGC activator BAY 60-2770, we investigated the infarct-limiting effects of targeting what may be regarded as a pathological state of the enzyme. Cohen et al.20 and Krieg et al.23 demonstrated that perfusion of the structurally similar BAY 58-2667 could limit infarct size in both the rat and the rabbit in a global model of ischaemia-reperfusion. Our results support this work and confirm that there indeed must be a component of the so-called pathological sGC present during early reperfusion. Infarct size limitation reported by Cohen et al.20 and Krieg et al.23 was much more marked than in the present study, matched by a larger percentage increase in cGMP. In contrast to our own studies; however, the basal concentrations of cGMP measured were much lower. Additionally, we perfused hearts with the sGC activator concomitantly with ODQ. These results confirm the haem-independent action of BAY 60-2770 and suggest that the concomitant therapy limited infarct size beyond the activator alone. More convincing were the cGMP measurements made in comparable experiments which showed that oxidizing the haem group with ODQ rendered the enzyme more sensitive to the activator, demonstrated by the larger elevation of total LV cGMP following concomitant perfusion.
To explore the benefits of targeting both redox states of sGC during early reperfusion, we co-perfused submaximal concentrations of both the sGC stimulator and the activator to ascertain whether targeting both redox states could afford greater infarct limitation than either treatment alone. Our results demonstrate that in terms of infarct limitation, concomitant perfusion of both compounds does not potentiate the protection observed by the other compound. Furthermore, infarct size was greater when both compounds were perfused together compared with the sGC activator alone. A possible explanation for these results could lie in physical or chemical interactions of the compounds at the target site on sGC. Although the sites of action of both compounds differ, they may impede each other's action when in close proximity. However, the subsequent results refute this speculation with elevation of LV cGMP measured in comparable experiments. Concentrations were significantly elevated above those of BAY 60-2270 only treated hearts, comparable with those of hearts perfused with the sGC stimulator BAY41-2272. These data suggest that both compounds were able to elicit a response in the presence of the other. Previous work from our laboratory demonstrated that the cGMP analogue 8-Br-cGMP had a deleterious effect on infarct size at high concentrations, suggesting that excess cGMP may inhibit the protective effect of lower concentrations.22
For the first time, we plotted total LV cGMP levels against infarct size across a range of corresponding treatment groups (Figure 5). Although there is a trend towards infarct limitation with increased total LV cGMP concentration, correlation analysis did not reach significance (P = 0.07). This data set suggests that affording protection goes beyond gross elevation of LV cGMP. Most surprising are the results for LV cGMP measurements in hearts perfused concomitantly with BAY 41-2272 and either l-NAME or C-PTIO. Although a modest protective state is achieved, cGMP levels are not elevated above baseline. This suggests that the gross elevation seen in BAY 41-2272 only treated hearts requires the presence of NO. Similarly, hearts protected with BAY 60-2770 did not show a significant elevation in total LV cGMP, whereas those perfused concomitantly with ODQ did.
There is a growing body of evidence to suggest that cGMP is compartmentalized within discrete subcellular domains, regulated by specific PDEs and guanylyl cyclases.28,29 We can only speculate that affording infarct limitation by targeting cGMP requires specific targeting of discrete pools and not gross LV elevation. What we may be missing in the current data set is that total LV cGMP may not be elevated, yet specific protective pools maybe, without net elevation. Conversely, the ability to resolve local protective cGMP elevation may be masked in groups where total LV cGMP is elevated. This has led us to make reference to the notion that there may be specific ‘cytoprotective cGMP pools’. This thesis is yet to be explored in the ischaemia-reperfusion setting as the ability to visualize/quantify changes in discrete subcellular cGMP is in its infancy. In addition, how the redox balance of sGC shifts during early reperfusion and how it affects distribution and production of cGMP would also be worthy of exploration and support this thesis.30
In conclusion, these studies demonstrate that targeting sGC during early reperfusion following simulated AMI is a tractable target to limit infarct size. Using cGMP elevating compounds provides a more specific target in comparison with NO donors which are associated with vascular tolerance and non-desirable effects.11,31 The current study corroborates in vitro mechanistic studies. These data do not directly support the hypothesis that targeting the so-called pathological state of sGC is a more attractive target during early reperfusion; nevertheless, quantifying the spatial differences of the sGC redox state, the extent of shift during the critical moments and the specific localized changes in cGMP concentration remains desirable.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: J.-P.S. is currently a full-time employee at Bayer Pharma AG. J.-P.S. holds more than 60 patent applications related to sGC stimulators, such as BAY 41-2272, and sGC activators, such as BAY 58-2667.
Funding
This work was supported by a postgraduate studentship awarded by Cardiff University. Funding to pay the Open Access publication charges for this article was provided by Cardiff University.
References
- 1.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Brit J Pharmacol. 2007;152:855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burley DS, Baxter GF. B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol. 2007;102:529–541. doi: 10.1007/s00395-007-0672-1. [DOI] [PubMed] [Google Scholar]
- 3.Yang XM, Philipp S, Downey JM, Cohen MV. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol. 2006;101:311–318. doi: 10.1007/s00395-006-0587-2. [DOI] [PubMed] [Google Scholar]
- 4.Ren B, Shen Y, Shao H, Qian J, Wu H, Jing H. Brain natriuretic peptide limits myocardial infarct size dependent of nitric oxide synthase in rats. Clin Chim Acta. 2007;377:83–87. doi: 10.1016/j.cca.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Yang X-M, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3–kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerzer R, Bohme E, Hofmann F, Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981;132:71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro LJ, Degnan JN, Baricos WH, Kadowitz PJ, Wolin MS. Activation of purified guanylate cyclase by nitric oxide requires heme. Comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung . Biochim Biophys Acta. 1982;718:49–59. doi: 10.1016/0304-4165(82)90008-3. [DOI] [PubMed] [Google Scholar]
- 8.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, Arun Kumar HS, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Pyriochou A, Kotanidou A, Dalkas G, van Eickels M, Spyroulias G, et al. Soluble guanylyl cyclase activation by HMR-1766 (ataciguat) in cells exposed to oxidative stress. Am J Physiol Heart Circ Physiol. 2008;295:H1763–H1771. doi: 10.1152/ajpheart.51.2008. [DOI] [PubMed] [Google Scholar]
- 10.Roy B, Mo E, Vernon J, Garthwaite J. Probing the presence of the ligand-binding haem in cellular nitric oxide receptors. Br J Pharmacol. 2008;153:1495–1504. doi: 10.1038/sj.bjp.0707687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andelova E, Bartekova M, Pancza D, Styk J, Ravingerova T. The role of NO in ischemia/reperfusion injury in isolated rat heart. Gen Physiol Biophys. 2005;24:411–426. [PubMed] [Google Scholar]
- 12.Schindler U, Strobel H, Schönafinger K, Linz W, Löhn M, Martorana PA, et al. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol. 2006;69:1260–1268. doi: 10.1124/mol.105.018747. [DOI] [PubMed] [Google Scholar]
- 13.Stasch JP, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Minuth T, et al. Pharmacological actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41–8543: in vitro studies. Br J Pharmacol. 2002;135:333–343. doi: 10.1038/sj.bjp.0704484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- 15.Stasch JP, Dembowsky K, Perzborn E, Stahl E, Schramm M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41–8543: in vivo studies. Br J Pharmacol. 2002;135:344–355. doi: 10.1038/sj.bjp.0704483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker E, Alonso-Alija C, Apeler H, Gerzer R, Minuth T, Pleibeta U, et al. NO-independent regulatory site of direct sGC stimulators like YC-1 and BAY 41–2272. BMC Pharmacol. 2001;1:13. doi: 10.1186/1471-2210-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galle J, Zabel U, Hubner U, Hatzelmann A, Wagner B, Wanner C, et al. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straub A, Benet-Buckholz J, Frode R, Kern A, Kohlsdorfer C, Schmitt P, et al. Metabolites of orally active NO-independent pyrazolopyridine stimulators of soluble guanylate cyclase. Bioorg Med Chem. 2002;10:1711–1717. doi: 10.1016/s0968-0896(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 19.Straub A, Stasch JP, Alonso-Alija C, Benet-Buchholz J, Ducke B, Feurer A, et al. NO-independent stimulators of soluble guanylate cyclase. Bioorg Med Chem Lett. 2001;11:781–784. doi: 10.1016/s0960-894x(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MV, Yang X-M, Liu Y, Solenkova NV, Downey JM. Cardioprotective PKG-independent NO signaling at reperfusion. Am J Physiol-Heart C. 2010;299:H2028–H2036. doi: 10.1152/ajpheart.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ovize M, Baxter GF, Di Lisa F, Ferdinandy Pt, Garcia-Dorado D, Hausenloy DJ, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position Paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 22.D'Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, et al. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol. 2003;284:H1592–H1600. doi: 10.1152/ajpheart.00902.2002. [DOI] [PubMed] [Google Scholar]
- 23.Krieg T, Liu Y, Rutz T, Methner C, Yang XM, Dost T, et al. BAY 58–2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur Heart J. 2009;30:1607–1613. doi: 10.1093/eurheartj/ehp143. [DOI] [PubMed] [Google Scholar]
- 24.Salloum FN, Das A, Samidurai A, Hoke NN, Chau VQ, Ockaili RA, et al. Cinaciguat, a novel activator of soluble guanylate cyclase, protects against ischemia/reperfusion injury: role of hydrogen sulfide. Am J Physiol-Heart C. 2012;302:H1347–H1354. doi: 10.1152/ajpheart.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt P, Schramm M, Schroder H, Stasch JP. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol. 2003;468:167–174. doi: 10.1016/s0014-2999(03)01674-1. [DOI] [PubMed] [Google Scholar]
- 26.Toque HA, Mónica FZT, Morganti RP, De Nucci G, Antunes E. Mechanisms of relaxant activity of the nitric oxide-independent soluble guanylyl cyclase stimulator BAY 41–2272 in rat tracheal smooth muscle. Eur J Pharmacol. 2010;645:158–164. doi: 10.1016/j.ejphar.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Keefer LK, Nims RW, Davies KM, Wink DA. ‘NONOates’ (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 28.Stangherlin A, Zaccolo M. cGMP-cAMP interplay in cardiac myocytes: a local affair with far-reaching consequences for heart function. Biochem Soc Trans. 2012;40:11–14. doi: 10.1042/BST20110655. [DOI] [PubMed] [Google Scholar]
- 29.Castro LR, Schittl J, Fischmeister R. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010;107:1232–1240. doi: 10.1161/CIRCRESAHA.110.226712. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann LS, Schmidt PM, Keim Y, Hoffmann C, Schmidt HHHW, Stasch J-P. Fluorescence dequenching makes haem-free soluble guanylate cyclase detectable in living cells. PLoS One. 2011;6:e23596. doi: 10.1371/journal.pone.0023596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gori T, Parker JD. The puzzle of nitrate tolerance: pieces smaller than we thought? Circulation. 2002;106:2404–2408. doi: 10.1161/01.cir.0000036742.52907.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.